Performance of Alternative Methane Reforms Based on Experimental Kinetic Evaluation and Simulation in a Fixed Bed Reactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Numerical Method
3. Kinetics and Reactor Modeling
3.1. Kinetics of Reforming Processes
3.2. Modeling of the Fixed Bed Reactor
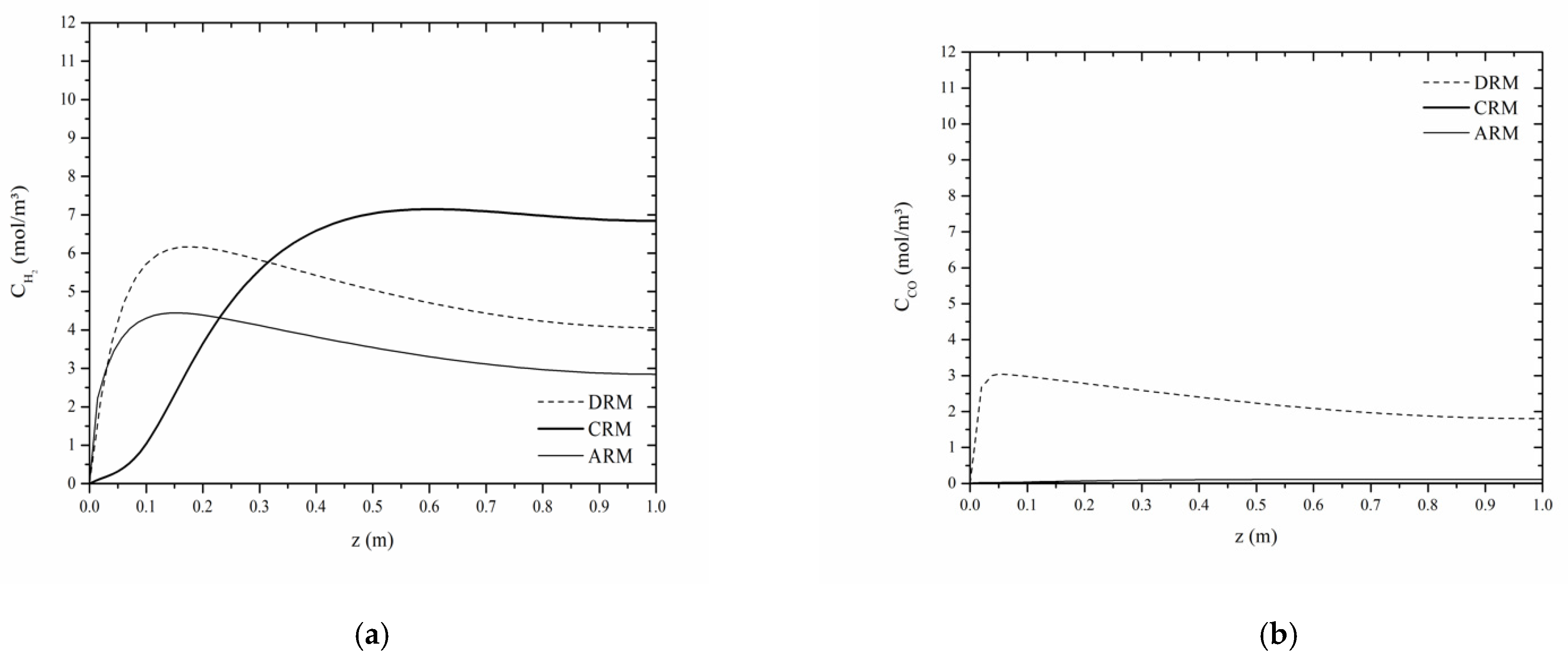
4. Results and Discussion
5. Conclusions
- The evolution of the concentration of reagents and products increase and are similar between them, with the reagents evolving in the reactor earlier, and the products afterwards;
- The reagent profiles decrease, while the product profiles increase, reaching higher levels of concentration in the outlet sector of the reactor;
- Carbon yields can be predicted at low levels, where the reaction steps involving its production are compensated by its consumption, according to interactions with O2, CO2 and H2O.
