Mass Spectrometry-Based Techniques for the Detection of Non-Intentionally Added Substances in Bioplastics
Abstract
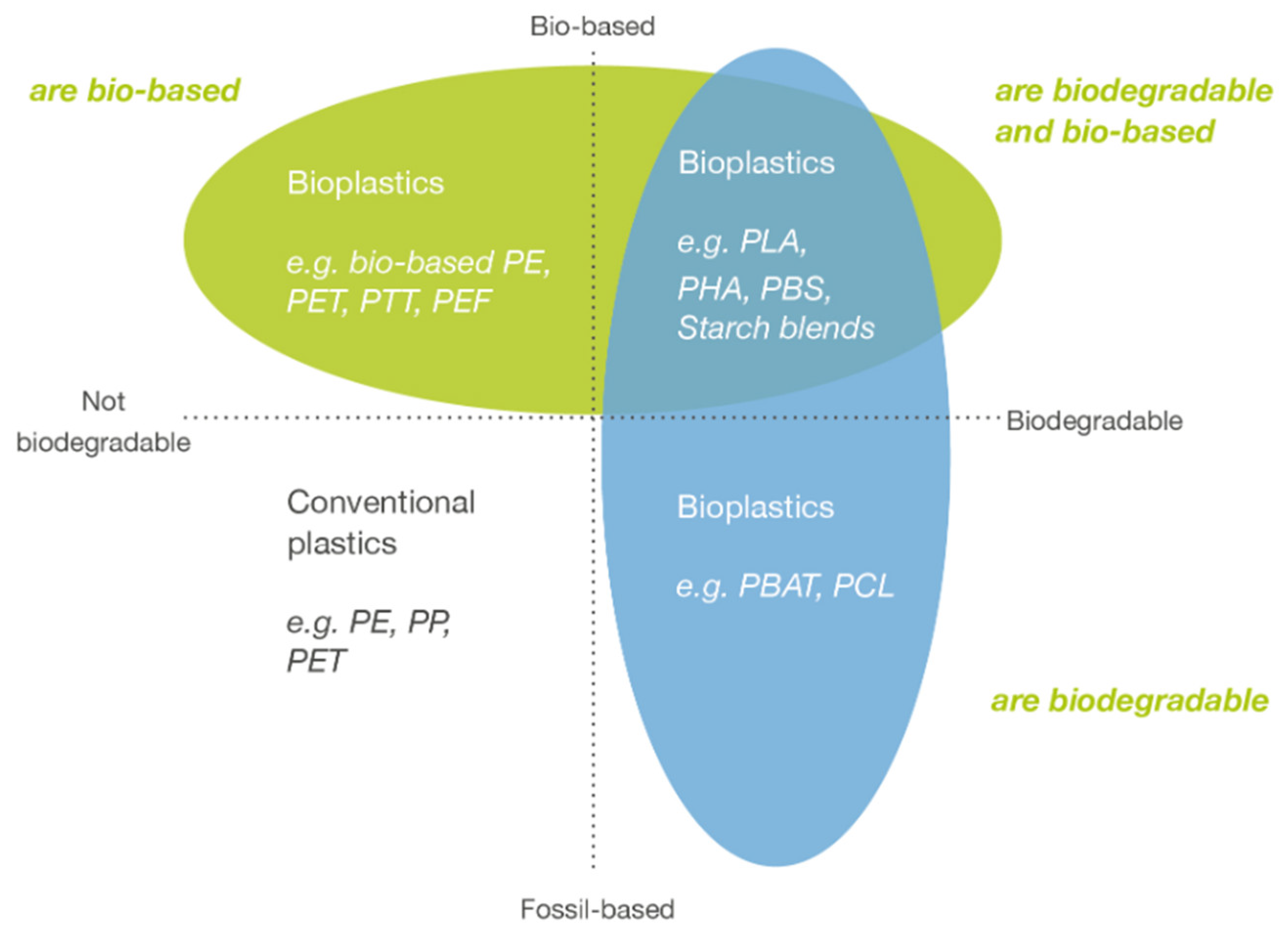
1. Bioplastics
2. Intentionally and Non-Intentionally Added Substances
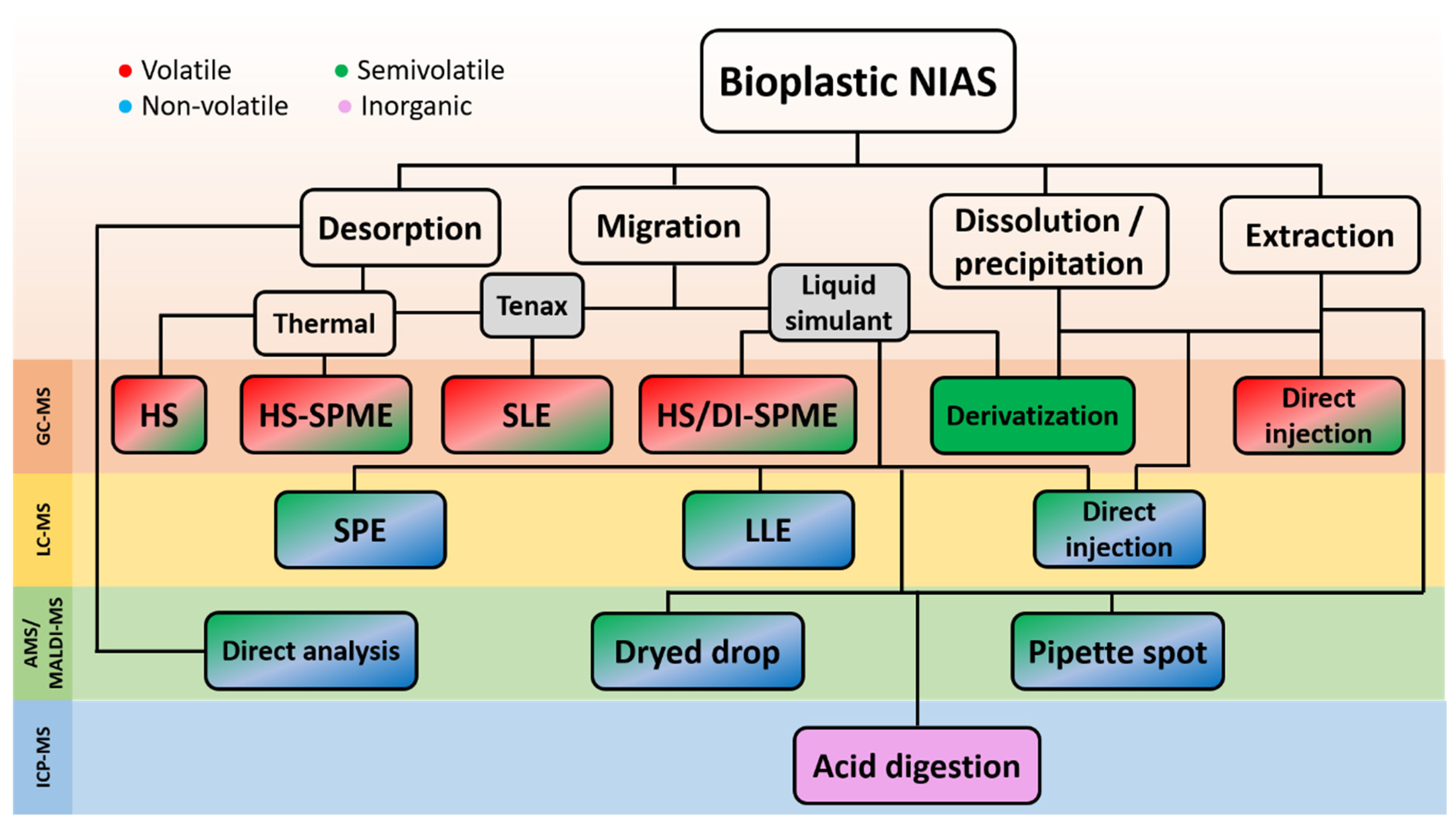
3. Migration Tests and Extraction Techniques
4. Untargeted Analysis of NIAS
5. Chromatographic Techniques for NIAS Identification
5.1. GC-MS Determination of Volatile and Semivolatile NIAS
5.2. LC-MS Determination of Non-Volatile NIAS
5.3. Complete Profiling of NIAS
6. Non-Chromatographic Techniques for NIAS Investigation
7. ICP-MS Determination of NIAS in Bioplastics
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | adipic acid |
| AIF | all ion fragmentation |
| AMS | ambient mass spectrometry |
| APGC | atmospheric pressure gas chromatography |
| ASAP | atmospheric solids analysis probe |
| BD | 1,4-butanediol |
| CCS | collision cross section |
| CID | collision-induced dissociation |
| DART | direct analysis in real time |
| DDA | data-dependent acquisition |
| DIA | data-independent acquisition |
| DI | direct immersion |
| DSPE | dispersive solid phase extraction |
| DVB/CAR/PDMS | divinylbenzene/carboxen/polydimethylsiloxane |
| EI | electron ionization sources |
| ESI | electrospray ionization |
| FCM | food contact material |
| FPSE | fabric phase sorptive extraction |
| FTICR | Fourier transformed ion cyclotron resonance |
| GC-MS | gas chromatography-mass spectrometry |
| GPC | gel permeation chromatography |
| HRMS | high-resolution mass spectrometry |
| HS | headspace extraction |
| IAS | intentionally added substances |
| ICP-MS | inductively coupled plasma-mass spectrometry |
