Abstract
Background: We present an interesting case involving a tumour comprising both basal cell tumour cells and sarcomatoid tumour cells. An 86-year-old woman presented with an erythematous lesion on her left cheek. Clinical and dermoscopic findings suggested BCC. Complete excision and histopathological examination revealed a BCC with a separate proliferation of atypical spindle and epithelioid cells. Immunohistochemical staining supported the diagnosis, with basaloid cells positive for CK5/6 and Ber-EP4 and sarcomatoid cells positive for CD10 and vimentin. Results: Histology and immunohistochemistry confirmed a basal cell carcinoma with sarcomatoid differentiation. The close proximity of sarcomatoid cells to the BCC component suggests a potential role of epithelial–mesenchymal interactions in tumour development. Further investigations into the exact origin of this tumour are required. Conclusion: Basal cell carcinoma with sarcomatoid differentiation is rare. This case highlights the importance of thorough histological and immunohistochemical evaluation. Further studies are necessary to better understand the pathogenesis of such collision tumours.
1. Introduction
We present a rare histopathological case of a tumour consisting of a basal cell carcinoma (BCC) and sarcomatoid tumour cells. Basal cell carcinoma histologically shows a proliferation of basaloid cells and embryologically originates from the ectoderm [1,2,3]. To date, only a few cases of basal cell carcinoma with sarcomatoid differentiation have been reported.
2. Histopathology Case
