Comparison of Conventional Extraction Techniques with Superheated Steam Distillation on Chemical Characterization and Biological Activities of Syzygium aromaticum L. Essential Oil
Abstract
:1. Introduction
2. Results and Discussion
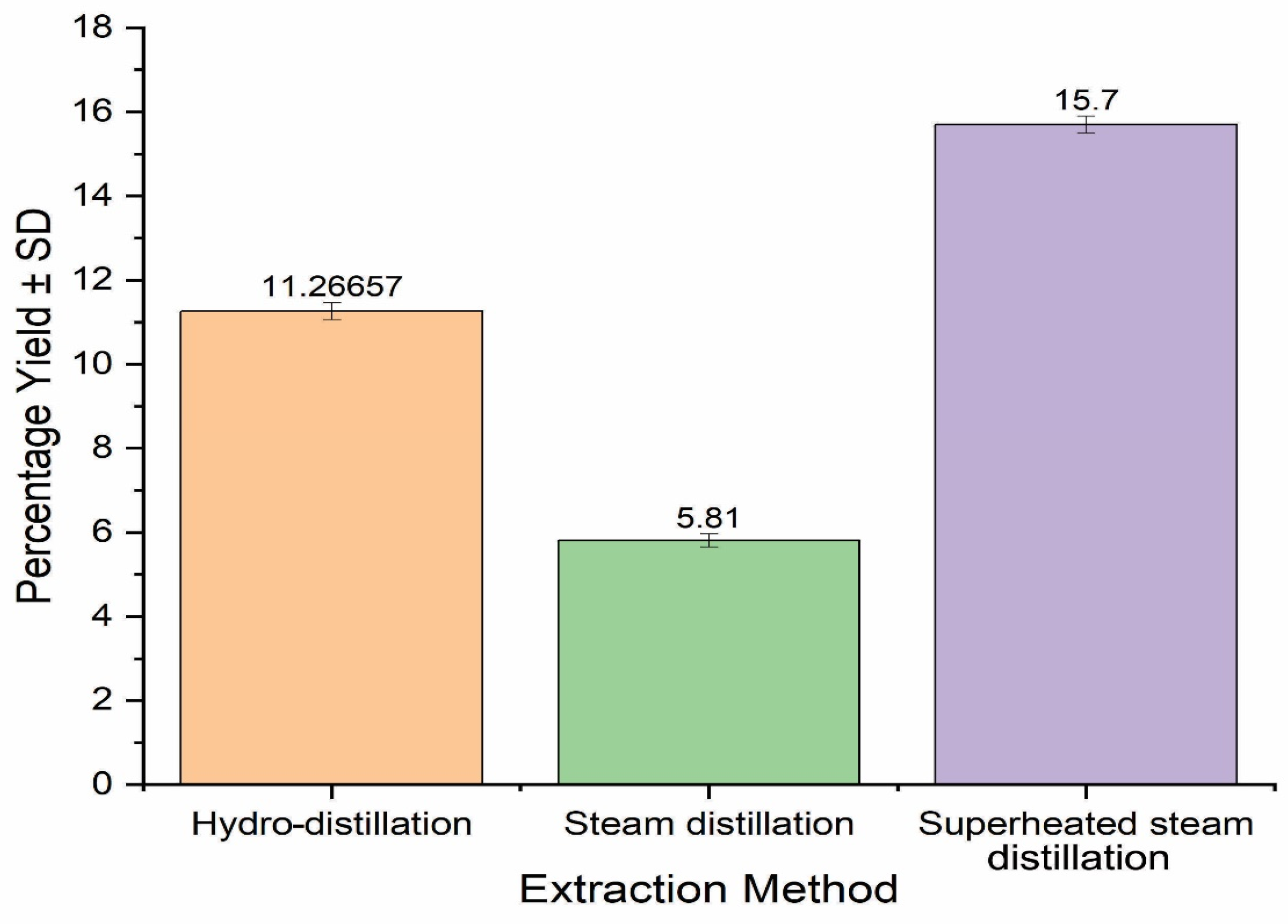
2.1. Essential Oil Yield
2.2. Antimicrobial Activity
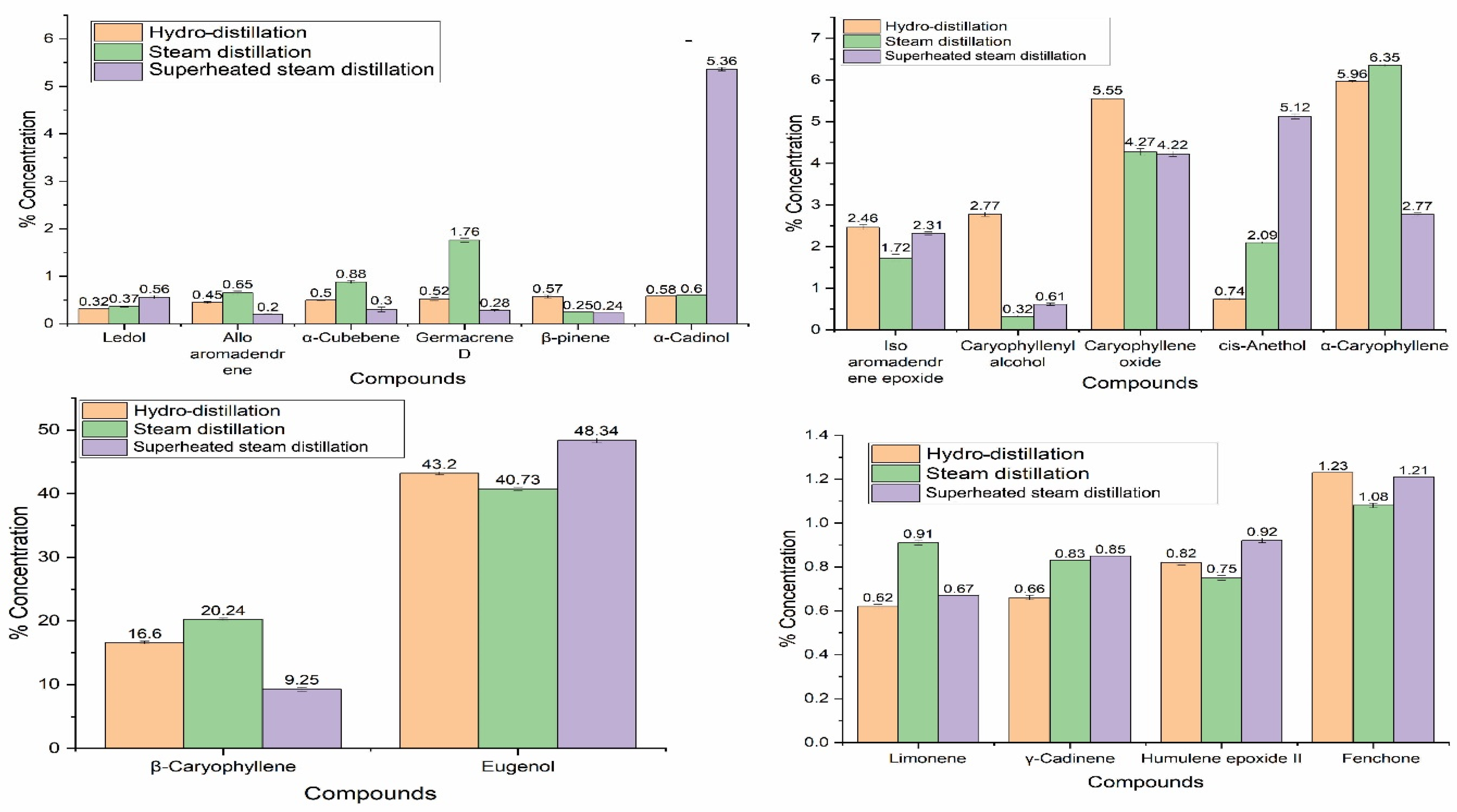
2.3. Chemical Composition of S. aromaticum EOs
2.4. Antioxidant Activity
2.5. Conclusions
3. Materials and Methods
3.1. Collection of Plant Materials
3.2. Extraction Methods
3.2.1. Hydro-Distillation
3.2.2. Steam Distillation
3.2.3. Superheated Steam Distillation
3.3. Antimicrobial Activity
3.3.1. Microbial Strains
3.3.2. Agar Well Diffusion Method
3.3.3. Resazurin Micro-Titer Plate Assay
3.3.4. Micro-Dilution Broth Susceptibility Assay
3.4. Gas Chromatography–Mass Spectrometry Analysis
3.5. Antioxidant Assays
3.5.1. DPPH Free Radical-Scavenging Activity
3.5.2. Reducing Power Ability (RPA)
3.5.3. Hydrogen Peroxide Scavenging Activity
3.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Otunola, G.A. Culinary spices in food and medicine: An overview of Syzygium aromaticum (L.) Merr. and LM Perry [Myrtaceae]. Front. Pharmacol. 2021, 12, 793200. [Google Scholar] [CrossRef] [PubMed]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Boughendjioua, H. Essential oil composition of Syzygium aromaticum (L.). IRJPMS 2018, 11, 26–28. [Google Scholar]
- Patra, J.K.; Baek, K.-H. Antibacterial activity and action mechanism of the essential oil from Enteromorpha linza L. against foodborne pathogenic bacteria. Molecules 2016, 21, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzales-Barron, U.; Thébault, A.; Kooh, P.; Watier, L.; Sanaa, M.; Cadavez, V. Strategy for systematic review of observational studies and meta-analysis modelling of risk factors for sporadic foodborne diseases. Microb. Risk Anal. 2021, 17, 100082. [Google Scholar] [CrossRef] [Green Version]
- Silva, F.; Domingues, F.C. Antimicrobial activity of coriander oil and its effectiveness as food preservative. Crit. Rev. Food Sci. Nutr. 2017, 57, 35–47. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. The inhibitory effect of plant essential oils on foodborne pathogenic bacteria in food. Crit. Rev. Food Sci. Nutr. 2019, 59, 3281–3292. [Google Scholar] [CrossRef]
- Sobiyi, O.; Sadgrove, N.; Magee, A.; Van Wyk, B.-E. The ethnobotany and major essential oil compounds of anise root (Annesorhiza species, Apiaceae). S. Afr. J. Bot. 2019, 126, 309–316. [Google Scholar] [CrossRef]
