Lipids Fraction from Caralluma europaea (Guss.): MicroTOF and HPLC Analyses and Exploration of Its Antioxidant, Cytotoxic, Anti-Inflammatory, and Wound Healing Effects
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material
2.2. Preparation of Lipids Extract
2.3. Animal Material
2.4. Ointment Preparation
2.5. Chemical Analysis of Lipids Compounds
2.5.1. Solvents and Reagents
2.5.2. Micro-TOF Analysis
2.5.3. HPLCMSD Analysis
2.6. Antioxidant Effect
2.6.1. Free Radical-Scavenging Capacity (DPPH)
2.6.2. Ferric Reducing Antioxidant Power (FRAP)
2.7. Cytotoxic Effect
2.8. Anti-Inflammatory Activity
2.9. Wound Healing Activity
2.10. Molecular Docking
2.11. Statistical Analysis
3. Results
3.1. Chemical Analysis
3.1.1. MicroTOF Analysis
3.1.2. HPLCMSD Analysis
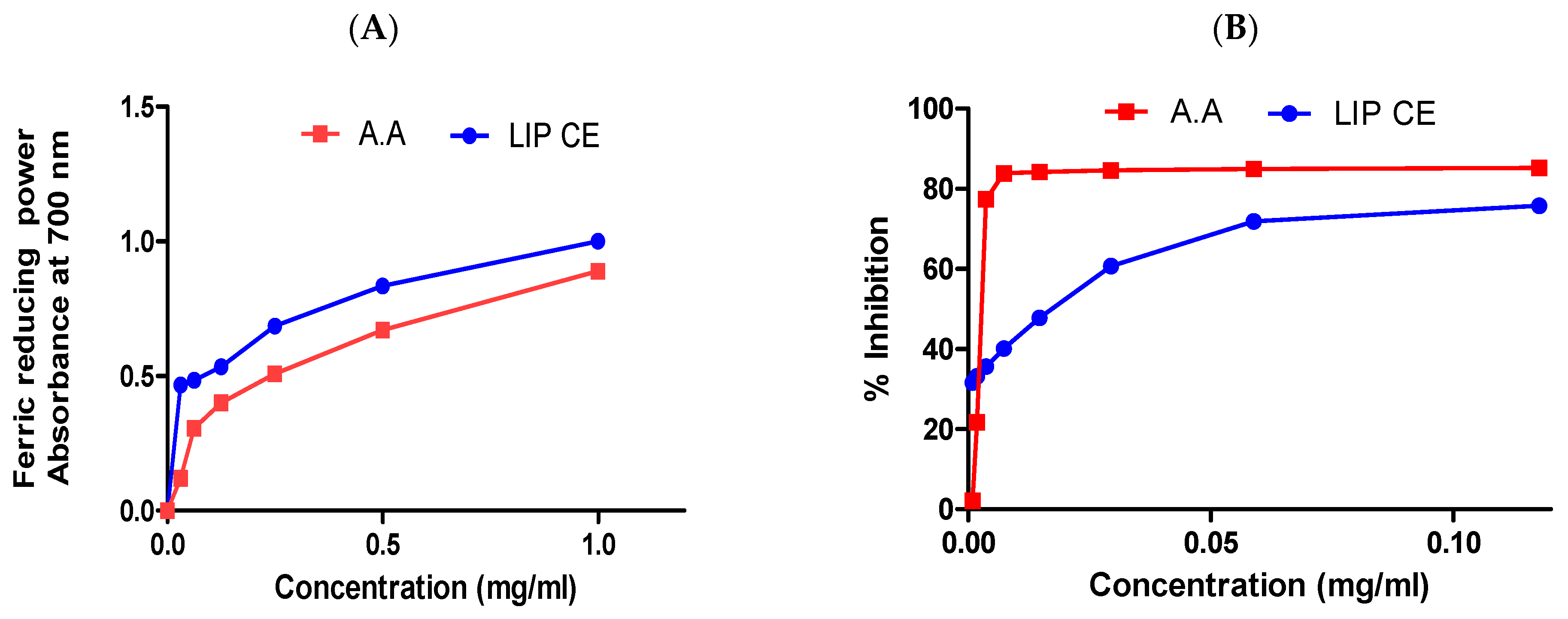
3.2. Antioxidant Activity
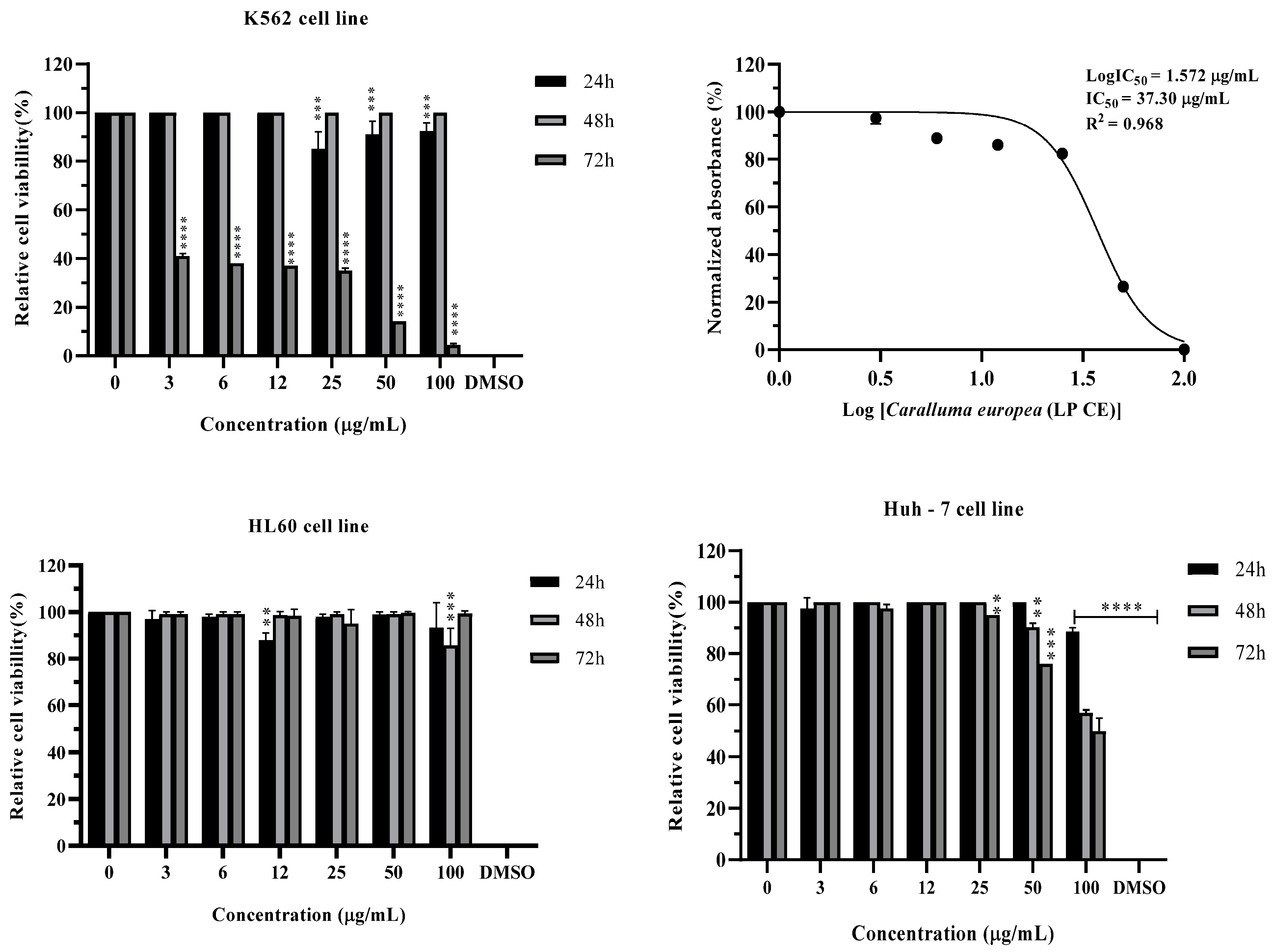
3.3. Cytotoxic Effect
3.4. Anti-Inflammatory Activity
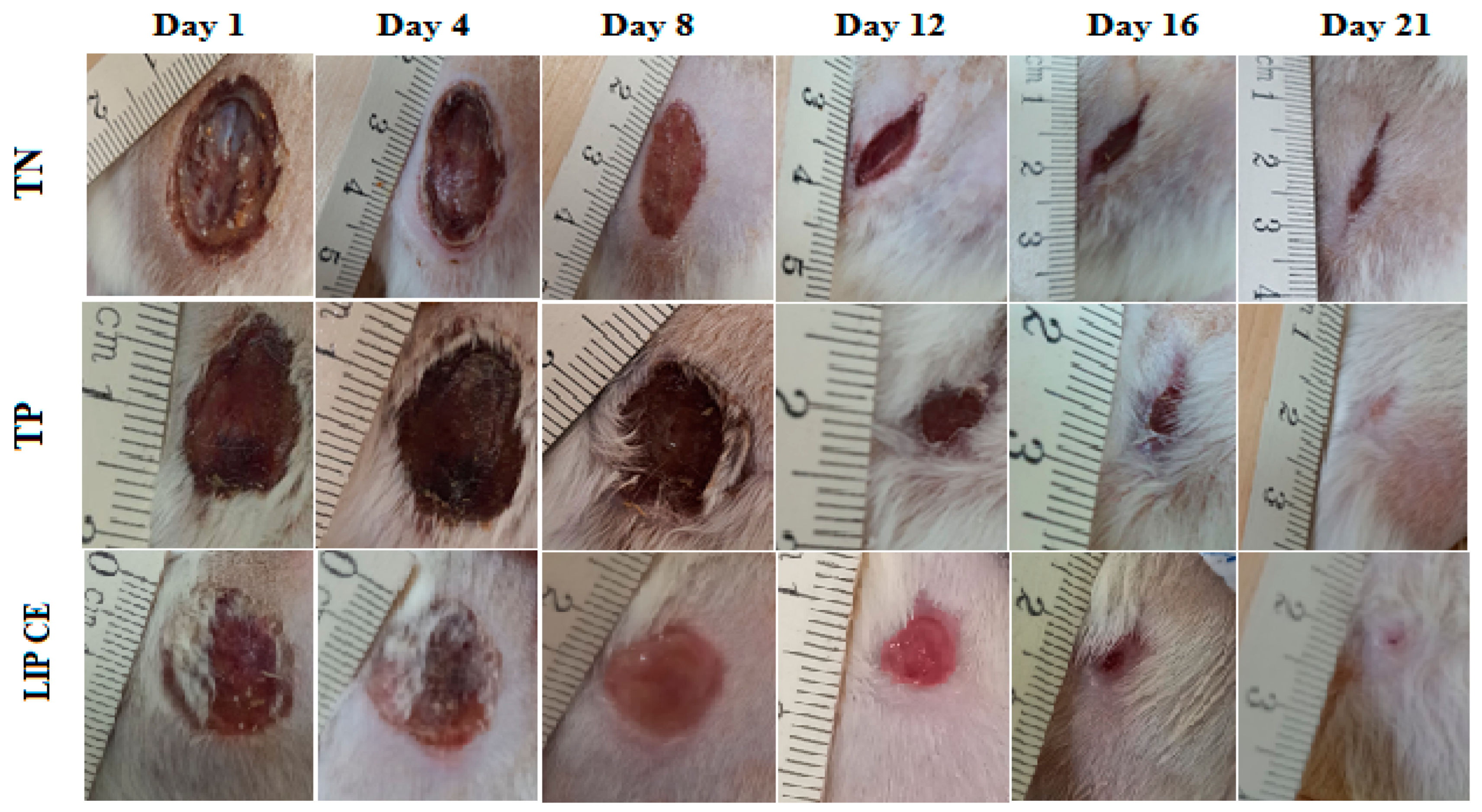
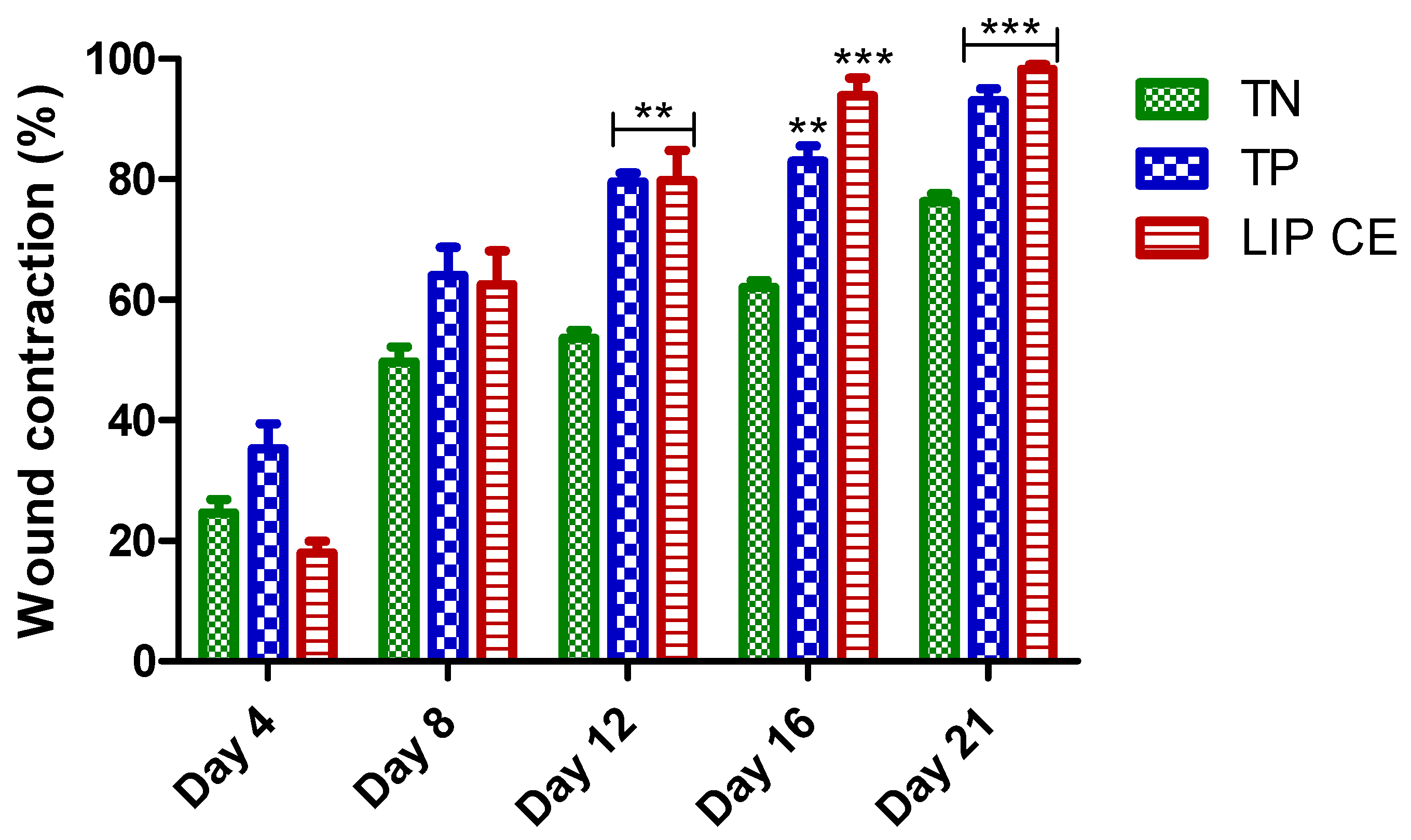
3.5. Wound Healing Activity
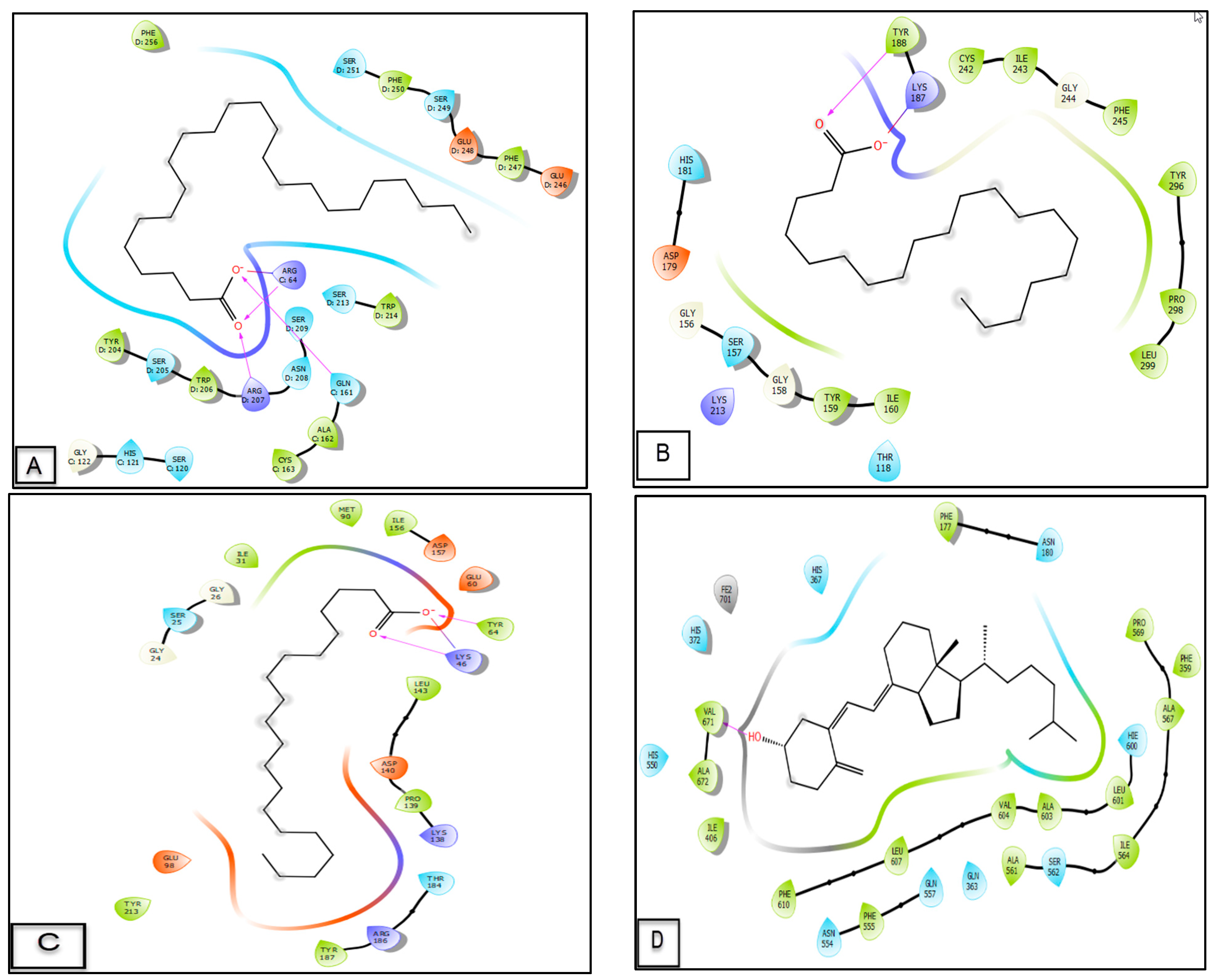
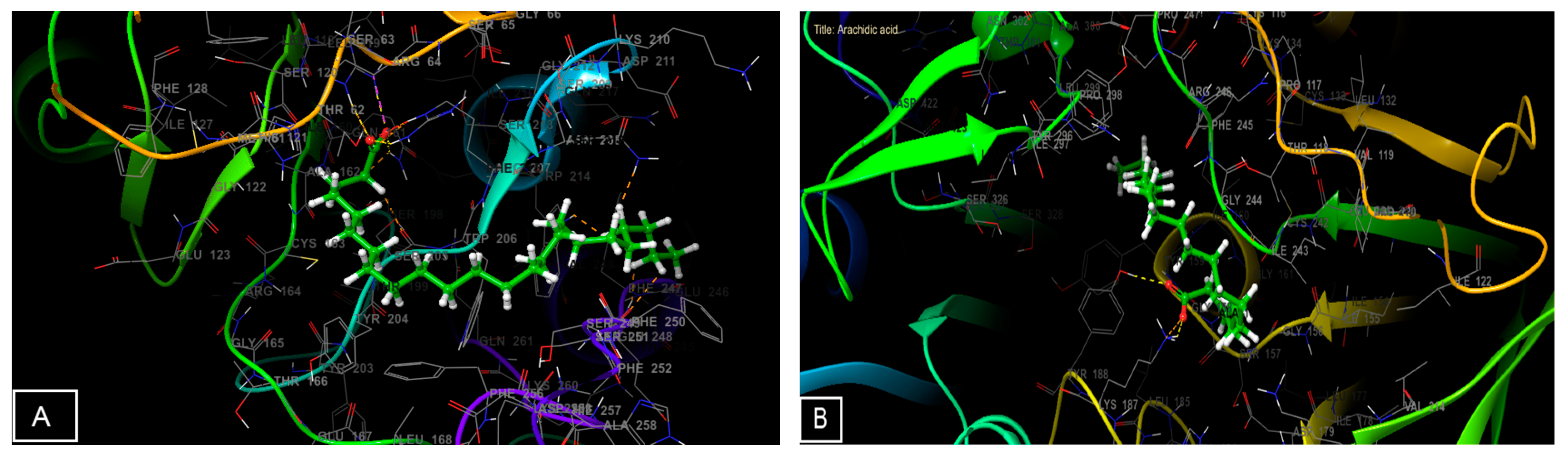
3.6. Molecular Docking Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Gómez, X.; Sanon, S.; Zambrano, K.; Asquel, S.; Bassantes, M.; Morales, J.E.; Otáñez, G.; Pomaquero, C.; Villarroel, S.; Zurita, A.; et al. Key points for the development of antioxidant cocktails to prevent cellular stress and damage caused by reactive oxygen species (ROS) during manned space missions. npj Microgravity 2021, 7, 35. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Ding, Y.; Tian, Y.; Yang, R.; Quan, M.; Tong, Z.; Zhang, X.; Luo, D.; Chi, Z.; Liu, C. Hydrolyzed low-molecular-weight polysaccharide from Enteromorpha prolifera exhibits high anti-inflammatory activity and promotes wound healing. Biomater. Adv. 2022, 133, 112637. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Anjali, R.; Beulaja, M.; Prabhu, N.M.; Koodalingam, A.; Saiprasad, G.; Chitra, P.; Arumugam, M. Synthesis, characterization, anti-proliferative and wound healing activities of silver nanoparticles synthesized from Caulerpa scalpelliformis. Process Biochem. 2019, 79, 135–141. [Google Scholar] [CrossRef]