- Methane is always consumed in the operations of the three reforms; this consumption occurs at the CRM via three reaction steps, with a predominance of the order of magnitude 102 of the specific reaction rates compared to the orders of 10−4 and 10−1 in the operations of the DRM and ARM reforms;
- Hydrogen production, considering the steps that involve consumption in each reform process, have their specific rates in the following orders of magnitude: 10−4 DRM, 10−1 RAM, and 102 RCM, considering, respectively, one step, three steps, and two reaction steps;
- The production of carbon monoxide occurs more quickly and at higher levels in DRM operations where its conversion is not verified, and the referred production occurs through two reaction steps.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Armor, J.N.; Martenak, D.J. Studying carbon formation at elevated pressure. Appl. Catal. A Gen. 2001, 206, 231–236. [Google Scholar] [CrossRef]
- Rostrupnielsen, J.; Hansen, J.H.H.T.A.B. CO2-Reforming of Methane over Transition Metals. J. Catal. 1993, 144, 38–49. [Google Scholar] [CrossRef]
- Tomishige, K.; Yamazaki, O.; Chen, Y.; Yokoyama, K.; Li, X.; Fujimoto, K. Development of ultra-stable Ni catalysts for CO2 reforming of methane. Catal. Today 1998, 45, 35–39. [Google Scholar] [CrossRef]
- Takenaka, S.; Ogihara, H.; Yamanaka, I.; Otsuka, K. Decomposition of methane over supported-Ni catalysts: Effects of the supports on the catalytic lifetime. Appl. Catal. A Gen. 2001, 217, 101–110. [Google Scholar] [CrossRef]
- Abreu, C.A.M.; Santos, D.A.; Pacífico, J.A.; Filho, N.M.L. Kinetic Evaluation of Methane−Carbon Dioxide Reforming Process Based on the Reaction Steps. Ind. Eng. Chem. Res. 2008, 47, 4617–4622. [Google Scholar] [CrossRef]
- Souza, A.E.A.M.; Maciel, L.J.L.; Cavalcanti-Filho, V.O.; Filho, N.M.L.; Abreu, C.A.M. Kinetic-Operational Mechanism to Autothermal Reforming of Methane. Ind. Eng. Chem. Res. 2011, 50, 2585–2599. [Google Scholar] [CrossRef]
- Maciel, L.J.L.; Souza, A.E.A.M.; Vasconcelos, S.M.; Knoechelmann, A.; Abreu, C.A.M. Dry reforming and partial oxidation of natural gas to syngas production. Stud. Surf. Sci. Catal. 2007, 167, 469–474. [Google Scholar]
- Singh, R.; Dhir, A.; Mohapatra, S.K.; Mahla, S.K. Dry reforming of methane using various catalysts in the process. Biomass Convers. Biorefinery 2020, 10, 567–587. [Google Scholar] [CrossRef]
- Song, Y.; Ozdemir, E.; Ramesh, S.; Adishev, A.; Subramanian, S.; Harale, A.; Albuali, M.; Fadhel, B.A.; Jamal, A.; Moon, D.; et al. Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science 2020, 367, 777–781. [Google Scholar] [CrossRef] [PubMed]
- Luneau, M.; Gianotti, E.; Guilhaume, N.; Landrivon, E.; Meunier, F.C.; Mirodatos, C.; Schuurman, Y. Experiments and Modeling of Methane Autothermal Reforming over Structured Ni–Rh-Based Si-SiC Foam Catalysts. Ind. Eng. Chem. Res. 2017, 56, 13165–13174. [Google Scholar] [CrossRef]
- Chen, J.; Li, L. Mechanism of the autothermal reforming reaction of methane on Pt(1 1 1) surfaces: A density functional theory study. Appl. Surf. Sci. 2021, 539, 148288. [Google Scholar] [CrossRef]
- Lino, A.V.P.; Assaf, E.M.; Assaf, J.M. Adjusting Process Variables in Methane Tri-reforming to Achieve Suitable Syngas Quality and Low Coke Deposition. Energy Fuels 2020, 34, 16522–16531. [Google Scholar] [CrossRef]