An 86-year-old woman presented to our dermatology department with a skin lesion on her left cheek that was clinically suspected to be BCC. According to her medical history, the lesion had progressed in the past few months. Clinical examination revealed an erythematous lesion approximately 1 cm in diameter. Dermoscopic examination of the lesion showed telangiectasia and a central scar. A complete excision and a histological analysis were performed. We found histopathological characteristics of conventional BCC (basaloid nests with peripheral palisading and cleft formation) (Figure 1 and Figure 2). In addition, other tumour components were found next to and between the basaloid cells. This second tumour component showed epithelioid and spindled cell proliferations (Figure 2). Immunohistochemical analyses were performed to further characterise this tumour. Immunohistochemically, basaloid tumour cells were CK5/6- and Ber-EP4-positive and CK20-negative. The epithelioid tumour cells were strongly CD10- and vimentin-positive and actin-, desmin-, CD34- and S100-negative. After histological and immunohistochemical correlation, the diagnosis of a basal cell carcinoma with sarcomatoid differentiation was made.
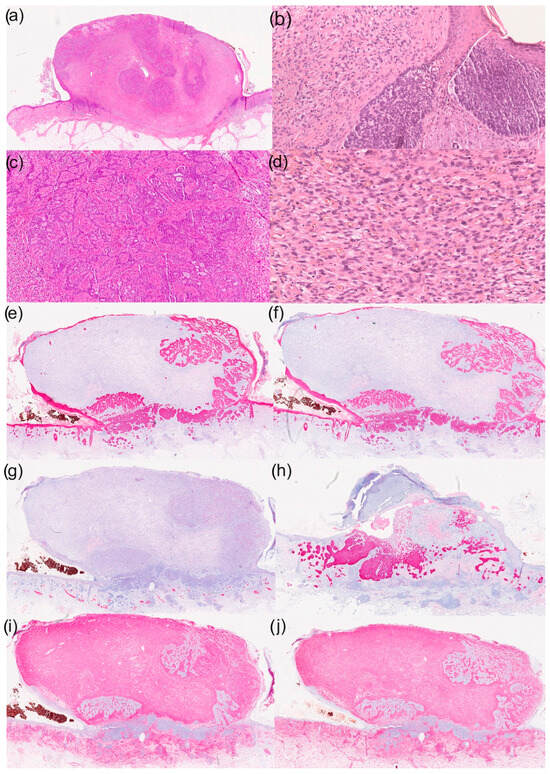
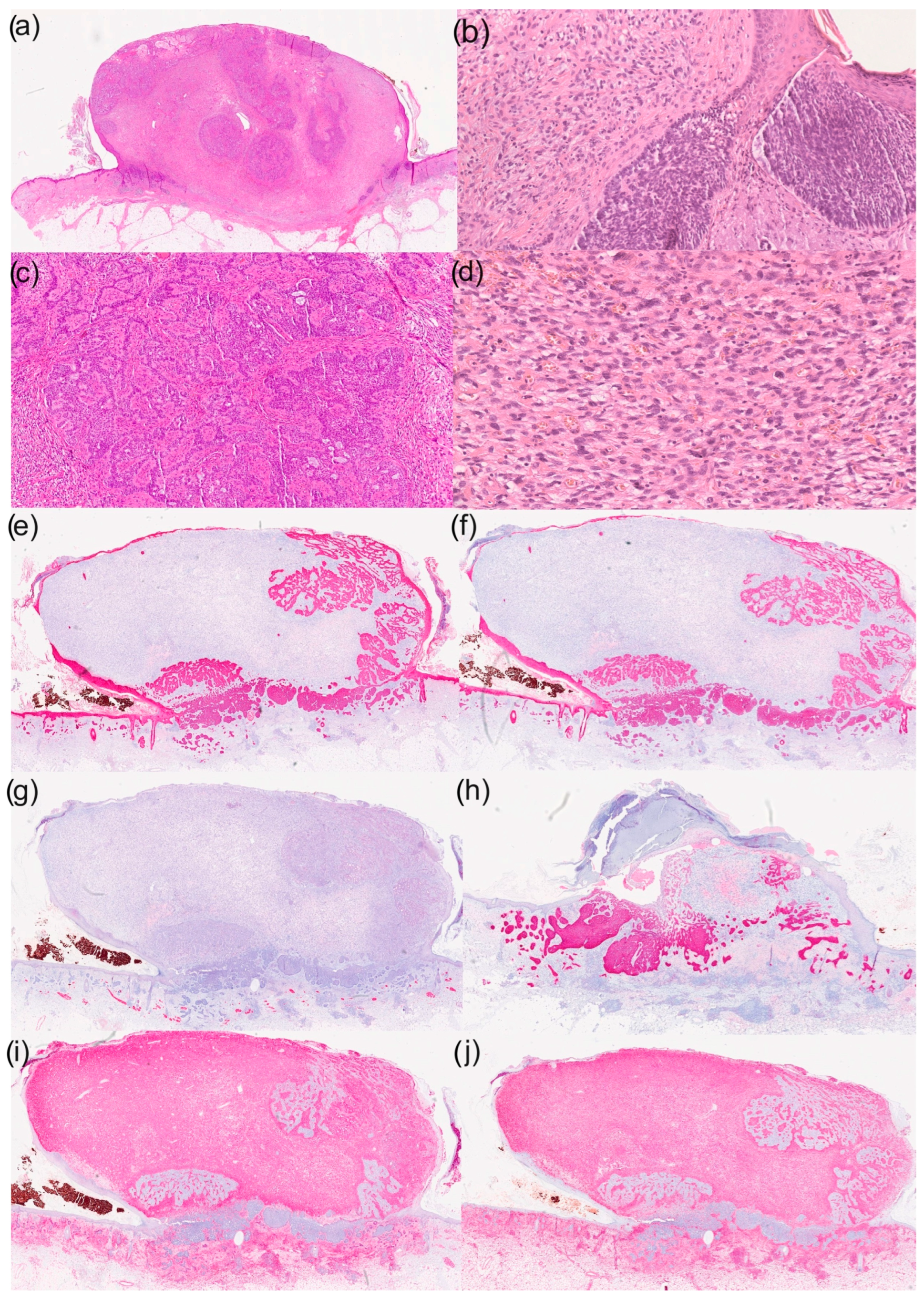
Figure 1.
Histological and immunohistochemical results of a basal cell carcinoma with sarcomatoid differentiation: (a) overview (haematoxylin–eosin staining; HE staining), (b) parts of the basal cell carcinoma with typical morphological characteristics (100× magnification, HE staining), (c) reticular growth pattern of basal cell carcinoma (50× magnification with HE staining), (d) cell- and vessel-rich stroma with epithelioid tumour cells (200× magnification with HE staining), (e) immunohistochemistry for CK5/6 (overview), (f) immunohistochemistry for CK MNF (overview), (g) immunohistochemistry for desmin (overview), (h) immunohistochemistry for Ber-EP4 (overview), (i) immunohistochemistry for CD10 (overview) and (j) immunohistochemistry for vimentin (overview).
