Bladder Substitution: The Role of Tissue Engineering and Biomaterials
Abstract
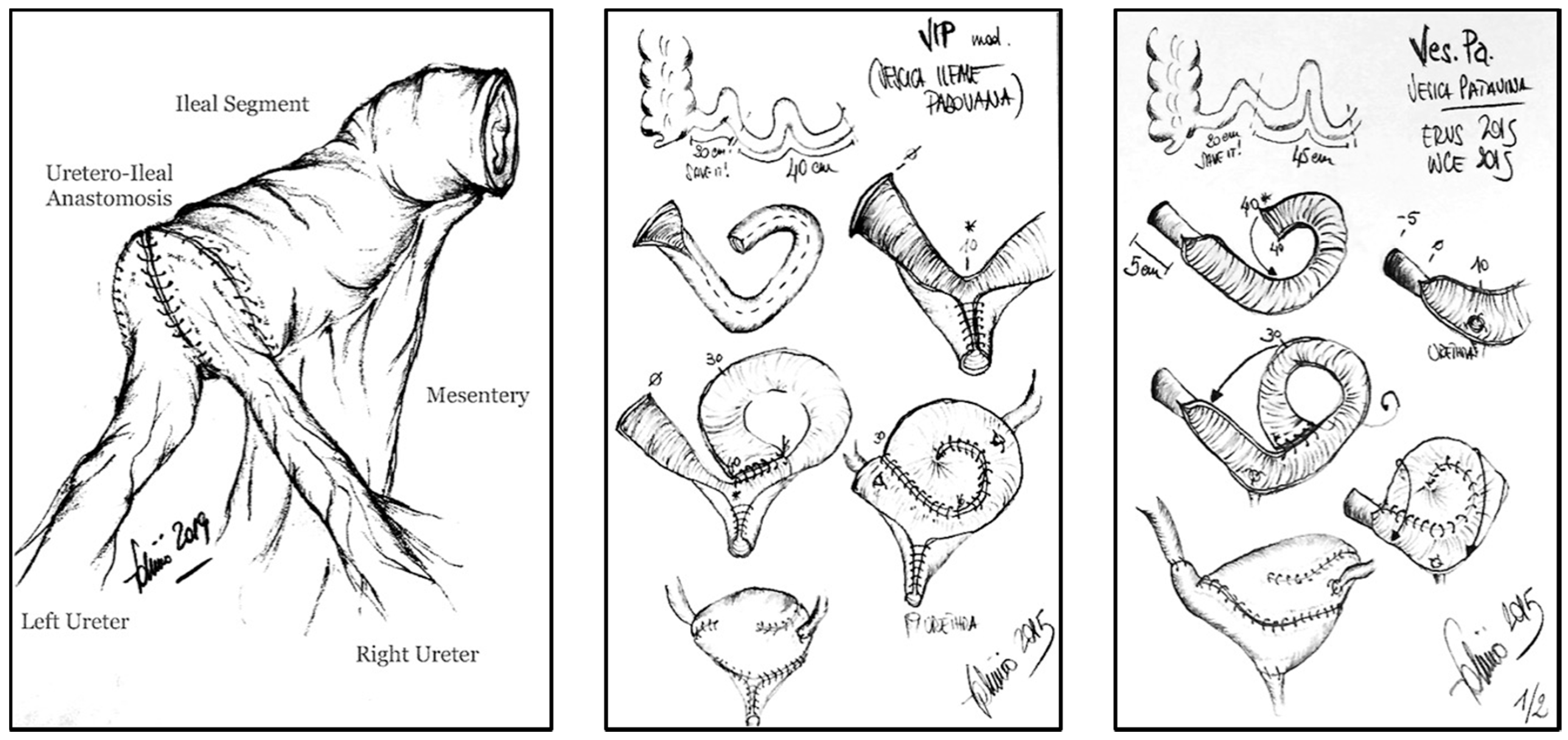
:1. Introduction: Clinical Context
2. Tissue Engineering in Urology
3. Tissue Engineered Urinary Scaffolds
3.1. Acellular Tissue Matrices
3.2. Naturally Derived Polymers
3.3. Synthetic Scaffolds
4. Seeded and Unseeded Scaffolds: The Choice of Cells Type
5. Mechanical Stimulation of Scaffold Using a Bioreactor
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Serrano-Aroca, Á.; Vera-Donoso, C.D.; Moreno-Manzano, V. Bioengineering approaches for bladder regeneration. Int. J. Mol. Sci. 2018, 19, 1796. [Google Scholar] [CrossRef] [Green Version]
- Witjes, J.A.; Lebret, T.; Compérat, E.M.; Cowan, N.C.; De Santis, M.; Bruins, H.M.; Hernández, V.; Espinos, E.L.; Dunn, J.; Rouanne, M.; et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur. Urol. 2016, 71, 462–475. [Google Scholar] [CrossRef]
- Bazargani, S.T.; Djaladat, H.; Ahmadi, H.; Miranda, G.; Cai, J.; Schuckman, A.K.; Daneshmand, S. Gastrointestinal complications following radical cystectomy using enhanced recovery protocol. Eur. Urol. Focus 2017, 4, 889–894. [Google Scholar] [CrossRef] [PubMed]
- Yuh, B.E.; Nazmy, M.; Ruel, N.H.; Jankowski, J.T.; Menchaca, A.R.; Torrey, R.R.; Linehan, J.A.; Lau, C.S.; Chan, K.G.; Wilson, T.G. Standardized analysis of frequency and severity of complications after robot-assisted radical cystectomy. Eur. Urol. 2012, 62, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Sloff, M.; De Vries, R.; Geutjes, P.; IntHout, J.; Ritskes-Hoitinga, M.; Oosterwijk, E.; Feitz, W. Tissue engineering in animal models for urinary diversion: A systematic review. PLoS ONE 2014, 9, e98734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloskowski, T.; Jundzill, A.; Kowalczyk, T.; Nowacki, M.; Bodnar, M.; Marszałek, A.; Pokrywczynska, M.; Frontczak-Baniewicz, M.; Kowalewski, T.; Chłosta, P.; et al. Ureter regeneration—The proper scaffold has to be defined. PLoS ONE 2014, 9, e106023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloskowski, T.; Kowalczyk, T.; Nowacki, M.; Drewa, T. Tissue engineering and ureter regeneration: Is it possible? Int. J. Artif. Organs 2013, 36, 392–405. [Google Scholar] [CrossRef] [PubMed]
- Ajalloueian, F.; Lemon, G.; Hilborn, J.; Chronakis, I.S.; Fossum, M. Bladder biomechanics and the use of scaffolds for regenerative medicine in the urinary bladder. Nat. Rev. Urol. 2018, 15, 155–174. [Google Scholar] [CrossRef]
- Johnson, S.C.; Smith, Z.L.; Sack, B.S.; Steinberg, G.D. Tissue engineering and conduit substitution. Urol. Clin. N. Am. 2018, 45, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. Organ engineering—Combining stem cells, biomaterials, and bioreactors to produce bioengineered organs for transplantation. BioEssays 2012, 35, 163–172. [Google Scholar] [CrossRef]
- Drewa, T. The artificial conduit for urinary diversion in rats: A preliminary study. Transplant. Proc. 2007, 39, 1647–1651. [Google Scholar] [CrossRef] [PubMed]