- Said Nasser Al-Owamri, F.; Saleh Abdullah Al Sibay, L.; Hemadri Reddy, S.; Althaf Hussain, S.; Subba Reddy Gangireddygari, V. Phytochemical, Antioxidant, hair growth and wound healing property of Juniperus excelsa, Olea oleaster and Olea europaea. J. King Saud Univ. Sci 2023, 35, 102446. [Google Scholar] [CrossRef]
- Hang, C.; Sun, H.; Zhang, A.; Yan, G.; Lu, S.; Wang, X. Recent Developments in the Field of Antioxidant Activity on Natural Products. Амурский Медицинский Журнал 2015, 2, 44–48. [Google Scholar]
- Builders, P. Herbal Medicine; IntechOpen: London, UK, 2019; 316p. [Google Scholar]
- Khalili Tilami, S.; Kouřimská, L. Assessment of the Nutritional Quality of Plant Lipids Using Atherogenicity and Thrombogenicity Indices. Nutrients 2022, 14, 3795. [Google Scholar] [CrossRef]
- Trela-Makowej, A.; Kruk, J.; Jemioła-Rzemińska, M.; Szymańska, R. Acylserotonins—A new class of plant lipids with antioxidant activity and potential pharmacological applications. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2021, 1866, 159044. [Google Scholar] [CrossRef]
- Toshkova-Yotova, T.; Georgieva, A.; Iliev, I.; Alexandrov, S.; Ivanova, A.; Pilarski, P.; Toshkova, R. Antitumor and antimicrobial activity of fatty acids from green microalga Coelastrella sp. BGV. S. Afr. J. Bot. 2022, 151, 394–402. [Google Scholar] [CrossRef]
- Aydar, E.F.; Mertdinç, Z.; Demircan, E.; Koca Çetinkaya, S.; Özçelik, B. Kidney bean (Phaseolus vulgaris L.) milk substitute as a novel plant-based drink: Fatty acid profile, antioxidant activity, in-vitro phenolic bio-accessibility and sensory characteristics. Innov. Food Sci. Emerg. Technol. 2023, 83, 103254. [Google Scholar] [CrossRef]