- Pokorný, J. Are natural antioxidants better–and safer–than synthetic antioxidants? Eur. J. Lipid Sci. Technol. 2007, 109, 629–642. [Google Scholar] [CrossRef]
- Brewer, M. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Amorati, R.; Foti, M.C.; Valgimigli, L. Antioxidant activity of essential oils. J. Agric. Food Chem. 2013, 61, 10835–10847. [Google Scholar] [CrossRef] [PubMed]
- Carvalho Jr, R.N.; Moura, L.S.; Rosa, P.T.; Meireles, M.A.A. Supercritical fluid extraction from rosemary (Rosmarinus officinalis): Kinetic data, extract’s global yield, composition, and antioxidant activity. J. Supercrit. Fluids 2005, 35, 197–204. [Google Scholar] [CrossRef]
- Dawra, M.; Nehme, N.; El Rayess, Y.; El Beyrouthy, M.; Taillandier, P.; Bouajila, J. Folk medicinal applications, phytochemical composition and biological activities of some Lebanese endemic plants. S. Afr. J. Bot. 2022, 150, 511–527. [Google Scholar] [CrossRef]
- Halim, N.A.A.; Abidin, Z.Z.; Siajam, S.I.; Hean, C.G.; Harun, M.R. Optimization studies and compositional analysis of subcritical water extraction of essential oil from Citrus hystrix DC. leaves. J. Supercrit. Fluids 2021, 178, 105384. [Google Scholar] [CrossRef]
- Ayub, M.A.; Hanif, M.A.; Blanchfield, J.; Zubair, M.; Abid, M.A.; Saleh, M.T. Chemical composition and antimicrobial activity of Boswellia serrata oleo-gum-resin essential oil extracted by superheated steam. Nat. Prod. Res. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ayub, M.A.; Hanif, M.A.; Sarfraz, R.A.; Shahid, M. Biological activity of Boswellia serrata Roxb. oleo gum resin essential oil: Effects of extraction by supercritical carbon dioxide and traditional methods. Int. J. Food Prop. 2018, 21, 808–820. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y.; Ma, H. Recent advances in the extraction of bioactive compounds with subcritical water: A review. Trends Food Sci. Technol. 2020, 95, 183–195. [Google Scholar] [CrossRef]
- Sadeghmousavi, S. Subcritical water extraction of fennel seeds essential oil and comparison with hydrodistillation. J. Food Sci. Technol. 2019, 16, 119–128. [Google Scholar]
