A Comparative Study of Improvement of Phycoremediation Using a Consortium of Microalgae in Municipal Wastewater Treatment Pond Systems as an Alternative Solution to Africa’s Sanitation Challenges
Abstract
:1. Introduction
2. Materials and Methods
2.1. Background
2.2. Physical and Chemical Sampling Analyses
2.3. Phytoplankton Sampling and Analyses
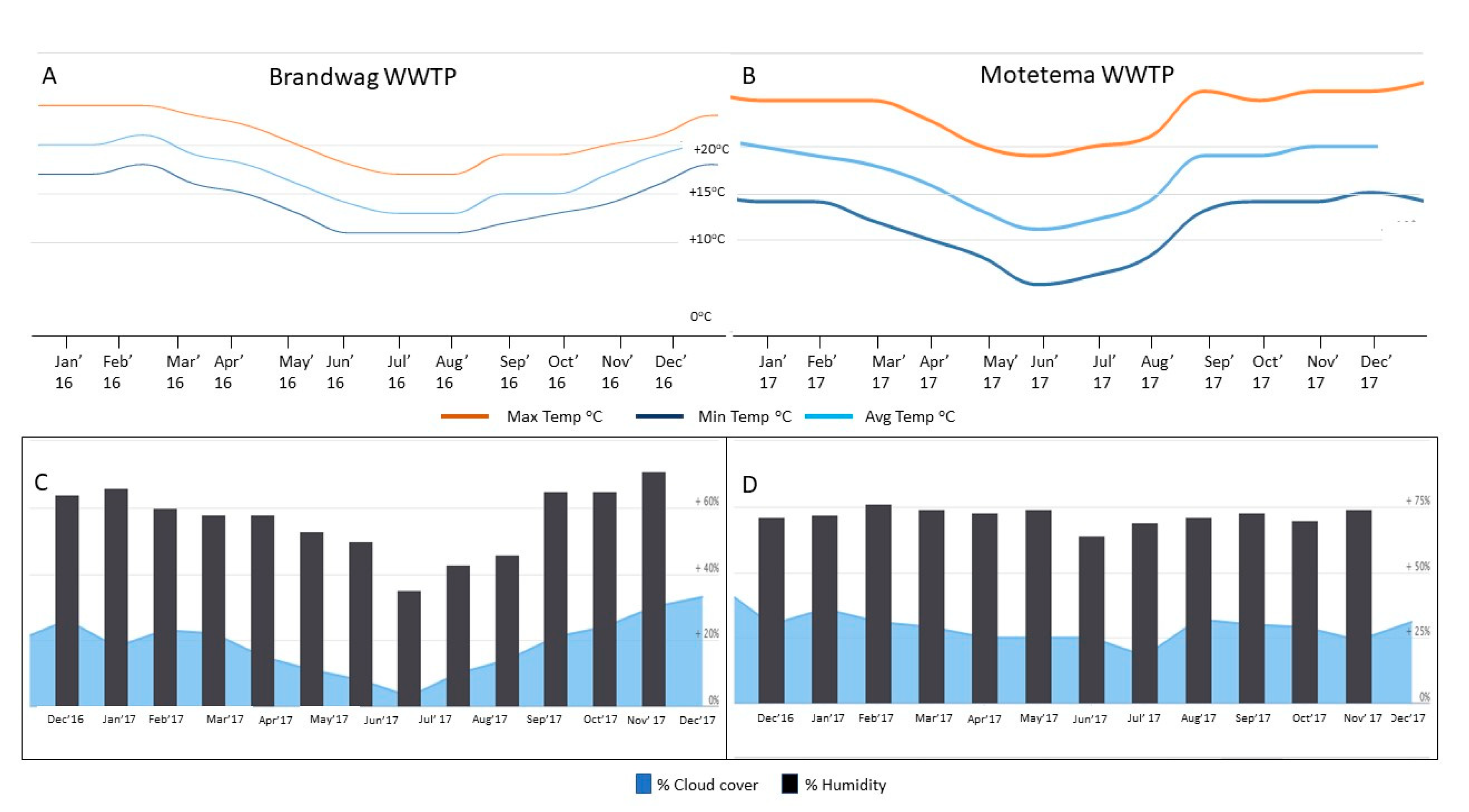
2.4. Weather Condition at Each WWTP Location
2.5. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oberholster, P.; Munyayi, R.C.; Turton, A.; Du Plessis, A. Overview. In Sanitation and Wastewater Atlas of Africa; AfDB: Abidjan, Ivory Coast; UNEP: Nairobi, Kenya; GRID-Arendal: Arendal, Norway, 2020; Volume 283, pp. 9–35. ISBN 978-82-7701-194-3. [Google Scholar]
- Weststrate, J.; Dijkstra, G.; Eshuis, J.; Gianoli, A.; Rusca, M. The sustainable development goal on water and sanitation: Learning from the Millennium Development Goals. Soc. Indic. Res. 2019, 143, 797–800. [Google Scholar] [CrossRef] [Green Version]
- Nikiema, J.; Figoli, A.; Weissenbacher, N.; Langergraber, G.; Marrot, B.; Moulin, P. Wastewater treatment practices in Africa—Experiences from seven countries. SSP 2013, 14, 26–34. Available online: https://hdl.handle.net/10568/40210 (accessed on 5 July 2010).
