Trans- and Multigenerational Effects of Isothiazolinone Biocide CMIT/MIT on Genotoxicity and Epigenotoxicity in Daphnia magna
Abstract
:1. Introduction
2. Materials and Methods
2.1. Daphnid Cultures
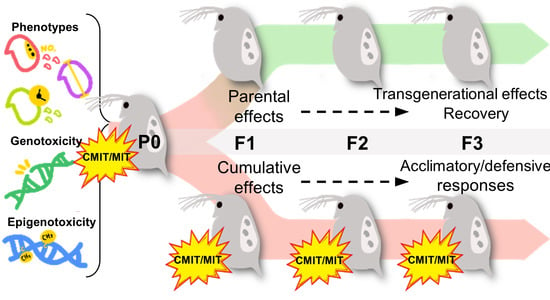
2.2. Exposure to Chemicals and Exposure Scenarios
2.3. Mortality and Reproduction Assay
2.4. Behavior Assay
2.5. Morphology and Body Size
2.6. Quantitative Mass Spectrometry (MS)-Based Proteomic and Bioinformatic Analyses
2.7. Comet Assay
2.8. Global DNA Methylation Measurement
2.9. Statistical Analysis
3. Results and Discussion
3.1. Environmental Concentrations of CMIT and MIT
3.2. Acute and Chronic Toxicity of CMIT/MIT in Daphnia magna
3.3. Protein Expression Alteration in Response to CMIT/MIT Exposure
3.4. Phenotypic Alterations under Parental and Multigenerational Exposure to CMIT/MIT: PE vs. ME
3.5. Genotoxic and Epigenotoxic Responses to Parental and Multigenerational Exposure to CMIT/MIT: PE vs. ME
- CMIT/MIT exposure caused deleterious effects on reproduction, and the proteomic analyses suggested that reproductive failure may have been due to the decreased expression of vitellogenin-related proteins in D. magna.
- Parental exposure to CMIT/MIT (PE) caused transgenerational effects on the time to first reproduction (F3) and parental effects (F1) on reproductive capacity and growth recovered after the termination of exposure (F3).
- Multigenerational exposure to CMIT/MIT (ME) caused an accumulative adverse effect on reproduction (F1), while acclimatory/defensive responses were observed under continued chemical exposure (F3).
- DNA damage was sustained and then decreased over generations in the ME scenario, indicating that DNA damage might be associated with reproductive toxicity and acclimatory/defensive responses to chemical exposure.
- DNA methylation increased in daphnids exposed to CMIT/MIT in P0, but it washed out across the subsequent generations under both the PE and ME scenarios. Namely, genotoxicity had a closer association with the inheritance of modified phenotypes, particularly reproduction, than epigenotoxicity.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.-R.; Yang, Y.-Y.; Liu, Y.-S.; Zhang, L.-J.; Zhao, J.-L.; Zhang, Q.-Q.; Zhang, M.; Zhang, J.-N.; Jiang, Y.-X.; Ying, G.-G. Biocides in wastewater treatment plants: Mass balance analysis and pollution load estimation. J. Hazard. Mater. 2017, 329, 310–320. [Google Scholar] [CrossRef]
- Liu, W.-R.; Zhao, J.-L.; Liu, Y.-S.; Chen, Z.-F.; Yang, Y.-Y.; Zhang, Q.-Q.; Ying, G.-G. Biocides in the Yangtze River of China: Spatiotemporal distribution, mass load and risk assessment. Environ. Pollut. 2015, 200, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Mardones, L.E.; Legnoverde, M.S.; Pereyra, A.M.; Basaldella, E.I. Long-lasting isothiazolinone-based biocide obtained by encapsulation in micron-sized mesoporous matrices. Prog. Org. Coatings 2018, 119, 155–163. [Google Scholar] [CrossRef]
- Silva, V.; Silva, C.; Soares, P.; Garrido, E.M.; Borges, F.; Garrido, J. Isothiazolinone Biocides: Chemistry, Biological, and Toxicity Profiles. Molecules 2020, 25, 991. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.M. The mechanism of action of isothiazolone biocides. In NACE-International Corrosion Conference Series; National Association of Corrosion Engineers International: Houston, Texas, USA, 2006. [Google Scholar]
- Rafoth, A.; Gabriel, S.; Sacher, F.; Brauch, H.-J. Analysis of isothiazolinones in environmental waters by gas chromatography–mass spectrometry. J. Chromatogr. A 2007, 1164, 74–81. [Google Scholar] [CrossRef]
- Thomsen, A.V.; Schwensen, J.F.; Bossi, R.; Banerjee, P.; Giménez-Arnau, E.; Lepoittevin, J.-P.; Lidén, C.; Uter, W.; White, I.R.; Johansen, J.D. Isothiazolinones are still widely used in paints purchased in five European countries: A follow-up study. Contact Dermat. 2017, 78, 246–253. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). EPA738-R-98-012, Reregistration Eligibility Decision (RED) Methylisothiazolinone, Office of Prevention, Pesticides and Toxic Substances (Washington, D. C., USA), October 1998. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P1009GN3.txt (accessed on 1 May 2022).