- Masondo, N.; Makunga, N. Advancement of analytical techniques in some South African commercialized medicinal plants: Current and future perspectives. S. Afr. J. Bot. 2019, 126, 40–57. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Waśkiewicz, A. Recent advances in supercritical fluid extraction of natural bioactive compounds from natural plant materials. Molecules 2020, 25, 3847. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, M.; Rahimi-Nasrabadi, M.; Pourmortazavi, S.M.; Wysokowski, M.; Jesionowski, T.; Ehrlich, H.; Mirsadeghi, S. Supercritical fluid extraction of essential oils. TrAC Trends Anal. Chem. 2019, 118, 182–193. [Google Scholar] [CrossRef]
- Naz, S.; Hanif, M.A.; Bhatti, H.N.; Ansari, T.M. Impact of supercritical fluid extraction and traditional distillation on the isolation of aromatic compounds from Cannabis indica and Cannabis sativa. J. Essent. Oil Bear. Plants 2017, 20, 175–184. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- de Vasconcelos Silva, M.G.; de Abreu Matos, F.J.; Roberto, P.; Lopes, O.; Silva, F.O.; Holanda, M.T. Composition of essential oils from three Ocimum species obtained by steam and microwave distillation and supercritical CO2 extraction. Arkivoc 2004, 6, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Jin, C.; Guo, J.; Zhu, H.; Wen, J. Optimization of superheated steam treatment conditions for wheat aleurone layer flour. Food Sci. Technol. 2021, 42, e71920. [Google Scholar] [CrossRef]
- Rouatbi, M.; Duquenoy, A.; Giampaoli, P. Extraction of the essential oil of thyme and black pepper by superheated steam. J. Food Eng. 2007, 78, 708–714. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Ozel, M.Z.; Kaymaz, H. Superheated water extraction, steam distillation and Soxhlet extraction of essential oils of Origanum onites. Anal. Bioanal. Chem. 2004, 379, 1127–1133. [Google Scholar] [CrossRef]
- Prakash, B.; Mishra, P.K.; Kedia, A.; Dubey, N. Antifungal, antiaflatoxin and antioxidant potential of chemically characterized Boswellia carterii Birdw essential oil and its in vivo practical applicability in preservation of Piper nigrum L. fruits. LWT-Food Sci. Technol. 2014, 56, 240–247. [Google Scholar] [CrossRef]
- Jayawardena, B.; Smith, R.M. Superheated water extraction of essential oils from Cinnamomum zeylanicum (L.). Phytochem. Anal. 2010, 21, 470–472. [Google Scholar] [CrossRef]