- Deghima, A.; Righi, N.; Daoud, I.; Ansorena, D.; Astiasarán, I.; Bedjou, F. Fatty acid composition, acute toxicity and anti-inflammatory activity of the n-hexane extract from Ranunculus macrophyllus Desf. roots. S. Afr. J. Bot. 2022, 148, 315–325. [Google Scholar] [CrossRef]
- Amrati, F.E.-Z.; Elmadbouh, O.H.M.; Chebaibi, M.; Soufi, B.; Conte, R.; Slighoua, M.; Saleh, A.; Al Kamaly, O.; Drioiche, A.; Zair, T.; et al. Evaluation of the toxicity of Caralluma europaea (C.E) extracts and their effects on apoptosis and chemoresistance in pancreatic cancer cells. J. Biomol. Struct. Dyn. 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Bourhia, M.; Slighoua, M.; Mohammad Salamatullah, A.; Alzahrani, A.; Ullah, R.; Bari, A.; Bousta, D. Traditional medicinal knowledge of plants used for cancer treatment by communities of mountainous areas of Fez-Meknes-Morocco. Saudi Pharm. J. 2021, 29, 1185–1204. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Bourhia, M.; Slighoua, M.; Ibnemoussa, S.; Bari, A.; Ullah, R.; Amaghnouje, A.; Di Cristo, F.; El Mzibri, M.; Calarco, A.; et al. Phytochemical Study on Antioxidant and Antiproliferative Activities of Moroccan Caralluma europaea Extract and Its Bioactive Compound Classes. Evid.-Based Complement. Altern. Med. 2020, 2020, 8409718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amrati, F.E.-Z.; Bourhia, M.; Slighoua, M.; Boukhira, S.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.E.; Grafov, A.; Bousta, D. Protective Effect of Chemically Characterized Polyphenol-Rich Fraction from Apteranthes europaea (Guss.) Murb. subsp. maroccana (Hook.f.) Plowes on Carbon Tetrachloride-Induced Liver Injury in Mice. Appl. Sci. 2021, 11, 554. [Google Scholar] [CrossRef]
- Ouassou, H.; Bouhrim, M.; Kharchoufa, L.; Imtara, H.; Daoudi, N.E.; Benoutman, A.; Bencheikh, N.; Ouahhoud, S.; Elbouzidi, A.; Bnouham, M. Caralluma europaea (Guss) N.E.Br.: A review on Ethnomedicinal uses, Phytochemistry, Pharmacological Activities, and Toxicology. J. Ethnopharmacol. 2021, 273, 113769. [Google Scholar] [CrossRef]
