Increased Occurrence of Cutaneous Leiomyomas and Dermatofibromas in Patients with Uterine Leiomyomas without Fumarate Hydratase Gene Mutations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Investigation
2.2. FH Mutation Analysis
2.3. Histological and Immunohistochemical Studies
2.4. Cell Culture
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
3.1. Clinical and Serological Data
3.2. Genomic Analysis and Frequency of Cutaneous Tumours
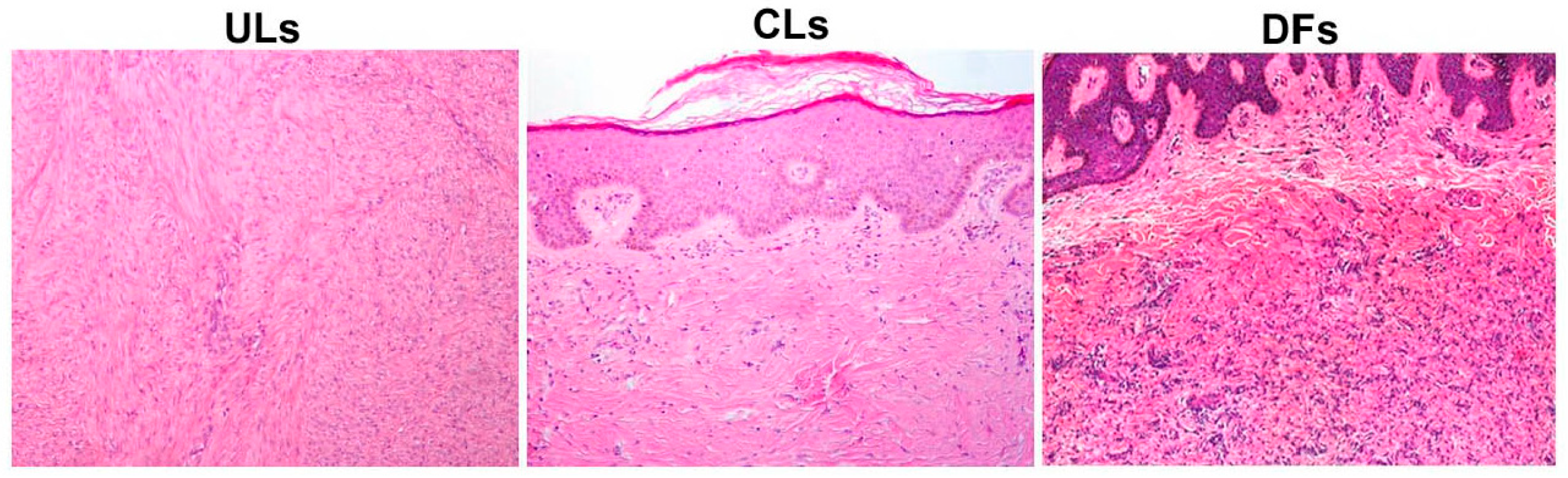
3.3. Microscopic and Immunohistochemical Analysis of the Dermis
3.4. 1,25(OH)2D3 and TGFβ-1 Have Opposite Effects on VDR, TGFβ-RII and α-SMA Expression In Vitro
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borahay, M.A.; Al-Hendy, A.; Kilic, G.S.; Boehning, D. Signaling Pathways in Leiomyoma: Understanding Pathobiology and Implications for Therapy. Mol. Med. 2015, 21, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Stewart, E.A.; Morton, C.C. Cutaneous and Uterine Leiomyomas. Mayo Clin. Proc. 2015, 7, 990. [Google Scholar] [CrossRef] [PubMed]
- Llamas-Velasco, M.; Requena, L.; Kutzner, H.; Schärer, L.; Rütten, A.; Hantschke, M.; Paredes, B.E.; Mentzel, T. Fumarate hydratase immunohistochemical staining may help to identify patients with multiple cutaneous and uterine leiomyomatosis (MCUL) and hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome. J. Cutan. Pathol. 2014, 41, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Lethaby, A.; Vollenhoven, B. Fibroids (uterine myomatosis, leiomyomas). BMJ Clin. Evid. 2015, 2015, 0814. [Google Scholar]
- Laughlin-Tommaso, S.K.; Stewart, E.A. Moving Toward Individualized Medicine for Uterine Leiomyomas. Obstet. Gynecol. 2018, 132, 961–971. [Google Scholar] [CrossRef]
- Dvorská, D.; Braný, D.; Danková, Z.; Halašová, E.; Višňovský, J. Molecular and clinical attributes of uterine leiomyomas. Tumour Biol. 2017, 39, 1010428317710226. [Google Scholar] [CrossRef] [Green Version]
- Malik, K.; Patel, P.; Chen, J.; Khachemoune, A. Leiomyoma cutis: A focused review on presentation, management, and association with malignancy. Am. J. Clin. Dermatol. 2015, 16, 35–46. [Google Scholar] [CrossRef]
- Bernett, C.N.; Mammino, J.J. Cutaneous Leiomyomas. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482166 (accessed on 29 August 2022).