- Glišić, S.B.; Milojević, S.Ž.; Dimitrijević, S.I.; Orlović, A.M.; Skala, D.U. Antimicrobial activity of the essential oil and different fractions of Juniperus communis L. and a comparison with some commercial antibiotics. J. Serb. Chem. Soc. 2007, 72, 311–320. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Franco-Vega, A.; Ramírez-Corona, N.; Palou, E.; López-Malo, A. Essential oils: Antimicrobial activities, extraction methods, and their modeling. Food Eng. Rev. 2015, 7, 275–297. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antibacterial activity of selected plant essential oils against Escherichia coli O157: H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayoola, G.; Lawore, F.; Adelowotan, T.; Aibinu, I.; Adenipekun, E.; Coker, H.; Odugbemi, T. Chemical analysis and antimicrobial activity of the essential oil of Syzigium aromaticum (clove). Afr. J. Microbiol. Res. 2008, 2, 162–166. [Google Scholar]
- Ogunwande, I.; Olawore, N.; Ekundayo, O.; Walker, T.; Schmidt, J.; Setzer, W. Studies on the essential oils composition, antibacterial and cytotoxicity of Eugenia uniflora L. Int. J. Aromather. 2005, 15, 147–152. [Google Scholar] [CrossRef]
- Fu, Y.; Zu, Y.; Chen, L.; Shi, X.; Wang, Z.; Sun, S.; Efferth, T. Antimicrobial activity of clove and rosemary essential oils alone and in combination. Phytother. Res. 2007, 21, 989–994. [Google Scholar] [CrossRef]
- Chaieb, K.; Hajlaoui, H.; Zmantar, T.; Kahla-Nakbi, A.B.; Rouabhia, M.; Mahdouani, K.; Bakhrouf, A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): A short review. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2007, 21, 501–506. [Google Scholar] [CrossRef]
- Rana, I.S.; Rana, A.S.; Rajak, R.C. Evaluation of antifungal activity in essential oil of the Syzygium aromaticum (L.) by extraction, purification and analysis of its main component eugenol. Braz. J. Microbiol. 2011, 42, 1269–1277. [Google Scholar] [CrossRef]
- Saricaoglu, F.T.; Turhan, S. Antimicrobial activity and antioxidant capacity of thyme, rosemary and clove essential oils and their mixtures. Int. J. Innov. Sci. Eng. Technol. 2018, 2, 25–33. [Google Scholar]
- Pinto, E.; Vale-Silva, L.; Cavaleiro, C.; Salgueiro, L. Antifungal activity of the clove essential oil from Syzygium aromaticum on Candida, Aspergillus and dermatophyte species. J. Med. Microbiol. 2009, 58, 1454–1462. [Google Scholar] [CrossRef]
- Nuñez, L.; D’Aquino, M. Microbicide activity of clove essential oil (Eugenia caryophyllata). Braz. J. Microbiol. 2012, 43, 1255–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuiyan, M.N.I.; Begum, J.; Nandi, N.C.; Akter, F. Constituents of the essential oil from leaves and buds of clove (Syzigium caryophyllatum (L.) Alston). Afr. J. Plant Sci. 2010, 4, 451–454. [Google Scholar]