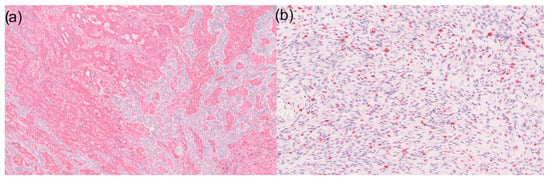
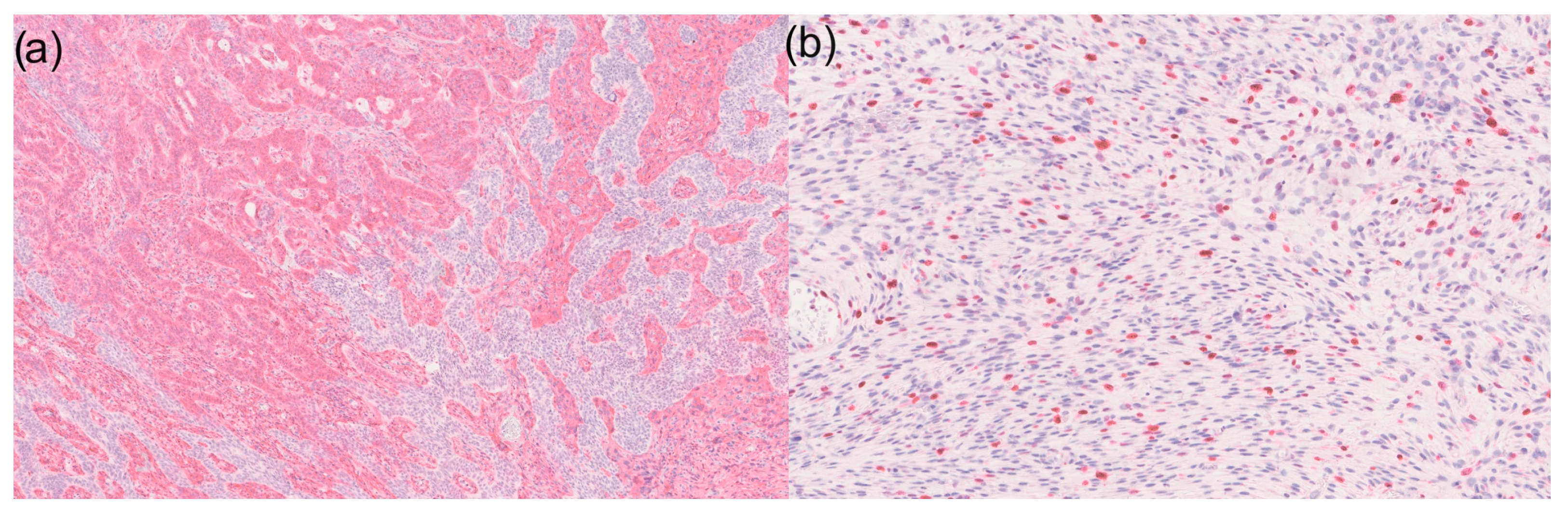
Figure 2.
(a) 50× magnification of a basal cell carcinoma with sarcomatoid differentiation in immunohistochemistry for vimentin: Sarcomatoid tumour components are visible on the left and basal cell carcinoma on the right side. However, it is noticeable that CD10-positive tumour cells can also be found within the basal cell carcinoma; (b) Ki67 expression shows high expression in the sarcomatoid tumour area (100× magnification).
3. Discussion and Conclusions
BCCs with sarcomatoid differentiation are a rare and heterogeneous tumour entity that has only been described in a few case reports and small case series to date. The studies published to date (Table 1) consistently show a biphasic architecture consisting of an epithelial BCC component and a sarcomatoid, spindle cell or heterologously differentiated mesenchymal component. Histologically, the mesenchymal components vary from undifferentiated spindle cell proliferations to osteosarcoma-like differentiation [2] to atypical fibroxanthoma-like patterns [4]. Immunohistochemically, the mesenchymal components sometimes show coexpression of epithelial markers (e.g., pancytokeratins, EMA) and mesenchymal markers (e.g., vimentin, smooth muscle actin), which supports the idea of translinear or dedifferentiating development. In individual cases, molecular abnormalities such as a PTCH1 mutation [5] or an EWSR1-PBX1 gene fusion [3] have been detected.