| IM | ion mobility |
| LA | lactic acid |
| LC-MS | liquid chromatography-mass spectrometry |
| LRMS | low-resolution mass spectrometry |
| LTQ-Orbitrap | linear ion trap-Orbitrap |
| LLE | liquid-liquid extraction |
| MALDI | matrix assisted laser ablation |
| MHS | multiple headspace |
| NIAS | non-intentionally added substances |
| NMR | nuclear magnetic resonance |
| OML | overall migration limit |
| PBAT | polybutylene adipate terephthalate |
| PBS | polybutylene succinate |
| PDMS | polydimethylsiloxane |
| PE | polyethylene |
| PET | polyethylene terephthalate |
| PHA | polyhydroxyalkanoates |
| PHB | polyhydroxybutyrate |
| PHT | phthalic acid |
| PL | polylimonene |
| PLA | polylactic acid |
| PP | polypropylene |
| P&T | purge and trap |
| PVA | polyvinyl alcohol |
| Q | single quadrupole |
| Q-Orbitrap | quadrupole-Orbitrap |
| QqQ | triple quadrupole |
| QToF | quadrupole-time of flight |
| SPE | solid phase extraction |
| TD | direct thermal desorption |
| THP | terephthalic acid |
| VOC | volatile organic compound |
| SPME | solid phase microextraction |
| SWATH | sequential windowed acquisition of all theoretical MS |
| UHPLC | ultra-high pressure liquid chromatography |
| ZnO NP | zinc oxide nanoparticle |
References
- Abrha, H.; Cabrera, J.; Dai, Y.; Irfan, M.; Toma, A.; Jiao, S.; Liu, X. Bio-Based Plastics Production, Impact and End of Life: A Literature Review and Content Analysis. Sustainability 2022, 14, 4855. [Google Scholar] [CrossRef]
- Plastics Europe; European Association of Plastics Recycling and Recovery Organisations. Plastics—The Facts 2022; PlasticsEurope AISBL: Brussels Belgium, 2022. [Google Scholar]