- Lino, A.V.P.; Calderon, Y.N.C.; Mastelaro, V.R.; Assaf, E.M.; Assaf, J.M. Syngas for Fischer-Tropsch synthesis by methane tri-reforming using nickel supported on MgAl2O4 promoted with Zr, Ce and Ce-Zr. Appl. Surf. Sci. 2019, 481, 747–760. [Google Scholar] [CrossRef]
- Chen, L.; Gangadharan, P.; Lou, H.H. Sustainability assessment of combined steam and dry reforming versus tri-reforming of methane for syngas production. Asia Pac. J. Chem. Eng. 2018, 13, e2168. [Google Scholar] [CrossRef]
- Dias, A.C.J.; Assaf, J.M. The advantages of air addition on the methane steam reforming over Ni/γ-Al2O3. J. Power Sources 2004, 137, 264–268. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, G.; Dong, H.; Yang, W. Effect of carbon dioxide on the reaction performance of partial oxidation of methane over a LiLaNiO/γ-Al2O3 catalyst. Appl. Catal. A Gen. 2000, 202, 141–146. [Google Scholar] [CrossRef]
- Larentis, A.L.; de Resende, N.S.; Salim, V.M.M.; Pinto, J.C. Modeling and optimization of the combined carbon dioxide reforming and partial oxidation of natural gas. Appl. Catal. A Gen. 2001, 215, 211–224. [Google Scholar] [CrossRef]
- Lu, Y.; Xue, J.; Yu, C.; Liu, Y.; Shen, S. Mechanistic investigation on the partial oxidation of methane to syngas over a nickel-on-alumina catalyst. Appl. Catal. A Gen. 1998, 174, 121–128. [Google Scholar] [CrossRef]
- Hong-Tao, J.; Hui-Quan, L.; Yi, Z. Tri-reforming of methane to syngas over Ni/Al2O3-Thermal distribution in the catalyst bed. J. Fuel Chem. Technol. 2007, 35, 72–78. [Google Scholar]
- Lee, S.-H.; Cho, W.; Ju, W.-S.; Cho, B.-H.; Lee, Y.-C.; Baek, Y.-S. Tri-reforming of CH4 using CO2 for production of synthesis gas to dimethyl ether. Catal. Today 2003, 87, 133–137. [Google Scholar] [CrossRef]
- Seo, Y.S.; Shirley, A.; Kolaczkowski, S.T. Evaluation of thermodynamically favorable operating conditions for production of hydrogen in three different reforming technologies. J. Power Sources 2002, 108, 213–225. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Process; John Wiley & Sons: Hoboken, NJ, USA, 1984. [Google Scholar]
- Villermaux, J. Génie de la Reaction Chimique: Conception et Fonctionement des Reactors; Technique et Documentation (Lavoisier), 2ͣ triage: Paris, France, 1982. [Google Scholar]
- Valentini, A.; Carreno, N.L.V.; Leite, E.R.; Goncalves, R.F.; Soledade, L.E.B.; Maniette, Y.; Longo, E.; Probst, L.F.D. Improved activity and stability of Ce-promoted Ni/gamma-Al2O3 catalysts for carbon dioxide reforming of methane. Lat. Am. Appl. Res. 2004, 34, 165–172. [Google Scholar]
- Maluf, S.S.; Assaf, E.M.; Assaf, J.M. Catalisadores Ni/Al2O3 promovidos com molibdênio para a reação de reforma a vapor de metano. Quím. Nova 2003, 26, 181–187. [Google Scholar] [CrossRef][Green Version]


| Step (i) | Chemical Equation | Reaction |
|---|---|---|
| 1 | CH4 → C + 2H2 | Methane cracking |
| 2 | C + CO2 → 2CO | Boudouard reverse reaction |
| 3 | CO2 + H2 → CO + H2O | Reverse water gas shift reaction |
| Step (i) | Chemical Equation | Reaction |
|---|---|---|
| 1 | CH4 + 1/2O2 → CO + 2H2 | Partial oxidation of methane |
| 2 | CH4 + 2H2O → CO2 + 4H2 | Steam reforming of methane |