- European ChemicalAgency (ECHA). CLH-O-0000001412-86-106/F, RAC EC Number: 55965-84-9, Committee for Risk Assessment RAC Opinion on 5-Chloro-2-Methyl-4-Isothiazolin-3-One [EC no. 247-500-7] and 2-Methyl-4-Isothiazolin-3-One [EC no. 220-239-6] (3:1), March 2016. Available online: https://echa.europa.eu/information-on-chemicals/biocidal-active-substances/-/disas/factsheet/1373/PT02 (accessed on 1 May 2022).
- Paijens, C.; Frère, B.; Caupos, E.; Moilleron, R.; Bressy, A. Determination of 18 Biocides in Both the Dissolved and Particulate Fractions of Urban and Surface Waters by HPLC-MS/MS. Water Air Soil Pollut. 2020, 231, 210. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, M. In-vitro DNA and cell damage induced by 5-chloro-2-methyl-4-isothiazolin-3-one (CMIT) and suppressive effect of selected phytochemicals against CMIT toxicity. J. Environ. Biol. 2019, 40, 335–341. [Google Scholar] [CrossRef]
- Do, V.Q.; Seo, Y.-S.; Park, J.-M.; Yu, J.; Duong, M.T.H.; Nakai, J.; Kim, S.-K.; Ahn, H.-C.; Lee, M.-Y. A mixture of chloromethylisothiazolinone and methylisothiazolinone impairs rat vascular smooth muscle by depleting thiols and thereby elevating cytosolic Zn2+ and generating reactive oxygen species. Arch. Toxicol. 2020, 95, 541–556. [Google Scholar] [CrossRef]
- Muyssen, B.T.; Janssen, C.R. Multi-generation cadmium acclimation and tolerance in Daphnia magna Straus. Environ. Pollut. 2004, 130, 309–316. [Google Scholar] [CrossRef]
- Chatterjee, N.; Choi, S.; Kwon, O.K.; Lee, S.; Choi, J. Multi-generational impacts of organic contaminated stream water on Daphnia magna: A combined proteomics, epigenetics and ecotoxicity approach. Environ. Pollut. 2019, 249, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, S.; Ploessl, F.; Bracher, F.; Laforsch, C. Single and combined toxicity of pharmaceuticals at environmentally relevant concentrations in Daphnia magna—A multigenerational study. Chemosphere 2010, 79, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 2018, 4, dvy016. [Google Scholar] [CrossRef] [PubMed]
- European Chemical Agency (ECHA). Guidance on Information Requirements and Chemical Safety Assessment, Chapter R.7c: Endpoint Specific Guidance (Version 3.0), June 2017. Available online: https://echa.europa.eu/documents/10162/13632/information_requirements_r7c_en.pdf/e2e23a98-adb2-4573-b450-cc0dfa7988e5 (accessed on 1 May 2022).
- Jeremias, G.; Gonçalves, F.J.M.; Pereira, J.L.; Asselman, J. Prospects for incorporation of epigenetic biomarkers in human health and environmental risk assessment of chemicals. Biol. Rev. 2020, 95, 822–846. [Google Scholar] [CrossRef] [PubMed]
- Mirbahai, L.; Chipman, J.K. Epigenetic memory of environmental organisms: A reflection of lifetime stressor exposures. Mutat. Res. Toxicol. Environ. Mutagen. 2014, 764–765, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Burggren, W. Epigenetic Inheritance and Its Role in Evolutionary Biology: Re-Evaluation and New Perspectives. Biology 2016, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Effertz, C.; Müller, S.; von Elert, E. Differential Peptide Labeling (iTRAQ) in LC–MS/MS Based Proteomics in Daphnia Reveal Mechanisms of an Antipredator Response. J. Proteome Res. 2014, 14, 888–896. [Google Scholar] [CrossRef]
- Vandegehuchte, M.B.; Lemière, F.; Vanhaecke, L.; Vanden Berghe, W.; Janssen, C.R. Direct and transgenerational impact on Daphnia magna of chemicals with a known effect on DNA methylation. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2010, 151, 278–285. [Google Scholar] [CrossRef]
- Harris, K.D.M.; Bartlett, N.J.; Lloyd, V.K. Daphnia as an Emerging Epigenetic Model Organism. Genet. Res. Int. 2012, 2012, 147892. [Google Scholar] [CrossRef]
- Chatterjee, N.; Gim, J.; Choi, J. Epigenetic profiling to environmental stressors in model and non-model organisms: Ecotoxicology perspective. Environ. Health Toxicol. 2018, 33, e2018015. [Google Scholar] [CrossRef]
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test, OECD Guidel. Test. Og Chem. Sect. 2004, 2, 1–12. Available online: http://www.oecd-ilibrary.org/content/book/9789264069947-en (accessed on 1 May 2022).