- Merino, D.; Quilez-Molina, A.I.; Perotto, G.; Bassani, A.; Spigno, G.; Athanassiou, A. A Second Life for Fruit and Vegetable Waste: A Review on Bioplastic Films and Coatings for Potential Food Protection Applications. Green Chem. 2022, 24, 4703–4727. [Google Scholar] [CrossRef]
- Horodytska, O.; Cabanes, A.; Fullana, A. Non-Intentionally Added Substances (NIAS) in Recycled Plastics. Chemosphere 2020, 251, 126373. [Google Scholar] [CrossRef] [PubMed]
- Gerassimidou, S.; Lanska, P.; Hahladakis, J.N.; Lovat, E.; Vanzetto, S.; Geueke, B.; Groh, K.J.; Muncke, J.; Maffini, M.; Martin, O.V.; et al. Unpacking the Complexity of the PET Drink Bottles Value Chain: A Chemicals Perspective. J. Hazard. Mater. 2022, 430, 128410. [Google Scholar] [CrossRef] [PubMed]
- European Council. Directive (Eu) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. Off. J. Eur. Union 2019, 155, 1–19. [Google Scholar]
- Falua, K.J.; Pokharel, A.; Babaei-Ghazvini, A.; Ai, Y.; Acharya, B. Valorization of Starch to Biobased Materials: A Review. Polymers 2022, 14, 2215. [Google Scholar] [CrossRef]
- Ghosh, K.; Jones, B.H. Roadmap to Biodegradable Plastics-Current State and Research Needs. ACS Sustain. Chem. Eng. 2021, 9, 6170–6187. [Google Scholar] [CrossRef]
- Lambert, S.; Wagner, M. Environmental Performance of Bio-Based and Biodegradable Plastics: The Road Ahead. Chem. Soc. Rev. 2017, 46, 6855–6871. [Google Scholar] [CrossRef]
- Rujnić-Sokele, M.; Pilipović, A. Challenges and Opportunities of Biodegradable Plastics: A Mini Review. Waste Manag. Res. 2017, 35, 132–140. [Google Scholar] [CrossRef]
- European Bioplastics What Are Bioplastics. Available online: https://www.european-bioplastics.org/bioplastics/ (accessed on 20 February 2023).