- Dra, L.A.; Rodrigues, M.J.; da Rosa Neng, N.; Nogueira, J.M.F.; Elamine, Y.; Aghraz, A.; Markouk, M.; Larhsini, M.; Custódio, L. Exploring Caralluma europaea (Guss.) N.E.Br. as a potential source of bioactive molecules: In vitro antioxidant and antidiabetic properties, and phenolic profile of crude extracts and fractions. Ind. Crops Prod. 2019, 139, 111527. [Google Scholar] [CrossRef]
- Formisano, C.; Senatore, F.; Della Porta, G.; Scognamiglio, M.; Bruno, M.; Maggio, A.; Rosselli, S.; Zito, P.; Sajeva, M. Headspace Volatile Composition of the Flowers of Caralluma europaea N.E.Br. (Apocynaceae). Molecules 2009, 14, 4597–4613. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- National Research Council Committee. Guide for the Care and Use of Laboratory Animals, The National Academies Collection: Reports Funded by National Institutes of Health, 8th ed.; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Mssillou, I.; Agour, A.; Slighoua, M.; Chebaibi, M.; Amrati, F.E.-Z.; Alshawwa, S.Z.; Kamaly, O.A.; El Moussaoui, A.; Lyoussi, B.; Derwich, E. Ointment-Based Combination of Dittrichia viscosa L. and Marrubium vulgare L. Accelerate Burn Wound Healing. Pharmaceuticals 2022, 15, 289. [Google Scholar] [CrossRef]
- Jaklová Dytrtová, J.; Moslova, K.; Jakl, M.; Sirén, H.; Riekkola, M.-L. Fluorescein isothiocyanate stability in different solvents. Monatsh Chem. 2021, 152, 1299–1306. [Google Scholar] [CrossRef]
- Seal, T. Quantitative HPLC analysis of phenolic acids, flavonoids and ascorbic acid in four different solvent extracts of two wild edible leaves, Sonchus arvensis and Oenanthe linearis of North-Eastern region in India. J. Appl. Pharm. Sci. 2016, 6, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on products of browning reaction—Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef] [Green Version]