- Kropp, B.P.; Rippy, M.K.; Badylak, S.; Adams, M.C.; Keating, M.A.; Rink, R.C.; Thor, K.B. Regenerative urinary bladder augmentation using small intestinal submucosa: Urodynamic and histopathologic assessment in long-term canine bladder augmentations. J. Urol. 1996, 155, 2098–2104. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Bharadwaj, S.; Atala, A.; Zhang, Y. Human urine-derived stem cells seeded in a modified 3D porous small intestinal submucosa scaffold for urethral tissue engineering. Biomaterials 2011, 32, 1317–1326. [Google Scholar] [CrossRef]
- Campodonico, F.; Benelli, R.; Michelazzi, A.; Ognio, E.; Toncini, C.; Maffezzini, M. Bladder cell culture on small intestinal submucosa as bioscaffold: Experimental study on engineered urothelial grafts. Eur. Urol. 2004, 46, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bharadwaj, S.; Lee, S.J.; Atala, A.; Zhang, Y. Optimization of a natural collagen scaffold to aid cell–matrix penetration for urologic tissue engineering. Biomaterials 2009, 30, 3865–3873. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.-B.; Song, C.; Li, Y.-W.; Yang, S.-X.; Meng, L.-C.; Li, X.-H. Tissue-engineered conduit using bladder acellular matrix and bladder epithelial cells for urinary diversion in rabbits. Chin. Med. J. 2013, 126, 335–339. [Google Scholar] [PubMed]
- Micol, L.; da Silva, L.F.A.; Geutjes, P.J.; Oosterwijk, E.; Hubbell, J.; Feitz, W.F.; Frey, P. In-vivo performance of high-density collagen gel tubes for urethral regeneration in a rabbit model. Biomaterials 2012, 33, 7447–7455. [Google Scholar] [CrossRef]
- Sayeg, K.; Freitas-Filho, L.G.; Waitzberg, A.F.L.; Arias, V.E.A.; Laks, M.; Egydio, F.M.; Oliveira, A.S. Integration of collagen matrices into the urethra when implanted as onlay graft. Int. Braz. Urol. 2013, 39, 414–423. [Google Scholar] [CrossRef] [Green Version]
- Pinnagoda, K.; Larsson, H.M.; Vythilingam, G.; Vardar, E.; Engelhardt, E.-M.; Thambidorai, R.C.; Hubbell, J.A.; Frey, P. Engineered acellular collagen scaffold for endogenous cell guidance, a novel approach in urethral regeneration. Acta Biomater. 2016, 43, 208–217. [Google Scholar] [CrossRef]
- Aufderklamm, S.; Vaegler, M.; Kelp, A.; Maurer, S.; Gustafsson, L.; Mundhenk, J.; Daum, L.; Stenzl, A.; Sievert, K.-D.; Amend, B.; et al. Collagen cell carriers seeded with human urothelial cells for urethral reconstructive surgery: First results in a xenograft minipig model. World J. Urol. 2016, 35, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Song, L.; Wang, J.; Fan, S.; Zhang, Y.; Xu, Y. Evaluation of stretched electrospun silk fibroin matrices seeded with urothelial cells for urethra reconstruction. J. Surg. Res. 2013, 184, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Algarrahi, K.; Franck, D.; Ghezzi, C.; Cristofaro, V.; Yang, X.; Sullivan, M.P.; Chung, Y.G.; Affas, S.; Jennings, R.; Kaplan, D.L.; et al. Acellular bi-layer silk fibroin scaffolds support functional tissue regeneration in a rat model of onlay esophagoplasty. Biomaterials 2015, 53, 149–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, B.; Zhang, Y.; Zhou, L.; Sun, Z.; Zheng, J.; Chen, Y.; Dai, Y. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng. Part C Methods 2010, 16, 1201–1211. [Google Scholar] [CrossRef]