- Bradley, E.L. Report FD 10/04 FSA PROJECT A03070 Biobased Materials Used in Food Contact Applications: An Assessment of the Migration Potential; The Food and Environment Research Agency: London, UK, 2010. [Google Scholar]
- Mora-Sandí, A.; Ramírez-González, A.; Castillo-Henríquez, L.; Lopretti-Correa, M.; Vega-Baudrit, J.R. Persea Americana Agro-Industrial Waste Biorefinery for Sustainable High-Value-Added Products. Polymers 2021, 13, 1727. [Google Scholar] [CrossRef]
- Gao, Z.; Yu, H.; Li, M.; Li, X.; Lei, J.; He, D.; Wu, G.; Fu, Y.; Chen, Q.; Shi, H. A Battery of Baseline Toxicity Bioassays Directed Evaluation of Plastic Leachates—Towards the Establishment of Bioanalytical Monitoring Tools for Plastics. Sci. Total Environ. 2022, 828, 154387. [Google Scholar] [CrossRef]
- Curto, M.; Le Gall, M.; Catarino, A.I.; Niu, Z.; Davies, P.; Everaert, G.; Dhakal, H.N. Long-Term Durability and Ecotoxicity of Biocomposites in Marine Environments: A Review. RSC Adv. 2021, 11, 32917–32941. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Filipe, D.A.; Paço, A.; Duarte, A.C.; Rocha-Santos, T.; Silva, A.L.P. Are Biobased Plastics Green Alternatives?—A Critical Review. Int. J. Environ. Res. Public Health 2021, 18, 7729. [Google Scholar] [CrossRef]
- Liao, J.; Chen, Q. Biodegradable Plastics in the Air and Soil Environment: Low Degradation Rate and High Microplastics Formation. J. Hazard. Mater. 2021, 418, 126329. [Google Scholar] [CrossRef] [PubMed]
- Manfra, L.; Marengo, V.; Libralato, G.; Costantini, M.; De Falco, F.; Cocca, M. Biodegradable Polymers: A Real Opportunity to Solve Marine Plastic Pollution? J. Hazard. Mater. 2021, 416, 125763. [Google Scholar] [CrossRef] [PubMed]
- Uribe-Echeverría, T.; Beiras, R. Acute Toxicity of Bioplastic Leachates to Paracentrotus Lividus Sea Urchin Larvae. Mar. Environ. Res. 2022, 176, 105605. [Google Scholar] [CrossRef]
- Wei, X.F.; Bohlén, M.; Lindblad, C.; Hedenqvist, M.; Hakonen, A. Microplastics Generated from a Biodegradable Plastic in Freshwater and Seawater. Water Res. 2021, 198, 117123. [Google Scholar] [CrossRef]
- Wei, X.F.; Capezza, A.J.; Cui, Y.; Li, L.; Hakonen, A.; Liu, B.; Hedenqvist, M.S. Millions of Microplastics Released from a Biodegradable Polymer during Biodegradation/Enzymatic Hydrolysis. Water Res. 2022, 211, 118068. [Google Scholar] [CrossRef]
- Yu, W.; Chen, J.; Zhang, S.; Zhao, Y.; Fang, M.; Deng, Y.; Zhang, Y. Extraction of Biodegradable Microplastics from Tissues of Aquatic Organisms. Sci. Total Environ. 2022, 838, 156396. [Google Scholar] [CrossRef]