- Amrati, F.E.-Z.; Bourhia, M.; Saghrouchni, H.; Slighoua, M.; Grafov, A.; Ullah, R.; Ezzeldin, E.; Mostafa, G.A.; Bari, A.; Ibenmoussa, S.; et al. Caralluma europaea (Guss.) N.E.Br.: Anti-Inflammatory, Antifungal, and Antibacterial Activities against Nosocomial Antibiotic-Resistant Microbes of Chemically Characterized Fractions. Molecules 2021, 26, 636. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, S.; Diao, Y.; Zhang, J.; Lv, D. Fatty acid extracts from Lucilia sericata larvae promote murine cutaneous wound healing by angiogenic activity. Lipids Health Dis. 2010, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Aye, M.M.; Aung, H.T.; Sein, M.M.; Armijos, C. A Review on the Phytochemistry, Medicinal Properties and Pharmacological Activities of 15 Selected Myanmar Medicinal Plants. Molecules 2019, 24, 293. [Google Scholar] [CrossRef] [Green Version]
- Augustus, G.D.P.S.; Seiler, G.J. Phytochemicals of selected plant species of the Apocynaceae and Asclepiadaceae from Western Ghats, Tamil Nadu, India. Biomass Bioenergy 2011, 35, 3012–3017. [Google Scholar] [CrossRef]
- Alqahtani, S.S.; Makeen, H.A.; Menachery, S.J.; Moni, S.S. Documentation of bioactive principles of the flower from Caralluma retrospiciens (Ehrenb) and in vitro antibacterial activity—Part B. Arab. J. Chem. 2020, 13, 7370–7377. [Google Scholar] [CrossRef]
- Ugwah-Oguejiofor, C.J.; Amuda, M.B.; Abubakar, K.; Ugwah, O.M.; Ofokansi, M.N.; Mshelia, H.E. An experimental evaluation of anticonvulsant activity of aqueous extract of Caralluma dalzielii N.E. Brown. Phytomed. Plus 2023, 3, 100401. [Google Scholar] [CrossRef]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty acid synthesis is a target for antibacterial activity of unsaturated fatty acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.; Yuan, C.; Ramaswamy, H.S.; Ren, Y.; Ren, X. Antioxidant capacity and hepatoprotective activity of myristic acid acylated derivative of phloridzin. Heliyon 2019, 5, e01761. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, Z.; Hekmatdoost, A.; Nourian, M. Antioxidant efficacy of vitamin D. J. Parathyr. Dis. 2016, 5, 11–16. [Google Scholar]