- Oberpenning, F.; Meng, J.; Yoo, J.J.; Atala, A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat. Biotechnol. 1999, 17, 149–155. [Google Scholar] [CrossRef]
- Lai, J.-Y.; Yoon, C.Y.; Yoo, J.J.; Wulf, T.; Atala, A. Phenotypic and functional characterization of in vivo tissue engineered smooth muscle from normal and pathological bladders. J. Urol. 2002, 168, 1853–1858. [Google Scholar] [CrossRef]
- Nakanishi, Y.; Chen, G.; Komuro, H.; Ushida, T.; Kaneko, S.; Tateishi, T.; Kaneko, M. Tissue-engineered urinary bladder wall sing PLGA mesh-collagen hybrid scaffolds: A omparison study of collagen sponge and gel as a caffold. J. Pediatr. Surg. 2003, 38, 1781–1784. [Google Scholar] [CrossRef]
- Atala, A.; Freeman, M.R.; Vacanti, J.P.; Shepard, J.; Retik, A.B. Implantation in vivo and retrieval of artificial structures consisting of rabbit and human urothelium and human bladder muscle. J. Urol. 1993, 150, 608–612. [Google Scholar] [CrossRef]
- Atala, A.; Bauer, S.B.; Soker, S.; Yoo, J.J.; Retik, A.B. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006, 367, 1241–1246. [Google Scholar] [CrossRef]
- Crapo, P.M.; Gilbert, T.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, M.H.; Chen, J.; Kirilak, Y.; Willers, C.; Xu, J.; Wood, D. Porcine small intestine submucosa (SIS) is not an acellular collagenous matrix and contains porcine DNA: Possible implications in human implantation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 73, 61–67. [Google Scholar] [CrossRef]
- Gilbert, T.; Freund, J.M.; Badylak, S.F. Quantification of DNA in biologic scaffold materials. J. Surg. Res. 2009, 152, 135–139. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.F.; Gilbert, T. Immune response to biologic scaffold materials. Semin. Immunol. 2008, 20, 109–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Record, R.D.; Hillegonds, D.; Simmons, C.; Tullius, R.; Rickey, F.; Elmore, D.; Badylak, S. In vivo degradation of 14C-labeled small intestinal submucosa (SIS) when used for urinary bladder repair. Biomaterials 2001, 22, 2653–2659. [Google Scholar] [CrossRef]
- Liao, W.; Yang, S.; Song, C.; Li, Y.; Meng, L.; Li, X.; Xiong, Y. Tissue-engineered tubular graft for urinary diversion after radical cystectomy in rabbits. J. Surg. Res. 2013, 182, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, J.; Kloskowski, T.; Stopel, M.; Gniadek, M.; Rasmus, M.; Balcerczyk, D.; Buhl, M.; Gagat, M.; Antosik, P.; Grzanka, D.; et al. The development of marine biomaterial derived from decellularized squid mantle for potential application as tissue engineered urinary conduit. Mater. Sci. Eng. C 2020, 119, 111579. [Google Scholar] [CrossRef]
- Bhargava, S.; Chapple, C.; Bullock, A.; Layton, C.; MacNeil, S. Tissue-engineered buccal mucosa for substitution urethroplasty. BJU Int. 2004, 93, 807–811. [Google Scholar] [CrossRef]