- Mentzel, T. Cutaneous mesenchymal tumours: An update. Pathology 2014, 46, 149–159. [Google Scholar] [CrossRef]
- Myers, D.J.; Fillman, E.P. Dermatofibroma. [Updated 24 October 2022]. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470538 (accessed on 24 August 2022).
- Kubo, M.; Ihn, H.; Yamane, K.; Tamaki, K. The expression levels and the differential expression of transforming growth factor-beta receptors in dermatofibroma and dermatofibrosarcoma protuberans. Br. J. Dermatol. 2006, 15, 919–925. [Google Scholar] [CrossRef]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part A: Renal, Penile, and Testicular Tumours. Eur Urol 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Bhola, P.T.; Gilpin, C.; Smith, A.; Graham, G.E. A retrospective review of 48 individuals, including 12 families, molecularly diagnosed with hereditary leiomyomatosis and renal cell cancer (HLRCC). Fam. Cancer 2018, 17, 615–620. [Google Scholar] [CrossRef]
- Diluvio, L.; Torti, C.; Terrinoni, A.; Candi, E.; Piancatelli, R.; Piccione, E.; Paternò, E.J.; Chimenti, S.; Orlandi, A.; Campione, E.; et al. Dermoscopy as an adjuvant tool for detecting skin leiomyomas in patient with uterine fibroids and cerebral cavernomas. BMC Dermatol. 2014, 14, 7. [Google Scholar] [CrossRef]
- Ciarmela, P.; Islam, M.S.; Reis, F.M.; Gray, P.C.; Bloise, E.; Petraglia, F.; Vale, W.; Castellucci, M. Growth factors and myometrium: Biological effects in uterine fibroid and possible clinical implications. Hum. Reprod. Update 2011, 17, 772–790. [Google Scholar] [CrossRef] [Green Version]
- Catherino, W.H.; Eltoukhi, H.M.; Al-Hendy, A. Racial and ethnic differences in the pathogenesis and clinical manifestations of uterine leiomyoma. Semin. Reprod. Med. 2013, 31, 370–379. [Google Scholar] [CrossRef] [Green Version]
- Halder, S.K.; Goodwin, J.S.; Al-Hendy, A. 1,25-Dihydroxyvitamin D3 reduces TGF-beta3-induced fibrosis-related gene expression in human uterine leiomyoma cells. J. Clin. Endocrinol. Metab. 2011, 96, E754–E762. [Google Scholar] [CrossRef]
- Brakta, S.; Diamond, J.S.; Al-Hendy, A.; Diamond, M.P.; Halder, S.K. Role of vitamin D in uterine fibroid biology. Fertil. Steril. 2015, 104, 698–706. [Google Scholar] [CrossRef] [Green Version]
- Leask, A.; Abraham, D.J. TGF-beta Signaling and the Fibrotic Response. J. FASEB 2004, 18, 816–827. [Google Scholar] [CrossRef]
- Ikushima, H.; Miyazono, K. TGFbeta signalling: A complex web in cancer progression. Nat. Rev. Cancer 2010, 10, 415–424. [Google Scholar] [CrossRef]
- Graycar, J.L.; Miller, D.A.; Arrick, B.A.; Lyons, R.M.; Moses, H.L.; Derynck, R. Human transforming growth factor-beta 3: Recombinant expression, purification, and biological activities in comparison with transforming growth factors-beta 1 and beta 2. Mol. Endocrinol. 1989, 3, 1977–1986. [Google Scholar] [CrossRef]