- Guzik, T.J.; Harrison, D.G. Vascular NADPH oxidases as drug targets for novel antioxidant strategies. Drug Discov. Today 2006, 11, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Eghbal, M.A.; Darabi, M.; Roshangar, L.; Asadi, M.; Zarghami, N.; Nouri, M. Improvement of Liver Cell Therapy in Rats by Dietary Stearic Acid. Iran Biomed. J. 2016, 20, 217–222. [Google Scholar]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [Green Version]
- Sawada, L.A.; Monteiro, V.S.D.C.; Rabelo, G.R.; Dias, G.B.; Da Cunha, M.; do Nascimento, J.L.M.; de Nazareth Tavares Bastos, G. Libidibia ferrea Mature Seeds Promote Antinociceptive Effect by Peripheral and Central Pathway: Possible Involvement of Opioid and Cholinergic Receptors. BioMed Res. Int. 2014, 2014, e508725. [Google Scholar] [CrossRef] [Green Version]
- Wisastra, R.; Dekker, F.J. Inflammation, Cancer and Oxidative Lipoxygenase Activity are Intimately Linked. Cancers 2014, 6, 1500–1521. [Google Scholar] [CrossRef] [Green Version]
- Naik, S. Wound, heal thyself. Nat. Med. 2018, 24, 1311–1312. [Google Scholar] [CrossRef]
- Mssillou, I.; Bakour, M.; Slighoua, M.; Laaroussi, H.; Saghrouchni, H.; Ez-Zahra Amrati, F.; Lyoussi, B.; Derwich, E. Investigation on wound healing effect of Mediterranean medicinal plants and some related phenolic compounds: A review. J. Ethnopharmacol. 2022, 298, 115663. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Zhao, D.; Zhang, C.; Liu, K.; He, Y.; Guan, F.; Yao, M. Nanocomposite multifunctional hyaluronic acid hydrogel with photothermal antibacterial and antioxidant properties for infected wound healing. Int. J. Biol. Macromol. 2023, 226, 870–884. [Google Scholar] [CrossRef] [PubMed]
- Amrati, F.E.-Z.; Chebaibi, M.; de Azevedo, R.G.; Conte, R.; Slighoua, M.; Mssillou, I.; Kiokias, S.; de Freitas Gomes, A.; Pontes, G.S.; Bousta, D. Phenolic Composition, Wound Healing, Antinociceptive, and Anticancer Effects of Caralluma europaea Extracts. Molecules 2023, 28, 1780. [Google Scholar] [CrossRef] [PubMed]





| Pic | Identified Compound | Formula | MW | Theoretical [M-H]− | Found [M-H]− | Error [ppm] |