- Oberholster, P.J.; Botha, A.-M.; Chamier, J.; De Klerk, A.J. Longitudinal trends in water chemistry and phytoplankton assemblage downstream of the Riverview WWTP in the Upper Olifants River. Ecohydrol. Hydrobiol. 2013, 13, 41–51. [Google Scholar] [CrossRef]
- Sasongko, N.A.; Noguchi, R.; Ito, J.; Demura, M.; Ichikawa, S.; Nakajima, M.; Watanabe, M.M. Engineering Study of a Pilot Scale Process Plant for Microalgae-Oil Production Utilizing Municipal Wastewater and Flue Gases: Fukushima Pilot Plant. Energies 2018, 11, 1693. [Google Scholar] [CrossRef] [Green Version]
- South Africa, Department of Water Affairs. Revision of General Authorisations in Terms of Section 39 of the National Water Act, 1998 (Act No. 36 of 1998), No. 665 of 2013; Department of Water Affairs: Pretoria, South Africa, 2013. [Google Scholar]
- Gray, N.F. (Ed.) How nature deals with waste. In Biology of Wastewater Treatment, 2nd ed.; Series on Environmental Science and Management; Imperial College Press: London, UK, 2004; Volume 4, pp. 1–131. [Google Scholar] [CrossRef]
- Mohammed, B. Design and performance evaluation of a wastewater treatment unit. AU JT 2006, 9, 193–198. [Google Scholar]
- Bucksteeg, K. German experiences with sewage treatment ponds. Water Sci. Technol. 1987, 19, 17–23. [Google Scholar] [CrossRef]
- Barceló, D.; Petrovic, M. Waste water treatment and reuse in the Mediterranean region. In The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2011; Volume 14. [Google Scholar]
- Bitton, G. Formula handbook for environmental engineers and scientists. In Environmental Science and Technology; A Wiley-Interscience Series of Texts and Monographs; Schoor, J.L., Zehnder, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 1998; Volume 117. [Google Scholar]
- Oberholster, P.J.; Cheng, P.-H.; Genthe, B.; Steyn, M. The environmental feasibility of low-cost algae-based sewage treatment as a climate change adaption measure in rural areas of SADC countries. J. Appl. Phycol. 2019, 31, 355–639. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for biogeochemical cycling of carbon, nitrogen and phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Otoo, M.; Drechsel, P. (Eds.) Resource Recovery from Waste: Business Models for Energy, Nutrient and Water Reuse in Low- and Middle-Income Countries; Routledge—Earthscan: Oxon, UK, 2018; Available online: https://hdl.handle.net/10568/93011 (accessed on 5 July 2010).