- Brahmatewari, J.; Serafini, A.; Serralta, V.; Mertz, P.M.; Eaglstein, W.H. The effects of topical transforming growth factor-beta2 and anti-transforming growth factor-beta2,3 on scarring in pigs. J. Cutan. Med. Surg. 2000, 4, 126–131. [Google Scholar] [CrossRef]
- Chang, Z.; Kishimoto, Y.; Hasan, A.; Welham, N.V. TGF-β3 modulates the inflammatory environment and reduces scar formation following vocal fold mucosal injury in rats. Dis. Model. Mech. 2014, 7, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Attisano, L.; Wrana, J.L. Mads and Smads in TGF beta signaling. Curr. Opin. Cell Biol. 1998, 10, 188–194. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Cheng, T.; Luan, Q.; Liao, T.; Nie, C.L.; Zheng, X.; Xie, X.G.; Gao, W.Y. Vitamin D: A novel therapeutic approach for keloid, an in vitro analysis. Br. J. Dermatol. 2011, 164, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.D.; Hill, M.C.; Schectman, J.M. Vitamin D and the risk of uterine fibroids. Epidemiology 2013, 24, 447–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, W.J.; Andrici, J.; Maclean, F.; Madadi-Ghahan, R.; Farzin, M.; Sioson, L.; Toon, C.W.; Clarkson, A.; Watson, N.; Pickett, J.; et al. Fumarate Hydratase-deficient Uterine Leiomyomas Occur in Both the Syndromic and Sporadic Settings. Am. J. Surg. Pathol. 2016, 40, 599–607. [Google Scholar] [CrossRef] [Green Version]
- Passeri, D.; Doldo, E.; Tarquini, C.; Costanza, G.; Mazzaglia, D.; Agostinelli, S.; Campione, E.; Di Stefani, A.; Giunta, A.; Bianchi, L.; et al. Loss of CRABP-II Characterizes Human Skin Poorly Differentiated Squamous Cell Carcinomas and Favors DMBA/TPA-Induced Carcinogenesis. J. Investig. Dermatol. 2016, 136, 1255–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlandi, A.; Francesconi, A.; Marcellini, M.; Ferlosio, A.; Spagnoli, L.G. Role of ageing and coronary atherosclerosis in the development of cardiac fibrosis in the rabbit. Cardiovasc. Res. 2004, 64, 544–552. [Google Scholar] [CrossRef] [Green Version]
- Bläuer, M.; Rovio, P.H.; Ylikomi, T.; Heinonen, P.K. Vitamin D inhibits myometrial and leiomyoma cell proliferation in vitro. Fertil. Steril. 2009, 91, 1919–1925. [Google Scholar] [CrossRef]
- Orlandi, A.; Ropraz, P.; Gabbiani, G. Proliferative activity and alpha-smooth muscle actin expression in cultured rat aortic smooth muscle cells are differently modulated by transforming growth factor-beta 1 and heparin. Exp. Cell Res. 1994, 214, 528–536. [Google Scholar] [CrossRef]
- Doldo, E.; Costanza, G.; Ferlosio, A.; Pompeo, E.; Agostinelli, S.; Bellezza, G.; Mazzaglia, D.; Giunta, A.; Sidoni, A.; Orlandi, A. High expression of cellular retinol binding protein-1 in lung adenocarcinoma is associated with poor prognosis. Genes Cancer 2015, 6, 490–502. [Google Scholar] [CrossRef] [Green Version]