- OECD. Test No. 211: Daphnia magna Reproduction Test, in OECD Guidelines for the Testing of Chemicals, Section 2: Effects on Biotic Systems. 2012. Available online: https://www.oecd.org/chemicalsafety/test-no-211-daphnia-magna-reproduction-test-9789264185203-en.htm (accessed on 1 May 2022).
- Lee, S.-B.; Choe, Y.; Chon, T.-S.; Kang, H.Y. Analysis of zebrafish (Danio rerio) behavior in response to bacterial infection using a self-organizing map. BMC Veter-Res. 2015, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Rivera, G.; Dagnac, T.; Lores, M.; Garcia-Jares, C.; Sanchez-Prado, L.; Lamas, J.P.; Llompart, M. Determination of isothiazolinone preservatives in cosmetics and household products by matrix solid-phase dispersion followed by high-performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1270, 41–50. [Google Scholar] [CrossRef]
- Baranowska, I.; Wojciechowska, I. The determination of preservatives in cosmetics and environmental waters by HPLC. Pol. J. Environ. Stud. 2013, 22, 1609–1625. [Google Scholar]
- Paijens, C.; Bressy, A.; Frère, B.; Tedoldi, D.; Mailler, R.; Rocher, V.; Neveu, P.; Moilleron, R. Urban pathways of biocides towards surface waters during dry and wet weathers: Assessment at the Paris conurbation scale. J. Hazard. Mater. 2020, 402, 123765. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Zawadzka, K.; Lisowska, K. Occurrence of methylisothiazolinone in water and soil samples in Poland and its biodegradation by Phanerochaete chrysosporium. Chemosphere 2020, 254, 126723. [Google Scholar] [CrossRef] [PubMed]
- Belaid, C.; Sbartai, I. Assessing the effects of Thiram to oxidative stress responses in a freshwater bioindicator cladoceran (Daphnia magna). Chemosphere 2020, 268, 128808. [Google Scholar] [CrossRef]
- Cai, M.; Liu, Z.; Chen, M.; Zhang, M.; Jiao, Y.; Chen, Q.; Zhao, Y. Comparative proteomic analysis of senescence in the freshwater cladoceran Daphnia pulex. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 239, 110352. [Google Scholar] [CrossRef]
- Jeong, S.W.; Lee, S.M.; Yum, S.S.; Iguchi, T.; Seo, Y.R. Genomic expression responses toward bisphenol-A toxicity in Daphnia magna in terms of reproductive activity. Mol. Cell. Toxicol. 2013, 9, 149–158. [Google Scholar] [CrossRef]
- YKato, Y.; Tokishita, S.-I.; Ohta, T.; Yamagata, H. A vitellogenin chain containing a superoxide dismutase-like domain is the major component of yolk proteins in cladoceran crustacean Daphnia magna. Gene 2004, 334, 157–165. [Google Scholar] [CrossRef]
- Ho, D.H.; Burggren, W.W. Epigenetics and transgenerational transfer: A physiological perspective. J. Exp. Biol. 2010, 213, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.B.; Pomati, F.; Eggen, R.I. The toxicity of chemical pollutants in dynamic natural systems: The challenge of integrating environmental factors and biological complexity. Sci. Total. Environ. 2013, 449, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Vandegehuchte, M.B.; Vandenbrouck, T.; De Coninck, D.; De Coen, W.M.; Janssen, C.R. Gene transcription and higher-level effects of multigenerational Zn exposure in Daphnia magna. Chemosphere 2010, 80, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Lee, M.J.; Yu, S.H.; Kim, S.D. The individual and population effects of tetracycline on Daphnia magna in multigenerational exposure. Ecotoxicology 2012, 21, 993–1002. [Google Scholar] [CrossRef]
- Hanazato, T. Pesticide effects on freshwater zooplankton: An ecological perspective. Environ. Pollut. 2000, 112, 1–10. [Google Scholar] [CrossRef]
- Kahrilas, G.A.; Blotevogel, J.; Stewart, P.S.; Borch, T. Biocides in Hydraulic Fracturing Fluids: A Critical Review of Their Usage, Mobility, Degradation, and Toxicity. Environ. Sci. Technol. 2015, 49, 16–32. [Google Scholar] [CrossRef]