In the literature, a distinction is made between a collision tumour and a tumour with biphasic components. A collision tumour is defined as the coexistence of two or more histologically distinct tumours at the same anatomic site with clearly demarcated borders [6]. Our case most likely represents a biphasic, sarcomatoid BCC, which is a tumour composed of a malignant epithelial component intimately associated with a malignant mesenchymal component.
Basal cell carcinoma is the most common malignant skin tumour and originates from the basal cell layer of the epidermis. Embryologically, the basal cell layer and the epidermis develop from the ectoderm [7]. Interestingly, in the area of the stroma of the BCC, CD10- and vimentin-positive sarcomatoid tumour cells were found in our case. CD10- and vimentin-positive tumour cells were also found between the reticular basaloid cell proliferates (Figure 2). The expression of CD10 in the stroma of BCCs varies depending on the histological subtype [8]. Whether the sarcomatoid component originates from the stromal mesenchymal cells of a BCC or is the result of metaplastic transformation of the carcinomatous component remains speculative [1,2,3,9]. Furthermore, the origin of this rare tumour could be explained by the embryogenesis of the skin. During embryogenesis, the dermis develops primarily from the mesoderm [10]. Sarcomatoid tumour cells are dermal, suggesting a mesenchymal origin. However, the ectodermal (epithelial) component is also known to be able to induce the mesoderm [10]. These epithelial–mesenchymal interactions influence the extracellular matrix and microarchitecture at the dermal–epidermal junction. This interaction may be the origin of BCC with sarcomatoid differentiation. This hypothesis cannot be proven based on our observations, but further research should be conducted to support or refute this hypothesis. Examination of the cases suggests that BCC with sarcomatoid differentiation arises from a clonal epithelial precursor that undergoes dedifferentiation. This hypothesis is supported by immunohistochemical evidence of identical marker expression (e.g., p53) in both components [11]. Several case reports have histologically demonstrated the transition from epithelial to mesenchymal components [2,12], which could indicate epithelial–mesenchymal transition. The detection of a gene fusion [3] raises the question of whether true myoepithelial differentiation is present in individual cases.
Basal cell carcinoma with sarcomatoid differentiation is a potentially aggressive tumour [11,13]. From a therapeutic perspective, complete surgical excision appears to remain the central approach. The published cases with sufficient follow-up show that no recurrences or metastases occurred following the removal of the entire tumour, even in cases of heterologous differentiation. However, the number of cases is small and further follow-up is required to definitively evaluate the actual recurrence and metastasis potential of these rare tumours.
In conclusion, a basal cell carcinoma with sarcomatoid differentiation is rare and its origin should be investigated further.

Table 1.
A brief overview of BCC with sarcomatoid differentiation in the literature.
Table 1.
A brief overview of BCC with sarcomatoid differentiation in the literature.
| Author (Year) | Article Type, Number of Cases | Histology | Conclusion |
|---|---|---|---|
| Menamin et al. (2016) [2] | Case series, n = 11 |
|
|
| Mestre-Alagarda et al. (2019) [3] | Case report, n = 1 |
|
|
| Kiuru et al. (2014) [5] | Case report, n = 1 |
|
|
| Rose et al. (2008) [12] | Case series, n = 5 |
|
|
| Bigby et al. (2005) [11] | Case report, n = 1 |
|
|
| Inaloz et al. (2003) [4] | Case report, n = 1 |
|
|
BCC, basal cell carcinoma.
Author Contributions
Conceptualisation, N.A.R., M.D., N.O., E.S. and M.S.; methodology, N.A.R., M.D., N.O., E.S. and M.S.; software, N.A.R., M.D., N.O., E.S. and M.S.; validation, N.A.R., M.D., N.O., E.S. and M.S.; formal analysis, N.A.R., M.D., N.O., E.S. and M.S.; investigation, N.A.R., M.D., N.O., E.S. and M.S.; resources, N.A.R., M.D., N.O., E.S. and M.S.; data curation, N.A.R., M.D., N.O., E.S. and M.S.; writing—original draft preparation, N.A.R., M.D., N.O., E.S. and M.S.; writing—review and editing, N.A.R., M.D., N.O., E.S. and M.S.; visualisation, N.A.R., M.D., N.O., E.S. and M.S.; supervision, N.A.R. and M.D.; project administration, N.A.R. and M.D.; funding acquisition, N.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We acknowledge support by the Open Access Publication Funds of the Ruhr-Universität Bochum.