- Kang, D.; Kim, K. Real Wastewater Treatment Using a Moving Bed and Wastewater-Borne Algal–Bacterial Consortia with a Short Hydraulic Retention Time. Processes 2021, 9, 116. [Google Scholar] [CrossRef]
- Dębowski, M.; Rusanowska, P.; Zieliński, M.; Dudek, M.; Romanowska-Duda, Z. Biomass Production and Nutrient Removal by Chlorella vulgaris from Anaerobic Digestion Effluents. Energies 2018, 11, 1654. [Google Scholar] [CrossRef] [Green Version]
- Mohsenpour, S.F.; Hennige, S.; Willoughby, A.A.; Adeloye, A.; Gutierrez, T. Integrating micro-algae into wastewater treatment: A review. Sci. Total Environ. 2021, 752, 142–168. [Google Scholar] [CrossRef]
- Dębowski, M.; Zieliński, M.; Kazimierowicz, J.; Kujawska, N.; Talbierz, S. Microalgae Cultivation Technologies as an Opportunity for Bioenergetic System Development—Advantages and Limitations. Sustainability 2020, 12, 9980. [Google Scholar] [CrossRef]
- Mambo, P.M.; Westensee, D.K.; Zuma, B.M.; Cowan, A.K. The Belmont Valley integrated algae pond system in retrospect. Water SA 2014, 40, 385–394. [Google Scholar] [CrossRef] [Green Version]
- Oswald, W.J. Ponds in the 21st century. Water Sci. Technol. 1995, 31, 1–8. [Google Scholar] [CrossRef]
- Renuka, N.; Sood, A.; Prasanna, R.; Ahluwalia, A.S. Phycoremediation of wastewaters: A synergistic approach using microalgae for bioremediation and biomass generation. Int. J. Environ. Sci. Technol. 2015, 12, 1443–1460. [Google Scholar] [CrossRef] [Green Version]
- Van den Berg, M.F.; Botha, A.M.; Bierman, A.; Oberholster, P. Assessing domestic wastewater effluent with a battery of bioassays after treatment with a specific consortium of microalgae and different flocculation methods. Water Air Soil Pollut. 2020, 231, 257. [Google Scholar] [CrossRef]
- Masaraure, R. The Development of an Approach for the Production and Use of Algae to Treat Urban Wastewater. 2016. Available online: https://repository.up.ac.za/bitstream/handle/2263/57260/Masaraure_Development_2016.pdf?isAllowed=y&sequence=1 (accessed on 5 July 2010).
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectrometry. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Pollution Control Federation. Standard Methods for the Examination of Water and Wastewater, 19th ed.; APHA, AWWA, and WPCF: Washington, DC, USA, 1992. [Google Scholar]
- Taylor, J.C.; Harding, W.R.; Archibald, C.G.M. An Illustrated Guide to Some Common Diatom Species from South Africa; WRC Report, No. TT 282/07; Water Research Commission: Pretoria, South Africa, 2007; pp. 1–178. [Google Scholar]
- Truter, E. An Aid to the Identification of the Dominant and Commonly Occurring Genera of Algae Observed in Some South African Impoundments; Department of Water Affairs, Hydrological Research Institute: Pretoria, South Africa, 1987. Available online: https://www.dws.gov.za/iwqs/reports/tr/TR_135_Truter_1987_An_aid_to_the_identification_of_algae_in_South_African_impoundments_s.pdf (accessed on 5 July 2010).
- Van Vuuren, S.; Taylor, J.; Gerber, A.; Van Ginkel, C. Easy Identification of the Most Common Freshwater Algae: A Guide for the Identification of Microscopic Algae in South African Freshwaters; North-West University and Department of Water Affairs and Forestry: Pretoria, South Africa, 2006. Available online: https://www.dws.gov.za/iwqs/eutrophication/NEMP/Janse_van_Vuuren_2006_Easy_identification_of_the_most_common_freshwater_algae.pdf (accessed on 5 July 2010).
- Wehr, J.D.; Sheath, R.G. Freshwater habitats of algae. In Freshwater Algae of North America: Ecology and Classification; Wehr, J.D., Sheath, R.G., Eds.; Academic Press: Cambridge, MA, USA, 2003; pp. 11–57. [Google Scholar]
- American Public Health Association. Standard Methods for Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2006. [Google Scholar]
- Berger, W.H.; Parker, F.L. Diversity of planktonic foraminifera in deep-sea sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef]
- World Weather Online. 2021. Available online: https://www.worldweatheronline.com/ (accessed on 17 July 2021).