- Silva, A.R.R.; Cardoso, D.N.; Cruz, A.; Pestana, J.L.; Mendo, S.; Soares, A.M.; Loureiro, S. Multigenerational effects of carbendazim in Daphnia magna. Environ. Toxicol. Chem. 2016, 36, 383–394. [Google Scholar] [CrossRef]
- Plaire, D.; Bourdineaud, J.-P.; Alonzo, A.; Camilleri, V.; Garcia-Sanchez, L.; Adam-Guillermin, C.; Alonzo, F. Transmission of DNA damage and increasing reprotoxic effects over two generations of Daphnia magna exposed to uranium. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2013, 158, 231–243. [Google Scholar] [CrossRef]
- Parisot, F.; Bourdineaud, J.-P.; Plaire, D.; Adam-Guillermin, C.; Alonzo, F. DNA alterations and effects on growth and reproduction in Daphnia magna during chronic exposure to gamma radiation over three successive generations. Aquat. Toxicol. 2015, 163, 27–36. [Google Scholar] [CrossRef]
- A Atienzar, F.; Jha, A.N. The random amplified polymorphic DNA (RAPD) assay to determine DNA alterations, repair and transgenerational effects in B(a)P exposed Daphnia magna. Mutat. Res. Mol. Mech. Mutagen. 2004, 552, 125–140. [Google Scholar] [CrossRef]
- Atienzar, F.A.; Cheung, V.V.; Jha, A.N.; Depledge, M.H. Fitness Parameters and DNA Effects Are Sensitive Indicators of Copper-Induced Toxicity in Daphnia magna. Toxicol. Sci. 2001, 59, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, J.; Head, J.A. Effects of in ovo exposure to benzo[k]fluoranthene (BkF) on CYP1A expression and promoter methylation in developing chicken embryos. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2018, 204, 88–96. [Google Scholar] [CrossRef]
- Bester, K.; Vollertsen, J.; Bollmann, U.E. Water Driven Leaching of Biocides from Paints and Renders (Pesticide Research No. 156, 2014); Danish Ministry of Environment: Copenhagen, Denmark, 2014. [Google Scholar]
- Lee, J.H.; Paek, J.H.; Park, H.N.; Park, S.; Kang, H. Screening and detection of methylisothiazolinone and chloromethylisothiazolinone in cosmetics by UPLC-MS/MS. Anal. Sci. Technol. 2020, 33, 125–133. [Google Scholar] [CrossRef]
- Zhong, H.; Li, Z.; Chen, S.; Zeng, Y.; Zheng, J.; Zeng, Y.; Li, D. Simultaneous quantitative analysis of six isothiazolinones in water-based adhesive used for food contact materials by high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS/MS). Molecules 2019, 24, 3894. [Google Scholar] [CrossRef] [PubMed]






| Nominal Concentration (mg/L) | Measured Concentration (mg/L) | ||
|---|---|---|---|
| CMIT/MIT Mixture (3:1) | Day 0 | Day 7 | |
| Standard solution | 14,000 | - | CMIT: 11,300 MIT: 3000 |
| Stock solutions | 1.4 | CMIT: 0.99 ± 0.17 MIT: 0.35 ± 0.06 | CMIT: 1.03 ± 0.24 MIT: 0.37 ± 0.04 |
| 0.14 | CMIT: 0.10 ± 0.01 MIT: 0.04 ± 0.01 | CMIT: 0.13 ± 0.02 MIT: 0.04 ± 0.01 | |
| Detection limit (mg/L) | ≥CMIT: 0.100, MIT: 0.030 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Choi, J. Trans- and Multigenerational Effects of Isothiazolinone Biocide CMIT/MIT on Genotoxicity and Epigenotoxicity in Daphnia magna. Toxics 2023, 11, 388. https://doi.org/10.3390/toxics11040388
Kim J, Choi J. Trans- and Multigenerational Effects of Isothiazolinone Biocide CMIT/MIT on Genotoxicity and Epigenotoxicity in Daphnia magna. Toxics. 2023; 11(4):388. https://doi.org/10.3390/toxics11040388
Chicago/Turabian StyleKim, Jiwan, and Jinhee Choi. 2023. "Trans- and Multigenerational Effects of Isothiazolinone Biocide CMIT/MIT on Genotoxicity and Epigenotoxicity in Daphnia magna" Toxics 11, no. 4: 388. https://doi.org/10.3390/toxics11040388
APA StyleKim, J., & Choi, J. (2023). Trans- and Multigenerational Effects of Isothiazolinone Biocide CMIT/MIT on Genotoxicity and Epigenotoxicity in Daphnia magna. Toxics, 11(4), 388. https://doi.org/10.3390/toxics11040388