- Costanza, G.; Doldo, E.; Ferlosio, A.; Tarquini, C.; Passeri, D.; Cascella, R.; Bavetta, M.; Di Stefani, A.; Bonifati, C.; Agostinelli, S.; et al. Expression and potential role of cellular retinol binding protein I in psoriasis. Oncotarget 2018, 9, 36736–36749. [Google Scholar] [CrossRef] [Green Version]
- Mentzel, T.; Wiesner, T.; Cerroni, L.; Hantschke, M.; Kutzner, H.; Rütten, A.; Häberle, M.; Bisceglia, M.; Chibon, F.; Coindre, J.M. Malignant dermatofibroma: Clinicopathological, immunohistochemical, and molecular analysis of seven cases. Mod. Pathol. 2013, 26, 256–267. [Google Scholar] [CrossRef] [Green Version]
- Beatrous, S.V.; Riahi, R.R.; Grisoli, S.B.; Cohen, P.R. Associated conditions in patients with multiple dermatofibromas: Case reports and literature review. Dermatol. Online J. 2017, 23, 13030/qt8zv852d8. [Google Scholar] [CrossRef]
- Paffoni, A.; Somigliana, E.; Vigano’, P.; Benaglia, L.; Cardellicchio, L.; Pagliardini, L.; Papaleo, E.; Candiani, M.; Fedele, L. Vitamin D status in women with uterine leiomyomas. J. Clin. Endocrinol. Metab. 2013, 98, E1374–E1378. [Google Scholar] [CrossRef] [Green Version]
- Wise, L.A.; Ruiz-Narváez, E.A.; Haddad, S.A.; Rosenberg, L.; Palmer, J.R. Polymorphisms in vitamin D-related genes and risk of uterine leiomyomata. Fertil. Steril. 2014, 102, 503–510.e1. [Google Scholar] [CrossRef] [Green Version]
- Zerr, P.; Vollath, S.; Palumbo-Zerr, K.; Tomcik, M.; Huang, J.; Distler, A.; Beyer, C.; Dees, C.; Gela, K.; Distler, O.; et al. Vitamin D receptor regulates TGF-β signalling in systemic sclerosis. Ann. Rheum. Dis. 2015, 74, e20. [Google Scholar] [CrossRef]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. Vitamin D3 inhibits expression and activities of matrix metalloproteinase-2 and -9 in human uterine fibroid cells. Hum. Reprod. 2013, 28, 2407–2416. [Google Scholar] [CrossRef] [Green Version]
- Halder, S.K.; Osteen, K.G.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 reduces extracellular matrix-associated protein expression in human uterine fibroid cells. Biol. Reprod. 2013, 89, 150. [Google Scholar] [CrossRef]
- Meredith, A.; Boroomand, S.; Carthy, J.; Luo, Z.; McManus, B. 1,25 Dihydroxyvitamin D3 Inhibits TGFβ1-Mediated Primary Human Cardiac Myofibroblast Activation. PLoS ONE 2015, 10, e0128655. [Google Scholar] [CrossRef]
- Shany, S.; Sigal-Batikoff, I.; Lamprecht, S. Vitamin D and Myofibroblasts in Fibrosis and Cancer: At Cross-purposes with TGF-β/SMAD Signaling. Anticancer. Res. 2016, 36, 6225–6234. [Google Scholar] [CrossRef] [Green Version]
- Halder, S.K.; Sharan, C.; Al-Hendy, A. 1,25-dihydroxyvitamin D3 treatment shrinks uterine leiomyoma tumors in the Eker rat model. Biol. Reprod. 2012, 86, 116. [Google Scholar] [CrossRef] [PubMed]
- Penn, J.W.; Grobbelaar, A.O.; Rolfe, K.J. The role of the TGF-β family in wound healing, burns and scarring: A review. Int. J. Burn. Trauma 2012, 2, 18–28. [Google Scholar]