- Chinnasamy, S.; Bhatnagar, A.; Claxton, R.; Das, K.C. Biomass and bioenergy production potential of microalgae consortium in open and closed bioreactors using untreated carpet industry effluent as growth medium. Bioresour. Technol. 2010, 101, 3097–3105. [Google Scholar] [CrossRef]
- Ruiz-Martinez, A.; Garcia, N.M.; Romero, I.; Seco, A.; Ferrer, J. Microalgae cultivation in wastewater: Nutrient removal from anaerobic membrane bioreactor effluent. Bioresour. Technol. 2012, 126, 247–253. [Google Scholar] [CrossRef]
- Silva-Benavides, A.M.; Torzillo, G. Nitrogen and phosphorus removal through laboratory batch cultures of microalgae Chlorella vulgaris and cyanobacterium Planktothrix isothrix grown as monoalgal and as co-cultures. J. Appl. Phycol. 2011, 24, 267–276. [Google Scholar] [CrossRef]
- González-Camejo, J.; Aparicio, S.; Ruano, M.V.; Borrás, L.; Barat, R.; Ferrer, J. Effect of ambient temperature variations on an indigenous microalgae-nitrifying bacteria culture dominated by Chlorella. Bioresour. Technol. 2019, 290, 121788. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Culturing microalgae in outdoor ponds. In Algal Culturing Techniques; Andersen, R.A., Ed.; Academic Press: Cambridge, MA, USA, 2005; Chapter 14; pp. 205–206. [Google Scholar]
- Larsdotter, K. Wastewater treatment with microalgae—A literature review. Vatten 2006, 62, 31–38. Available online: https://www.tidskriftenvatten.se/wp-content/uploads/2017/04/48_article_2125.pdf (accessed on 5 July 2010).
- Falkowski, P.G.; Raven, J.A. Aquatic Photosynthesis, 2nd ed.; Princeton University Press: Princeton, NJ, USA, 2007. [Google Scholar]
- Mahapatra, D.M.; Chanakya, H.N.; Ramachandra, T.V. Treatment efficacy of algae-based sewage treatment plants. Environ. Monit. Assess. 2013, 185, 7145–7164. [Google Scholar] [CrossRef] [PubMed]
- Ndlela, L.L.; Oberholster, P.J.; Van Wyk, J.H.; Cheng, P.H. An overview of cyanobacterial bloom occurrences and research in Africa over the last decade. Harmful Algae 2016, 60, 11–26. [Google Scholar] [CrossRef]
- Vasconcelos, V.M. Toxic cyanobacteria (blue−green−algae) in Portuguese fresh-waters. Arch. Hydrobiol. 1994, 130, 449–451. [Google Scholar] [CrossRef]
- AlMomani, F.; Örmeci, B. Assessment of algae-based wastewater treatment in hot climate regions: Treatment performance and kinetics. Process Saf. Environ. Prot. 2020, 141, 140–149. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhan, J.-J.; Hong, Y. The effects of temperature on the growth, lipid accumulation and nutrient removal characteristics of Chlorella sp. HQ. Desalin. Water Treat. 2016, 57, 10403–10408. [Google Scholar] [CrossRef]
- Shi, C.; Shi, X. Characterization of three genes encoding the subunits of light-independent protochlorophylide reductase in Chlorella protothecoides CS-41. Biotechnol. Prog. 2016, 22, 1050–1055. [Google Scholar] [CrossRef]
- Mara, D. Domestic Wastewater Treatment in Developing Countries; Earthscan: London, UK, 2003; Available online: https://www.pseau.org/outils/ouvrages/earthscan_ltd_domestic_wastewater_treatment_in_developing_countries_2003.pdf (accessed on 10 February 2005).
- Cassidy, K.O. Evaluating Algal Growth at Different Temperature. Master’s Thesis, University of Kentucky, Lexington, KY, USA, 2011. Available online: https://uknowledge.uky.edu/bae_etds/3 (accessed on 22 August 2021).
- Latala, A. Effects of salinity, temperature and light on the growth and morphology of green planktonic algae. Oceanologia 1991, 31, 119–138. Available online: http://www.iopan.gda.pl/oceanologia/OC_31/OC_31_119-138.pdf (accessed on 22 August 2021).