- Fu, Q.; Deng, C.-L.; Liu, W.; Cao, Y.-L. Urethral replacement using epidermal cell-seeded tubular acellular bladder collagen matrix. BJU Int. 2007, 99, 1162–1165. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, Y.-M.; Song, L.-J.; Fu, Q.; Cui, L.; Yin, S. Urethral Reconstruction Using Oral Keratinocyte Seeded Bladder Acellular Matrix Grafts. J. Urol. 2008, 180, 1538–1542. [Google Scholar] [CrossRef]
- Dorin, R.P.; Pohl, H.; De Filippo, R.E.; Yoo, J.J.; Atala, A. Tubularized urethral replacement with unseeded matrices: What is the maximum distance for normal tissue regeneration? World J. Urol. 2008, 26, 323–326. [Google Scholar] [CrossRef]
- Feng, C.; Xu, Y.-M.; Fu, Q.; Zhu, W.-D.; Cui, L. Reconstruction of three-dimensional neourethra using lingual keratinocytes and corporal smooth muscle cells seeded acellular corporal spongiosum. Tissue Eng. Part A 2011, 17, 3011–3019. [Google Scholar] [CrossRef]
- Li, H.; Xu, Y.; Xie, H.; Li, C.; Song, L.; Feng, C.; Zhang, Q.; Xie, M.; Wang, Y.; Lv, X. Epithelial-differentiated adipose-derived stem cells seeded bladder acellular matrix grafts for urethral reconstruction: An animal model. Tissue Eng. Part A 2014, 20, 774–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, S.J.; Kim, D.H.; Jo, J.K.; Chung, J.H.; Lee, J.Y.; Park, S.Y.; Kim, Y.T.; Park, H.K.; Choi, H.Y.; Moon, H.S. Bladder reconstruction using bovine pericardium in a case of enterovesical fistula. Korean J. Urol. 2011, 52, 150–153. [Google Scholar] [CrossRef] [Green Version]
- Davis, N.F.; Callanan, A.; McGuire, B.B.; Flood, H.D.; McGloughlin, T. Evaluation of viability and proliferative activity of human urothelial cells cultured onto xenogenic tissue-engineered extracellular matrices. Urology 2011, 77, 1007.e1–1007.e7. [Google Scholar] [CrossRef] [PubMed]
- Caione, P.; Boldrini, R.; Salerno, A.; Nappo, S.G. Bladder augmentation using acellular collagen biomatrix: A pilot experience in exstrophic patients. Pediatr. Surg. Int. 2012, 28, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Geutjes, P.; Roelofs, L.; Hoogenkamp, H.; Walraven, M.; Kortmann, B.; De Gier, R.; Farag, F.; Tiemessen, D.; Sloff, M.; Oosterwijk, E.; et al. Tissue engineered tubular construct for urinary diversion in a preclinical porcine model. J. Urol. 2012, 188, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Sloff, M.; Simaioforidis, V.; Tiemessen, D.M.; Janke, H.P.; Kortmann, B.B.; Roelofs, L.A.; Geutjes, P.J.; Oosterwijk, E.; Feitz, W.F. Tubular constructs as artificial urinary conduits. J. Urol. 2016, 196, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- De Filippo, R.; Yoo, J.J.; Atala, A. Urethral replacement using cell seeded tubularized collagen matrices. J. Urol. 2002, 168, 1783–1789. [Google Scholar] [CrossRef]