- Raina, V.; Srivastava, S.; Aggarwal, K.; Syamasundar, K.; Kumar, S. Essential oil composition of Syzygium aromaticum leaf from Little Andaman, India. Flavour Fragr. J. 2001, 16, 334–336. [Google Scholar] [CrossRef]
- Srivastava, A.; Srivastava, S.; Syamsundar, K. Bud and leaf essential oil composition of Syzygium aromaticum from India and Madagascar. Flavour Fragr. J. 2005, 20, 51–53. [Google Scholar] [CrossRef]
- Xu, J.-G.; Liu, T.; Hu, Q.-P.; Cao, X.-M. Chemical composition, antibacterial properties and mechanism of action of essential oil from clove buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- Kaur, K.; Kaushal, S.; Rani, R. Chemical composition, antioxidant and antifungal potential of clove (Syzygium aromaticum) essential oil, its major compound and its derivatives. J. Essent. Oil Bear. Plants 2019, 22, 1195–1217. [Google Scholar] [CrossRef]
- Chienthavorn, O.; Insuan, W. Superheated water extraction of lime peel: A comparison with conventional methods. Anal. Lett. 2004, 37, 2393–2409. [Google Scholar] [CrossRef]
- Venditti, A. What is and what should never be: Artifacts, improbable phytochemicals, contaminants and natural products. Nat. Prod. Res. 2020, 34, 1014–1031. [Google Scholar] [CrossRef]
- Özkan, A.; Erdoğan, A. A comparative evaluation of antioxidant and anticancer activity of essential oil from Origanum onites (Lamiaceae) and its two major phenolic components. Turk. J. Biol. 2011, 35, 735–742. [Google Scholar] [CrossRef]
- Ghadermazi, R.; Keramat, J.; Goli, S. Antioxidant activity of clove (Eugenia caryophyllata Thunb), oregano (Oringanum vulgare L.) and sage (Salvia officinalis L.) essential oils in various model systems. Int. Food Res. J. 2017, 24, 1628. [Google Scholar]
- Alfikri, F.N.; Pujiarti, R.; Wibisono, M.G.; Hardiyanto, E.B. Yield, quality, and antioxidant activity of clove (Syzygium aromaticum L.) bud oil at the different phenological stages in young and mature trees. Scientifica 2020, 9701701. [Google Scholar] [CrossRef]
- Azeem, M.; Zaman, T.; Abbasi, A.M.; Abid, M.; Mozūratis, R.; Alwahibi, M.S.; Elshikh, M.S. Pesticidal potential of some wild plant essential oils against grain pests Tribolium castaneum (Herbst, 1797) and Aspergillus flavus (Link, 1809). Arab. J. Chem. 2022, 15, 103482. [Google Scholar] [CrossRef]