| Country | Total Number | First Most Used Technology | Second Most Used Technology | Third Most Used Technology | Feed Flow Rate (m3/h) |
|---|---|---|---|---|---|
| Burkina Faso | 2 | 100% pond systems | N/A | N/A | 96 (for the largest pond system) |
| Ghana | 19 1 (4 ponds under construction) | 42% are pond systems | 26% are activated sludge (AS) or aerated tanks | 16% are anaerobic digesters | 1–25 |
| Senegal | 9 | 56% are ponds | 44% are AS | N/A | N/A |
| Algeria | 123 (96 under construction, of which 60 are AS and 36 are pond systems) | 55% are ponds | 45% are AS | N/A | 8–2750 |
| Egypt | >99 | Between 65% and 85% are AS | About 10% are pond systems | Others | _ |
| Morocco | >100 | >77% are pond systems | 5% are AS | Trickling filters | 12–4914 |
| Tunisia | 109 | 82% are AS | 13% are pond systems | Trickling filters and wetlands | 4–3250 |
| Parameters | Motetema Wastewater Pond System | Brandwag Wastewater Pond System | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Treatment Pond 6 (STDEV) | After Treatment Pond 6 (STDEV) | Reduction after Algae Treatment (%) | Before Treatment Pond 7 (Outflow) (STDEV) | After Treatment Pond 7 (Outflow) (STDEV) | Reduction after Algae Treatment (%) | Before Treatment Pond 5 (STDEV) | After treatment Pond 5 (STDEV) | Reduction after Algae Treatment (%) | Before Treatment Pond 6 (Outflow) (STDEV) | After Treatment Pond 6 (Outflow) (STDEV) | Reduction after Algae Treatment (%) | |
| Total nitrogen (mgL) | 50 (3) | 24 (7) | 52.00 | 41 (5) | 11 (4) | 73.1 | 70 (11) | 45 (7) | 43.0 | 28 (7) | 17 (4) | 35.4 |
| Total organic carbon (mgL) | 53 (10) | 32 (12) | 39.62 | 50 (4) | 23 (3) | 54.0 | 195 (18) | 69 (9) | 69.0 | 52 (11) | 42 (8) | 22.2 |
| Total chemical oxygen demand (mgL) | 140 (26) | 145 (46) | −3.57 | 122 (72) | 114 (42) | 6.6 | 572 (83) | 147 (23) | 75.0 | 235 (39) | 97 (11) | 60.0 |
| Total phosphorous (mgL) | 17 (2) | 19 (9) | 11.76 | 12 (2) | 6 (1) | 50.0 | 9.5 (4) | 2.7 (0.9) | 74.5 | 9 (3) | 2.2 (0.3) | 74.4 |
| Sulfate as SO4 dissolved (mgL) | 195 (90) | 73 (20) | 62.56 | 184 (85) | 78 (24) | 58.0 | 81 (10) | 113 (21) | −45.0 | 172 (13) | 127 (11) | 26.9 |
| Ortho Phosphate as P (mgL) | 12 (1) | 2.36 (0.79) | 80.33 | 8 (3) | 1.36 (0.7) | 83.0 | 5.8 (1.1) | 2.1 (0.6) | 77.0 | 3.7 (0.8) | 0.74 (0.4) | 87.0 |
| Ammonia as N (mgL) | 20 (13) | 9 (4) | 55.00 | 19 (2) | 0.1 (0.85) | 99.4 | 48 (11) | 32 (7) | 43.0 | 27 (9) | 23 (8) | 16.6 |
| Electrical conductivity (mSm) | 114 (10) | 185 (33) | −62.28 | 118 (2) | 160 (22) | −36.0 | 117 (11) | 125 (21) | −7.1 | 112 (10) | 128 (9) | −16.0 |
| pH (Lab) 20 °C | 8.1 (0.15) | 8.4 (0.06) | −3.70 | 8.2 (0.06) | 9.1 (0.49) | 10.9 | 7.7 (0.03) | 8.8 (0.03) | −14.1 | 8.2 (0.02) | 8.7 (0.18) | −6.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oberholster, P.J.; Steyn, M.; Botha, A.-M. A Comparative Study of Improvement of Phycoremediation Using a Consortium of Microalgae in Municipal Wastewater Treatment Pond Systems as an Alternative Solution to Africa’s Sanitation Challenges. Processes 2021, 9, 1677. https://doi.org/10.3390/pr9091677
Oberholster PJ, Steyn M, Botha A-M. A Comparative Study of Improvement of Phycoremediation Using a Consortium of Microalgae in Municipal Wastewater Treatment Pond Systems as an Alternative Solution to Africa’s Sanitation Challenges. Processes. 2021; 9(9):1677. https://doi.org/10.3390/pr9091677
Chicago/Turabian StyleOberholster, Paul J., Maronel Steyn, and Anna-Maria Botha. 2021. "A Comparative Study of Improvement of Phycoremediation Using a Consortium of Microalgae in Municipal Wastewater Treatment Pond Systems as an Alternative Solution to Africa’s Sanitation Challenges" Processes 9, no. 9: 1677. https://doi.org/10.3390/pr9091677
APA StyleOberholster, P. J., Steyn, M., & Botha, A.-M. (2021). A Comparative Study of Improvement of Phycoremediation Using a Consortium of Microalgae in Municipal Wastewater Treatment Pond Systems as an Alternative Solution to Africa’s Sanitation Challenges. Processes, 9(9), 1677. https://doi.org/10.3390/pr9091677