- Jia, W.; Tang, H.; Wu, J.; Hou, X.; Chen, B.; Chen, W.; Zhao, Y.; Shi, C.; Zhou, F.; Yu, W.; et al. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials 2015, 69, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Pederzoli, F.; Joice, G.; Salonia, A.; Bivalacqua, T.J.; Sopko, N.A. Regenerative and engineered options for urethroplasty. Nat. Rev. Urol. 2019, 16, 453–464. [Google Scholar] [CrossRef]
- Basu, J.; Jayo, M.J.; Ilagan, R.M.; Guthrie, K.I.; Sangha, N.; Genheimer, C.W.; Quinlan, S.F.; Payne, R.; Knight, T.; Rivera, E.; et al. Regeneration of Native-Like Neo-Urinary Tissue from Nonbladder Cell Sources. Tissue Eng. Part A 2012, 18, 1025–1034. [Google Scholar] [CrossRef]
- Raya-Rivera, A.; Esquiliano, D.R.; Yoo, J.J.; Lopez-Bayghen, E.; Soker, S.; Atala, A. Tissue-engineered autologous urethras for patients who need reconstruction: An observational study. Lancet 2011, 377, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Guo, X.; Zhao, W.; Niu, G.; Mo, X.; Fu, Q. Application of Wnt pathway inhibitor delivering scaffold for inhibiting fibrosis in urethra strictures: In vitro and in vivo study. Int. J. Mol. Sci. 2015, 16, 27659–27676. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Fu, Q.; Yoo, J.; Chen, X.; Chandra, P.; Mo, X.; Song, L.; Atala, A.; Zhao, W. 3D bioprinting of urethra with PCL/PLCL blend and dual autologous cells in fibrin hydrogel: An in vitro evaluation of biomimetic mechanical property and cell growth environment. Acta Biomater. 2017, 50, 154–164. [Google Scholar] [CrossRef]
- Drewa, T.; Sir, J.; Czajkowski, R.; Wozniak, A. Scaffold seeded with cells is essential in urothelium regeneration and tissue remodeling in vivo after bladder augmentation using in vitro engineered graft. Transplant. Proc. 2006, 38, 133–135. [Google Scholar] [CrossRef]
- Tian, H.; Bharadwaj, S.; Liu, Y.; Ma, P.X.; Atala, A.; Zhang, Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: Potential for urological tissue engineering. Tissue Eng. Part A 2010, 16, 1769–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.; Mandhani, A.; Bose, S.; Basu, B. Dynamically crosslinked polydimethylsiloxane-based polyurethanes with contact-killing antimicrobial properties as implantable alloplasts for urological reconstruction. Acta Biomater. 2021, 129, 122–137. [Google Scholar] [CrossRef]
- Brehmer, B.; Rohrmann, D.; Becker, C.; Rau, G.; Jakse, G. Different types of scaffolds for reconstruction of the urinary tract by tissue engineering. Urol. Int. 2007, 78, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.S.; Baskin, L.S.; Hayward, S.W.; Cunha, G.R. Regeneration of bladder urothelium, smooth muscle, blood vessels and nerves into an acellular tissue matrix. J. Urol. 1996, 156, 571–577. [Google Scholar] [CrossRef]
- Farhat, W.; Chen, J.; Erdeljan, P.; Shemtov, O.; Courtman, D.; Khoury, A.; Yeger, H. Porosity of porcine bladder acellular matrix: Impact of ACM thickness. J. Biomed. Mater. Res. 2003, 67, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Cartwright, L.M.; Shou, Z.; Yeger, H.; Farhat, W.A. Porcine bladder acellular matrix porosity: Impact of hyaluronic acid and lyophilization. J. Biomed. Mater. Res. Part A 2006, 77, 180–184. [Google Scholar] [CrossRef]