- Rashid, S.; Rather, M.A.; Shah, W.A.; Bhat, B.A. Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Willd. Food Chem. 2013, 138, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Dabur, R.; Ali, M.; Singh, H.; Gupta, J.; Sharma, G. A novel antifungal pyrrole derivative from Datura metel leaves. Int. J. Pharm. Sci. Res. 2004, 59, 568–570. [Google Scholar]
- Das, A.; Dey, S.; Sahoo, R.K.; Sahoo, S.; Subudhi, E. Antibiofilm and antibacterial activity of essential oil bearing Zingiber officinale Rosc.(Ginger) Rhizome against multi-drug resistant isolates. J. Essent. Oil-Bear. Plants 2019, 22, 1163–1171. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; Santos, T.C.d.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Habila, J.; Bello, I.; Dzikwi, A.; Musa, H.; Abubakar, N. Total phenolics and antioxidant activity of Tridax procumbens Linn. Afr. J. Pharm. Pharmacol. 2010, 4, 123–126. [Google Scholar]
- Bittencourt, V.; Bahiense, T.; Fernandes, E.; Souza, E.; Botelho, M.; Nogueira, N.; Bastos, G.; Fonseca, S.; Lemos, T.; Cavalcanti, S. essential oils–A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar]
| Microbial Strains | Hydro-Distilled EO | Steam Distilled EO | Superheated Steam Distilled EO | Positive Control |
|---|---|---|---|---|
| Minimum inhibitory concentration (mg/mL) | ||||
| Fusarium solani | 1.25 ± 0.02 a | 0.31 ± 0.04 b | 0.16 ± 0.02 c | 0.16 ± 0.02 d |
| Aspergillus niger | 5.00 ± 0.03 a | 2.50 ± 0.02 b | 1.25 ± 0.01 c | 0.63 ± 0.00 d |
| Alternaria alternate | 0.63 ± 0.02 a | 0.31 ± 0.02 b | 0.08 ± 0.01 c | 0.02 ± 0.00 d |
| Aspergillus flavus | 2.50 ± 0.03 a | 1.25 ± 0.02 b | 0.65 ± 0.03 c | 0.32 ± 0.02 d |
| Staphylococcus aureus | 1.25 ±0.01 a | 0.63 ± 0.01 b | 0.31 ± 0.00 c | 0.078 ± 0.01 d |
| Escherichia coli | 2.50 ± 0.03 a | 1.25 ± 0.02 b | 0.63 ± 0.01 c | 0.31 ± 0.00 d |
| Bacillus subtilis | 0.31 ± 0.02 a | 0.16 ± 0.03 b | 0.08 ± 0.00 c | 0.039 ± 0.00 d |
| Pastrulla multocida | 0.16 ± 0.01 a | 0.08 ± 0.04 b | 0.04 ± 0.03 c | 0.01 ± 0.00 d |
| Inhibition zone (mm) | ||||
| Fusarium solani | 13.86 ± 0.08 d | 14.28 ± 0.04 c | 15.72 ± 0.05 b | 34.74 ± 0.18 a |
| Aspergillus niger | 12.06 ± 0.05 d | 12.75 ± 0.07 c | 14.10 ± 0.07 b | 33.12 ± 0.23 a |
| Alternaria alternate | 17.14 ± 0.09 d | 17.36 ± 0.06 c | 19.48 ± 0.04 b | 38.28 ± 0.17 a |
| Aspergillus flavus | 13.16 ± 0.06 d | 13.89 ± 0.09 c | 15.24 ± 0.04 b | 34.32 ± 0.16 a |
| Staphylococcus aureus | 16.50 ± 0.11 d | 17.50 ± 0.15 c | 18.00 ± 0.20 b | 30.21 ± 0.17 a |
| Escherichia coli | 17.03 ± 0.05 d | 17.50 ± 0.07 c | 19.00 ± 0.07 b | 38.02 ± 0.23 a |
| Bacillus subtilis | 19.02 ± 0.15 d | 20.50 ± 0.13 c | 21.00 ± 0.18 b | 33.12 ± 0.12 a |