Conflicts of Interest
N.A. received funding, travel support and/or personal honoraria for lectures from Novartis Pharma, Janssen-Cilag GmbH, Recordati Rare Diseases Germany GmbH, Therakos (UK) Ltd. and Johnson & Johnson that were independent of the work submitted. E.S. has received lecture fees from Almirall, Leo, Pierre Favre and Philips. All other authors (N.O., M.S. and M.D.) declare no conflicts of interest.
References
- Barrabés-Torrella, C.; Arandes-Marcocci, J.; Armillas-Lliteras, L.; Posada-Caez, R.A.; Koptseva, I.; Fumagalli, C.; Piñol-Ballús, D.; Salleras-Redonnet, M. Sarcomatoid transformation of a basal cell carcinoma following treatment with hedgehog inhibitors. Br. J. Dermatol. 2023, 189, 470–472. [Google Scholar] [CrossRef] [PubMed]
- Mc Menamin, M.E.; Goh, S.G.N.; Poblet, E.; Gostelow, B.E.; Robson, A.; Calonje, E. Sarcomatoid basal cell carcinoma-predilection for osteosarcomatous differentiation: A series of 11 cases. Am. J. Surg. Pathol. 2006, 30, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Mestre-Alagarda, C.; Nieto, G.; Terrádez, L.; Monteagudo, C. Primary cutaneous biphasic sarcomatoid basal cell carcinoma with myoepithelial carcinoma differentiation: A new variant. J. Cutan. Pathol. 2019, 46, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Inaloz, H.S.; Ayyalaraju, R.S.; Holt, P.J.A.; Laidler, P. A case of sarcomatoid carcinoma of the skin. J. Eur. Acad. Dermatol. Venereol. 2003, 17, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Kiuru, M.; McDermott, G.; Coit, D.C.; Berger, M.F.; Busam, K.J. Basal cell carcinosarcoma with PTCH1 mutations in both epithelial and sarcomatoid primary tumor components and in the sarcomatoid metastasis. Am. J. Surg. Pathol. 2014, 38, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Bulte, C.A.; Hoegler, K.M.; Khachemoune, A. Collision tumors: A review of their types, pathogenesis, and diagnostic challenges. Dermatol. Ther. 2020, 33, e14236. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.A.; Hemmati-Brivanlou, A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature 1995, 376, 331–333. [Google Scholar] [CrossRef] [PubMed]
- Yada, K.; Kashima, K.; Daa, T.; Kitano, S.; Fujiwara, S.; Yokoyama, S. Expression of CD10 in basal cell carcinoma. Am. J. Dermatopathol. 2004, 26, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Espejo, C.; Herrera-Sabal, A.; Ríos-Martín, J.J.; Camacho-Martínez, F. Basal cell carcinoma with sarcomatoid features (sarcomatoid carcinoma): Report of a case and review of the literature. J. Dermatol. 2003, 30, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Sengel, P. Pattern formation in skin development. Int. J. Dev. Biol. 1990, 34, 33–50. [Google Scholar] [PubMed]
- Bigby, S.M.; Charlton, A.; Miller, M.V.; Zwi, L.J.; Oliver, G.F. Biphasic sarcomatoid basal cell carcinoma (carcinosarcoma): Four cases with immunohistochemistry and review of the literature. J. Cutan. Pathol. 2005, 32, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.F.; Merchant, W.; Stables, G.I.; Lyon, C.L.; Platt, A. Basal cell carcinoma with a sarcomatous component (carcinosarcoma): A series of 5 cases and a review of the literature. J. Am. Acad. Dermatol. 2008, 59, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.A.; Muller, S.; Chaudahri, P.J.; Carlson, J.A. Cutaneous carcinosarcoma: Adnexal vs. epidermal types define high- and low-risk tumors. Results of a meta-analysis. J. Cutan. Pathol. 2005, 32, 2–11. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Society of Dermatopathology. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).