- Kogan, E.A.; Ignatova, V.E.; Rukhadze, T.N.; Kudrina, E.A.; Ishchenko, A.I. A role of growth factors in development of various histological types of uterine leiomyoma. Arkh. Patol. 2005, 67, 34–38. [Google Scholar] [PubMed]
- Doherty, L.F.; Taylor, H.S. Leiomyoma-derived transforming growth factor-β impairs bone morphogenetic protein-2-mediated endometrial receptivity. Fertil. Steril. 2015, 103, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Imanishi, A.; Imanishi, H.; Yoshida, Y.; Okabayashi, A.; Tateishi, C.; Ikushima, H.; Nagasako, R.; Nakagawa, K.; Tsuruta, D. Upregulation of TGF-β1 and basic FGF in elastofibroma: An immunohistochemical analysis. Med. Mol. Morphol. 2016, 49, 83–88. [Google Scholar] [CrossRef]
- De Falco, M.; Staibano, S.; D’Armiento, F.P.; Mascolo, M.; Salvatore, G.; Busiello, A.; Carbone, I.F.; Pollio, F.; Di Lieto, A. Preoperative treatment of uterine leiomyomas: Clinical findings and expression of transforming growth factor-beta3 and connective tissue growth factor. J. Soc. Gynecol. Investig. 2006, 13, 297–303. [Google Scholar] [CrossRef]
- Yen-Ping Ho, J.; Man, W.C.; Wen, Y.; Polan, M.L.; Shih-Chu Ho, E.; Chen, B. Transforming growth interacting factor expression in leiomyoma compared with myometrium. Fertil. Steril. 2010, 94, 1078–1083. [Google Scholar] [CrossRef]
- Di, X.; Andrews, D.M.K.; Tucker, C.J.; Yu, L.; Moore, A.B.; Zheng, X.; Castro, L.; Hermon, T.; Xiao, H.; Dixon, D. A high concentration of genistein down-regulates activin A, Smad3 and other TGF-β pathway genes in human uterine leiomyoma cells. Exp. Mol. Med. 2012, 44, 281–292. [Google Scholar] [CrossRef]
- Lee, S.A.; Yang, H.W.; Um, J.Y.; Shin, J.M.; Park, I.H.; Lee, H.M. Vitamin D attenuates myofibroblast differentiation and extracellular matrix accumulation in nasal polyp-derived fibroblasts through smad2/3 signaling pathway. Sci. Rep. 2017, 7, 7299. [Google Scholar] [CrossRef]



| Variable | n = 71 | % | |
|---|---|---|---|
| Age (Mean ± SEM) | 46.5 (±16.5 years) | ||
| Body-mass index | Underweight | 19 | 26.76 |
| Normal weight | 38 | 53.52 | |
| Overweight | 14 | 19.72 | |
| Smoking (>10 cigarettes/day) | no | 30 | 42.25 |
| yes | 41 | 57.75 | |
| Parity | Nulliparous | 31 | 43.66 |
| Parous | 40 | 56.34 | |
| Mode of Delivery | Spontaneous | 20 | 28.17 |
| Operative | 2 | 2.82 | |
| Caesarean section | 18 | 25.35 | |
| Nulliparous | 31 | 43.66 | |
| Infertility | no | 51 | 71.83 |
| yes | 20 | 28.17 | |
| Period flow | Regular period | 19 | 26.76 |
| Irregular period | 24 | 33.80 | |
| Menopause | 18 | 25.35 | |
| Contraceptive use | 10 | 14.08 | |
| Uterine leiomyomas | no | 37 | 52.11 |
| yes | 34 | 47.89 | |
| Cutaneous leiomyomas | no | 63 | 88.73 |
| yes | 8 | 11.27 | |
| Cutaneous dermatofibromas | no | 44 | 61.97 |
| yes | 27 | 38.03 |
| ULs Group | Control Group | OR | 95% CI | |
|---|---|---|---|---|
| (n = 34) | (n = 37) | |||
| Anaemia (<12 g/dL) | ||||