| Pastrulla multocida | 24.14 ± 0.08 d | 24.94± 0.12 c | 26.87 ± 0.09 b | 38.52 ± 0.34 a |
| Components | RI A | Hydro-Distilled EO | Steam Distilled EO | Superheated Steam Distilled EO | Method of Identification |
|---|---|---|---|---|---|
| Monoterpene hydrocarbons | |||||
| α-Pinene | 939 | 0.12 ± 0.00 a | --- | --- | a, b |
| β-pinene | 980 | 0.57 ± 0.03 a | 0.25 ± 0.00 b | 0.24 ± 0.00 ab | a, b |
| Limonene | 1031 | 0.62 ± 0.01 c | 0.91 ± 0.03 a | 0.67 ± 0.00 b | a, b |
| Oxygenated monoterpene hydrocarbons | |||||
| Fenchone | 1094 | 1.23 ± 0.00 a | 1.08 ± 0.01 c | 1.21 ± 0.00 b | a, b |
| Cinnamaldehyde | 1232 | --- | --- | 5.71 ± 0.21 a | a, b |
| α-Citral | 1240 | --- | 0.25 ± 0.01 b | 0.34 ± 0.03 a | a, b |
| cis-Anethol | 1269 | 0.74 ± 0.03 c | 2.09 ± 0.12 b | 5.12 ± 0.26 a | a, b |
| Eugenol | 1356 | 43.20 ± 1.18 b | 40.73 ± 1.21 c | 48.34 ± 1.36 a | a, b |
| Sesquiterpene hydrocarbons | |||||
| α-Cubebene | 1351 | 0.50 ± 0.01 b | 0.88 ± 0.03 a | 0.30 ± 0.03 c | a, b |
| β-elemene | 1375 | --- | --- | 0.36 ± 0.03 a | a, b |
| Copaene | 1376 | --- | 3.41 ± 0.29 a | 0.55 ± 0.04 b | a, b |
| β-Cubebene | 1390 | --- | --- | 0.17 ± 0.01 a | a, b |
| α-Cadinene | 1409 | 1.13 ± 0.32 a | 0.90 ± 0.04 b | --- | a, b |
| Isocaryophillene | 1413 | 3.81 ± 0.29 a | 2.77 ± 0.15 b | --- | a, b |
| β-Caryophyllene | 1428 | 16.60 ± 1.18 b | 20.24 ± 1.22 a | 9.25 ± 0.32 c | a, b |
| α-Caryophyllene | 1444 | 5.96 ± 0.24 b | 6.35 ± 0.31 a | 2.77 ± 0.45 c | a, b |
| Alloaromadendrene | 1461 | 0.45 ± 0.02 b | 0.65 ± 0.04 a | 0.20 ± 0.01 c | a, b |
| Germacrene D | 1480 | 0.52 ± 0.03 b | 1.76 ± 0.65 a | 0.28 ± 0.02 c | a, b |
| α -Amorphene | 1485 | 1.03 ± 0.05 b | --- | 7.12 ± 0.36 a | a, b |
| α-Muurolene | 1499 | --- | 5.31 ± 0.24 a | --- | a, b |
| γ-Cadinene | 1513 | 0.66 ± 0.04 c | 0.83 ± 0.00 b | 0.85 ± 0.00 a | a, b |
| Cadinene | 1524 | 2.43 ± 0.08 b | 2.17 ± 0.05 a | --- | a, b |
| α-Calacorene | 1548 | --- | 0.31 ± 0.06 a | 0.66 ± 0.05 b | a, b |
| Isosativene | 1556 | --- | --- | 0.31 ± 0.03 a | a, b |
| Oxygenated sesquiterpene hydrocarbons | |||||
| Ledol | 1565 | 0.32 ± 0.01 c | 0.37 ± 0.02 b | 0.56 ± 0.03 a | a, b |
| Caryophyllenyl alcohol | 1568 | 2.77 ± 0.57 a | 0.32 ± 0.02 c | 0.61 ± 0.03 b | a, b |
| Isoaromadendrene epoxide | 1578 | 2.46 ± 0.06 a | 1.72 ± 0.09 c | 2.31 ± 0.04 b | a, b |
| Caryophyllene oxide | 1581 | 5.55 ± 0.46 a | 4.27 ± 0.08 b | 4.22 ± 0.06 c | a, b |