- Seifarth, V.; Gossmann, M.; Janke, H.P.; Grosse, J.O.; Becker, C.; Heschel, I.; Artmann, G.M.; Artmann, A.T. Development of a bioreactor to culture tissue engineered ureters based on the application of tubular OPTIMAIX 3D scaffolds. Urol. Int. 2015, 95, 106–113. [Google Scholar] [CrossRef]
- Farhat, W.A.; Yeger, H. Does mechanical stimulation have any role in urinary bladder tissue engineering? World J. Urol. 2008, 26, 301–305. [Google Scholar] [CrossRef]
- Wallis, M.C.; Yeger, H.; Cartwright, L.; Shou, Z.; Radisic, M.; Haig, J.; Suoub, M.; Antoon, R.; Farhat, W.A. Feasibility study of a novel urinary bladder bioreactor. Tissue Eng. Part A 2008, 14, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Hubschmid, U.; Leong-Morgenthaler, P.-M.; Basset-Dardare, A.; Ruault, S.; Frey, P. In vitro growth of human urinary tract smooth muscle cells on laminin and collagen type i-coated membranes under static and dynamic conditions. Tissue Eng. 2005, 11, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, F.M.; O’Brien, T.P.; Callanan, A.; Kavanagh, E.G.; Burke, P.E.; Grace, P.A.; McGloughlin, T.M. New pulsatile hydrostatic pressure bioreactor for vascular tissue-engineered constructs. Artif. Organs 2010, 34, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Ahvaz, H.H.; Soleimani, M.; Mobasheri, H.; Bakhshandeh, B.; Shakhssalim, N.; Soudi, S.; Hafizi, M.; Vasei, M.; Dodel, M. Effective combination of hydrostatic pressure and aligned nanofibrous scaffolds on human bladder smooth muscle cells: Implication for bladder tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2281–2290. [Google Scholar] [CrossRef] [PubMed]
- Tiemessen, D.; De Jonge, P.; Daamen, W.F.; Feitz, W.; Geutjes, P.; Oosterwijk, E. The effect of a cyclic uniaxial strain on urinary bladder cells. World J. Urol. 2017, 35, 1531–1539. [Google Scholar] [CrossRef] [Green Version]
- Adamowicz, J.; Pasternak, I.; Kloskowski, T.; Gniadek, M.; Van Breda, S.V.; Buhl, M.; Balcerczyk, D.; Gagat, M.; Grzanka, D.; Strupinski, W.; et al. Development of a conductive biocomposite combining graphene and amniotic membrane for replacement of the neuronal network of tissue-engineered urinary bladder. Sci. Rep. 2020, 10, 5824. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casarin, M.; Morlacco, A.; Dal Moro, F. Bladder Substitution: The Role of Tissue Engineering and Biomaterials. Processes 2021, 9, 1643. https://doi.org/10.3390/pr9091643
Casarin M, Morlacco A, Dal Moro F. Bladder Substitution: The Role of Tissue Engineering and Biomaterials. Processes. 2021; 9(9):1643. https://doi.org/10.3390/pr9091643
Chicago/Turabian StyleCasarin, Martina, Alessandro Morlacco, and Fabrizio Dal Moro. 2021. "Bladder Substitution: The Role of Tissue Engineering and Biomaterials" Processes 9, no. 9: 1643. https://doi.org/10.3390/pr9091643
APA StyleCasarin, M., Morlacco, A., & Dal Moro, F. (2021). Bladder Substitution: The Role of Tissue Engineering and Biomaterials. Processes, 9(9), 1643. https://doi.org/10.3390/pr9091643