- Cavazza, A.; Mattarozzi, M.; Franzoni, A.; Careri, M. A Spotlight on Analytical Prospects in Food Allergens: From Emerging Allergens and Novel Foods to Bioplastics and Plant-Based Sustainable Food Contact Materials. Food Chem. 2022, 388, 132951. [Google Scholar] [CrossRef]
- Bignardi, C.; Cavazza, A.; Laganà, C.; Salvadeo, P.; Corradini, C. Release of Non-Intentionally Added Substances (NIAS) from Food Contact Polycarbonate: Effect of Ageing. Food Control 2017, 71, 329–335. [Google Scholar] [CrossRef]
- Kato, L.S.; Conte-Junior, C.A. Safety of Plastic Food Packaging: The Challenges about Non-Intentionally Added Substances (NIAS) Discovery, Identification and Risk Assessment. Polymers 2021, 13, 2077. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Bartosova, Z.; Braun, K.; Oehlmann, J.; Völker, C.; Wagner, M. Plastic Products Leach Chemicals That InduceIn VitroToxicity under Realistic Use Conditions. Environ. Sci. Technol. 2021, 55, 11814–11823. [Google Scholar] [CrossRef]
- Geueke, B.; Groh, K.J.; Maffini, M.V.; Martin, O.V.; Boucher, J.M.; Chiang, Y.T.; Gwosdz, F.; Jieh, P.; Kassotis, C.D.; Łańska, P.; et al. Systematic Evidence on Migrating and Extractable Food Contact Chemicals: Most Chemicals Detected in Food Contact Materials Are Not Listed for Use. Crit. Rev. Food. Sci. Nutr. 2022, 1–11. [Google Scholar] [CrossRef]
- Martínez-Bueno, M.J.; Gómez Ramos, M.J.; Bauer, A.; Fernández-Alba, A.R. An Overview of Non-Targeted Screening Strategies Based on High Resolution Accurate Mass Spectrometry for the Identification of Migrants Coming from Plastic Food Packaging Materials. TrAC—Trends Anal. Chem. 2019, 110, 191–203. [Google Scholar] [CrossRef]
- Nerín, C.; Bourdoux, S.; Faust, B.; Gude, T.; Lesueur, C.; Simat, T.; Stoermer, A.; Van Hoek, E.; Oldring, P. Guidance in Selecting Analytical Techniques for Identification and Quantification of Non-Intentionally Added Substances (NIAS) in Food Contact Materials (FCMS). Food Addit. Contam. Part A 2022, 39, 620–643. [Google Scholar] [CrossRef] [PubMed]
- Nerin, C.; Alfaro, P.; Aznar, M.; Domeño, C. The Challenge of Identifying Non-Intentionally Added Substances from Food Packaging Materials: A Review. Anal. Chim. Acta 2013, 775, 14–24. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerin, C.; Dreolin, N.; Goshawk, J. The Detection and Elucidation of Oligomers Migrating from Biodegradable Multilayer Teacups Using Liquid Chromatography Coupled to Ion Mobility Time-of-Flight Mass Spectrometry and Gas Chromatography–Mass Spectrometry. Food. Chem. 2022, 374, 131777. [Google Scholar] [CrossRef]
- EFSA Food Contact Materials. Available online: https://food.ec.europa.eu/safety/chemical-safety/food-contact-materials_en (accessed on 5 January 2023).