| Humulene epoxide II | 1607 | 0.82 ± 0.03 b | 0.75 ± 0.04 c | 0.92 ± 0.05 a | a, b |
| Humulol | 1618 | 8.00 ± 0.05 a | --- | 0.69 ± 0.03 b | a, b |
| α-Cadinol | 1653 | 0.58 ± 0.00 c | 0.60 ± 0.00 b | 5.36 ± 0.34 a | a, b |
| β-Bisabolol | 1683 | --- | --- | 0.86 ± 0.04 a | a, b |
| Total monoterpene | 1.31 | 1.16 | 0.91 | ||
| Total oxygenated monoterpene | 45.17 | 45.15 | 60.72 | ||
| Total sesquiterpene | 33.09 | 45.58 | 22.82 | ||
| Total oxygenated sesquiterpenes | 20.02 | 8.03 | 15.53 | ||
| Overall total concentration | 99.97 | 99.92 | 99.98 | ||
| Extraction Methods | DPPH-FRSA (%) | Total Antioxidant Contents/FRPA | Hydrogen Peroxide FRSA (%) |
|---|---|---|---|
| Hydro-distillation | 74.48 ± 0.45 c | 1064.91 ± 1.46 b | 76.66 ± 0.64 c |
| Steam distillation | 71.14 ± 0.63 d | 549.15 ± 1.21 c | 73.33 ± 0.34 d |
| Superheated steam distillation | 77.47 ± 0.74 b | 1483.94 ± 2.34 a | 80.00 ± 0.94 b |
| Ascorbic acid | --- | --- | 95.33 ± 0.46 a |
| Gallic acid | 81.03 ± 0.54 a | --- | --- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayub, M.A.; Goksen, G.; Fatima, A.; Zubair, M.; Abid, M.A.; Starowicz, M. Comparison of Conventional Extraction Techniques with Superheated Steam Distillation on Chemical Characterization and Biological Activities of Syzygium aromaticum L. Essential Oil. Separations 2023, 10, 27. https://doi.org/10.3390/separations10010027
Ayub MA, Goksen G, Fatima A, Zubair M, Abid MA, Starowicz M. Comparison of Conventional Extraction Techniques with Superheated Steam Distillation on Chemical Characterization and Biological Activities of Syzygium aromaticum L. Essential Oil. Separations. 2023; 10(1):27. https://doi.org/10.3390/separations10010027
Chicago/Turabian StyleAyub, Muhammad Adnan, Gulden Goksen, Ambreen Fatima, Muhammad Zubair, Muhammad Amin Abid, and Małgorzata Starowicz. 2023. "Comparison of Conventional Extraction Techniques with Superheated Steam Distillation on Chemical Characterization and Biological Activities of Syzygium aromaticum L. Essential Oil" Separations 10, no. 1: 27. https://doi.org/10.3390/separations10010027
APA StyleAyub, M. A., Goksen, G., Fatima, A., Zubair, M., Abid, M. A., & Starowicz, M. (2023). Comparison of Conventional Extraction Techniques with Superheated Steam Distillation on Chemical Characterization and Biological Activities of Syzygium aromaticum L. Essential Oil. Separations, 10(1), 27. https://doi.org/10.3390/separations10010027