| 3 | CO2 + H2 → CO + H2O | Reverse reaction WGS |
| 4 | CH4 → C + 2H2 | Methane cracking |
| 5 | C + CO2 → 2CO | Boudouard reverse reaction |
| 6 | C + O2 → CO2 | Carbon gasification |
| Step (i) | Chemical Equation | Reaction |
|---|---|---|
| 1 | CH4 +5/8 O2 ↔ CO + 7/4 H2 + ¼H2O | Partial oxidation of methane |
| 2 | CH4 → C + 2H2 | Methane cracking |
| 3 | CO → 1/2C + 1/2CO2 | Boudouard reaction |
| 4 | CO2 + H2 → CO + H2O | Reverse reaction of WGS |
| Cat. Bed | Operation | Parameters |
|---|---|---|
| Ni (4.87%wt.)/γ-Al2O3 | Uo, 0.66 m s−1 | Dax, 7.89 × 10−4 m2 s−1 |
| ε, 0.67 | 1023 K | |
| ρcat, 2300 kg m−3 | 1.0 bar | |
| dpt, 2.0 × 10−3 m | ||
| wcat, 10.4 g |
| Reactant | DRM (mol m−3) | ATRM (mol m−3) | CRM (mol m−3) |
|---|---|---|---|
| CH4 | 11.4 | 20 | 43 |
| CO2 | 16.0 | - | 25 |
| O2 | - | 4.0 | 1.7 |
| H2O | - | 12 | 12 |
| J | Consumption | Production | RJ |
|---|---|---|---|
| CH4 | Step 1 k1 = 6.79 × 10−4 mol kg−1s−1 | - | r1 |
| CO | Step 2 k2 = 9.89 × 10−6 (m3)2 mol−1kg−1s−1 | Step 1 k1 = 6.79 × 10−4 mol kg−1s−1 | 2r1 + r3 |
| H2 | - | Steps 2, 3 k2 = 9.89 × 10−6 (m3)2 mol−1kg−1s−1 k3 = 3.94 × 10−4 m3 kg−1s−1 | 2r2 + r3 |
| J | Consumption | Production | RJ |
|---|---|---|---|
| CH4 | Steps 1, 2, 4 k1 = 1.31 × 10−1 mol/kg s k2 = 8.30 × 10−2 (m3)2/kg s mol k4 = 1.04 × 10−1 m3/kg s | - | −r1 − r2 − r4 |
| CO | Step 3 k3 = 4.11 × 10−5 m3/kg s | Steps 1, 5 k1 = 1.31 × 10−1 mol/kg.s k5 = 9.41 × 10−8 m3/kg s | 2r1 − r3 + r5 |
| H2 | Step 3 k3 = 4.11 × 10−5 m3/kg s | Steps 1, 2, 3, 4 k1 = 1.31 × 10−1 mol/kg s k2 = 8.30 × 10−2 (m3)2/kg s mol k4 = 1.04 × 10−1 m3/kg s | 2r1 + 4r2 − r3 + 2r4 |
| J | Consumption | Production | RJ |
|---|---|---|---|
| CH4 | Step 1 k1 = 6.79 × 10−4 mol kg−1s−1 | −r1 − r2 | |
| CO | Step 2 k2 = 9.89 × 10−6 (m3)2 mol−1kg−1s−1 | Step 1 k1 = 6.79 × 10−4 mol kg−1s−1 | r1 + r3 + 2r5 |
| H2 | - | Steps 2, 3 k2 = 9.89 × 10−6 (m3)2 mol−1kg−1s−1 k3 = 3.94 × 10−4 m3 kg−1s−1 | (7/4)r1 + 2r2 − r4 |
| Reform | Kinetic Selectivities Sip (%, i = H2, CO) | |
|---|---|---|
| Product | H2 | CO |
| DRM | 13.3 | 59.2 |
| ATRM | 31.6 | 28.3 |
| CRM | 86.1 | 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knoelchemann, A.; Sales, D.C.S.; Silva, M.A.M.; Abreu, C.A.M. Performance of Alternative Methane Reforms Based on Experimental Kinetic Evaluation and Simulation in a Fixed Bed Reactor. Processes 2021, 9, 1479. https://doi.org/10.3390/pr9081479
Knoelchemann A, Sales DCS, Silva MAM, Abreu CAM. Performance of Alternative Methane Reforms Based on Experimental Kinetic Evaluation and Simulation in a Fixed Bed Reactor. Processes. 2021; 9(8):1479. https://doi.org/10.3390/pr9081479
Chicago/Turabian StyleKnoelchemann, Augusto, Deivson C. S. Sales, Marcos A. M. Silva, and Cesar A. M. Abreu. 2021. "Performance of Alternative Methane Reforms Based on Experimental Kinetic Evaluation and Simulation in a Fixed Bed Reactor" Processes 9, no. 8: 1479. https://doi.org/10.3390/pr9081479
APA StyleKnoelchemann, A., Sales, D. C. S., Silva, M. A. M., & Abreu, C. A. M. (2021). Performance of Alternative Methane Reforms Based on Experimental Kinetic Evaluation and Simulation in a Fixed Bed Reactor. Processes, 9(8), 1479. https://doi.org/10.3390/pr9081479