| no | 15 (44.0) | 26 (71.0) | - | - |
| yes | 19 (56.0) | 11 (29.0) | 2.99 (p < 0.02) | 1.13–7.96 |
| Menometrorrhagia | ||||
| no | 12 (35.2) | 28 (75.7) | - | - |
| yes | 22 (64.7) | 9 (24.3) | 5.70 (p < 0.0006) | 2.04–15.96 |
| Headache | ||||
| no | 11 (32.4) | 22 (59.6) | - | - |
| yes | 23 (67.6) | 15 (40.5) | 3.07 (p < 0.02) | 1.16–8.12 |
| Vitamin D level (ng/mL) | ||||
| <10 | 20 (58.8) | 1 (2.7) | - | - |
| 11–20 | 7 (20.6) | 14 (37.8) | 40 (p < 0.001) | 4.42–362.39 |
| 21–50 | 7 (20.6) | 22 (59.5) | 62.9 (p < 0.001) | 7.10–556.69 |
| Family History of UL | ||||
| no | 12 (35.3) | 22 (59.6) | - | - |
| yes | 22 (64.7) | 15 (40.5) | 2.69 (p < 0.04) | 1.03–7.04 |
| ULs Group (n = 34) | Control Group (n = 37) | OR | 95%CI | |
|---|---|---|---|---|
| CLs | ||||
| no | 27 (79.4) | 36 (97.3) | - | - |
| yes | 7 (20.6) | 1 (2.7) | 0.13 (p < 0.03) | 0.11–1.14 |
| DFs | ||||
| no | 19 (55.9) | 34 (91.9) | - | - |
| yes | 15 (44.1) | 3 (8.1) | 7.93 (p < 0.001) | 2.02–31.03 |
| Nevi | ||||
| no | 18 (52.9) | 19 (51.4) | - | - |
| yes | 16 (47.1) | 18 (48.6) | 0.94 | 0.37–2.38 |
| Other | ||||
| no | 19 (55.9) | 18 (48.6) | - | - |
| yes | 15 (44.1) | 19 (51.4) | 0.75 | 0.29–1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campione, E.; Di Prete, M.; Costanza, G.; Saggini, A.; Agostinelli, S.; Terrinoni, A.; Centofanti, F.; Rapanotti, M.C.; Bianchi, L.; Ferlosio, A.; et al. Increased Occurrence of Cutaneous Leiomyomas and Dermatofibromas in Patients with Uterine Leiomyomas without Fumarate Hydratase Gene Mutations. Dermatopathology 2023, 10, 231-243. https://doi.org/10.3390/dermatopathology10030032
Campione E, Di Prete M, Costanza G, Saggini A, Agostinelli S, Terrinoni A, Centofanti F, Rapanotti MC, Bianchi L, Ferlosio A, et al. Increased Occurrence of Cutaneous Leiomyomas and Dermatofibromas in Patients with Uterine Leiomyomas without Fumarate Hydratase Gene Mutations. Dermatopathology. 2023; 10(3):231-243. https://doi.org/10.3390/dermatopathology10030032
Chicago/Turabian StyleCampione, Elena, Monia Di Prete, Gaetana Costanza, Andrea Saggini, Sara Agostinelli, Alessandro Terrinoni, Federica Centofanti, Maria Cristina Rapanotti, Luca Bianchi, Amedeo Ferlosio, and et al. 2023. "Increased Occurrence of Cutaneous Leiomyomas and Dermatofibromas in Patients with Uterine Leiomyomas without Fumarate Hydratase Gene Mutations" Dermatopathology 10, no. 3: 231-243. https://doi.org/10.3390/dermatopathology10030032
APA StyleCampione, E., Di Prete, M., Costanza, G., Saggini, A., Agostinelli, S., Terrinoni, A., Centofanti, F., Rapanotti, M. C., Bianchi, L., Ferlosio, A., Scioli, M. G., & Orlandi, A. (2023). Increased Occurrence of Cutaneous Leiomyomas and Dermatofibromas in Patients with Uterine Leiomyomas without Fumarate Hydratase Gene Mutations. Dermatopathology, 10(3), 231-243. https://doi.org/10.3390/dermatopathology10030032