|---|---|---|---|---|---|---|
| 1 | Myristic acid | C14H28O2 | 228.37 | 227.2017 | 227.2041 | 10.765767 |
| 2 | Palmitic acid | C16H32O2 | 256.40 | 255.2329 | 255.2355 | 9.975 |
| 3 | Linoleic acid | C18H32O2 | 280.45 | 279.2330 | 279.2353 | 8.402 |
| 4 | Stearic acid | C18H36O2 | 284.48 | 283.2643 | 283.2661 | 6.517 |
| 5 | Arachidic acid | C20H40O2 | 312.53 | 311.2956 | 311.2248 | −227.115 |
| 6 | Behenic acid | C22H44O2 | 340.58 | 339.3269 | 339.3299 | 8.977 |
| 7 | Lignoceric acid | C24H48O2 | 368.63 | 367.3582 | 367.3616 | 9.380 |
| 8 | Cholecalciferol (Vitamin D3) | C27H44O | 384.65 | 383.3319 | 383.3561 | 63.026 |
| Peak | Lipidic Compound | RT (min) | Formula |
|---|---|---|---|
| 1 | Stearic acid | 52.192 | C18H36O2 |
| 2 | Palmitic acid | 52.914 | C16H32O2 |
| 3 | Myristic acid | 54.186 | C14H28O2 |
| IC50 (µg/mL) | |||
|---|---|---|---|
| Human chronic myelogenous leukemia (K562 cell line) | Human hepatocellular carcinoma (Huh-7 cell line) | Human acute promyelocytic leukemia (HL60 cell line) | Normal cell line (Vero cells) |
| 37.30 *** | - | >100 | >100 |
| Treatment Group | Initial Diameter (cm) | Edema Diameter after the Injection of Carrageenan (cm)/Inhibition of Edema (%) | |||
|---|---|---|---|---|---|
| 3 h | 4 h | 5 h | 6 h | ||
| Vaseline | 2.370 ± 0.049 | 2.670 ± 0.037 | 2.870 ± 0.037 | 2.838 ± 0.066 | 2.570 ± 0.020 |
| Diclofenac® (1%) | 2.226 ± 0.035 | 2.424 ± 0.037 ** 34% | 2.358 ± 0.034 * 73.60% | 2.302 ± 0.028 83.76% | 2.256 ± 0.030 85% |
| Lipids CE (10%) | 2.417 ± 0.044 | 2.650 ± 0.050 * 22.33% | 2.567 ± 0.067 70% | 2.500 ± 0.057 82.27% | 2.450 ± 0.050 83.50% |
| Molecules | Glide G Score (Kcal/mol) | ||||
|---|---|---|---|---|---|
| 3GJQ | 2CDU | 6GZD | 1Q5K | 3V99 | |
| Arachidic acid | −5.652 | −3.479 | −2.853 | −2.968 | −4.542 |
| Behenic acid | −6.334 | −2.929 | −2.204 | −1.817 | −4.396 |
| Lignoceric acid | −6.453 | −1.641 | - | −1.876 | −2.177 |
| Linoleic acid | −4.141 | −2.478 | −1.26 | −1.207 | −1.992 |
| Myristic acid | −3.759 | - | - | 0.042 | −0.753 |
| Palmitic acid | −3.642 | - | - | −0.46 | −1.346 |
| Stearic acid | −3.7 | −0.753 | −0.636 | −0.269 | −0.637 |
| Vitamin D3 | −4.279 | - | - | −4.538 | −4.909 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amrati, F.E.-Z.; Slighoua, M.; Mssillou, I.; Chebaibi, M.; Galvão de Azevedo, R.; Boukhira, S.; Moslova, K.; Al Kamaly, O.; Saleh, A.; Correa de Oliveira, A.; et al. Lipids Fraction from Caralluma europaea (Guss.): MicroTOF and HPLC Analyses and Exploration of Its Antioxidant, Cytotoxic, Anti-Inflammatory, and Wound Healing Effects. Separations 2023, 10, 172. https://doi.org/10.3390/separations10030172
Amrati FE-Z, Slighoua M, Mssillou I, Chebaibi M, Galvão de Azevedo R, Boukhira S, Moslova K, Al Kamaly O, Saleh A, Correa de Oliveira A, et al. Lipids Fraction from Caralluma europaea (Guss.): MicroTOF and HPLC Analyses and Exploration of Its Antioxidant, Cytotoxic, Anti-Inflammatory, and Wound Healing Effects. Separations. 2023; 10(3):172. https://doi.org/10.3390/separations10030172
Chicago/Turabian StyleAmrati, Fatima Ez-Zahra, Meryem Slighoua, Ibrahim Mssillou, Mohamed Chebaibi, Renata Galvão de Azevedo, Smahane Boukhira, Karina Moslova, Omkulthom Al Kamaly, Asmaa Saleh, André Correa de Oliveira, and et al. 2023. "Lipids Fraction from Caralluma europaea (Guss.): MicroTOF and HPLC Analyses and Exploration of Its Antioxidant, Cytotoxic, Anti-Inflammatory, and Wound Healing Effects" Separations 10, no. 3: 172. https://doi.org/10.3390/separations10030172
APA StyleAmrati, F. E. -Z., Slighoua, M., Mssillou, I., Chebaibi, M., Galvão de Azevedo, R., Boukhira, S., Moslova, K., Al Kamaly, O., Saleh, A., Correa de Oliveira, A., Gomes, A. d. F., Soares Pontes, G., & Bousta, D. (2023). Lipids Fraction from Caralluma europaea (Guss.): MicroTOF and HPLC Analyses and Exploration of Its Antioxidant, Cytotoxic, Anti-Inflammatory, and Wound Healing Effects. Separations, 10(3), 172. https://doi.org/10.3390/separations10030172