- European Commission. European Union Commission Regulation (EU) No 10/2011 of 14 January 2011. Off. J. Eur. Union 2011, 12, 1–89. [Google Scholar]
- EFSA Panel on Food Contact Materials, Enzymes, Flavourings and Processing Aids (CEF). Recent Developments in the Risk Assessment of Chemicals in Food and Their Potential Impact on the Safety Assessment of Substances Used in Food Contact Materials. EFSA J. 2016, 14, 4357. [Google Scholar] [CrossRef]
- Martínez-Bueno, M.J.; Hernando, M.D.; Uclés, S.; Rajski, L.; Cimmino, S.; Fernández-Alba, A.R. Identification of Non-Intentionally Added Substances in Food Packaging Nano Films by Gas and Liquid Chromatography Coupled to Orbitrap Mass Spectrometry. Talanta 2017, 172, 68–77. [Google Scholar] [CrossRef]
- US Food & Drug Administration. CFR—Code of Federal Regulations Title 21; US Food & Drug Administration: Silver Spring, MD, USA, 2022. [Google Scholar]
- Gavriil, G.; Kanavouras, A.; Coutelieris, F.A. Food-Packaging Migration Models: A Critical Discussion. Crit. Rev. Food Sci. Nutr. 2018, 58, 2262–2272. [Google Scholar] [CrossRef]
- US Food & Drug Administration. Guidance for Industry: Preparation of Premarket Submissions for Food Contact Substances (Chemistry Recommendations); Center for Food Safety and Applied Nutrition: College Park, MD, USA, 2007. [Google Scholar]
- Osorio, J.; Dreolin, N.; Aznar, M.; Nerín, C.; Hancock, P. Determination of Volatile Non Intentionally Added Substances Coming from a Starch-Based Biopolymer Intended for Food Contact by Different Gas Chromatography-Mass Spectrometry Approaches. J. Chromatogr. A 2019, 1599, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Dierkes, G.; Ternes, T.A.; Völker, C.; Wagner, M. Benchmarking the in Vitro Toxicity and Chemical Composition of Plastic Consumer Products. Environ. Sci. Technol. 2019, 53, 11467–11477. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Dombrowski, A.; Völker, C.; Wagner, M. Are Bioplastics and Plant-Based Materials Safer than Conventional Plastics? In Vitro Toxicity and Chemical Composition. Environ. Int. 2020, 145, 106066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, C.; Ouyang, C.; Zeng, X.; Guo, Z.; Lai, F.; Li, J. Analysis of Oligomers in Poly (Butylene Succinate) and Poly (Butylene Adipate-Co-Terephthalate). Polym. Bull. 2022, 80, 4487–4502. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Nerín, C. Determination of Volatile Compounds and Their Sensory Impact in a Biopolymer Based on Polylactic Acid (PLA) and Polyester. Food Chem. 2019, 294, 171–178. [Google Scholar] [CrossRef]
- Aznar, M.; Ubeda, S.; Dreolin, N.; Nerín, C. Determination of Non-Volatile Components of a Biodegradable Food Packaging Material Based on Polyester and Polylactic Acid (PLA) and Its Migration to Food Simulants. J. Chromatogr. A 2019, 1583, 1–8. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Alfaro, P.; Nerín, C. Migration of Oligomers from a Food Contact Biopolymer Based on Polylactic Acid (PLA) and Polyester. Anal. Bioanal. Chem. 2019, 411, 3521–3532. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerín, C. UPLC–ESI-Q-TOF-MSE and GC–MS Identification and Quantification of Non-Intentionally Added Substances Coming from Biodegradable Food Packaging. Anal. Bioanal. Chem. 2015, 407, 6781–6790. [Google Scholar] [CrossRef]
- García Ibarra, V.; Rodríguez Bernaldo de Quirós, A.; Paseiro Losada, P.; Sendón, R. Non-Target Analysis of Intentionally and Non Intentionally Added Substances from Plastic Packaging Materials and Their Migration into Food Simulants. Food Packag. Shelf Life 2019, 21, 100325. [Google Scholar] [CrossRef]
- Okoffo, E.D.; Chan, C.M.; Rauert, C.; Kaserzon, S.; Thomas, K.V. Identification and Quantification of Micro-Bioplastics in Environmental Samples by Pyrolysis-Gas Chromatography-Mass Spectrometry. Environ. Sci. Technol. 2022, 56, 13774–13785. [Google Scholar] [CrossRef]
- Klein, K.; Hof, D.; Dombrowski, A.; Schweyen, P.; Dierkes, G.; Ternes, T.; Schulte-Oehlmann, U.; Oehlmann, J. Enhanced in Vitro Toxicity of Plastic Leachates after UV Irradiation. Water Res. 2021, 199, 117203. [Google Scholar] [CrossRef] [PubMed]
- Asensio, E.; Montañés, L.; Nerín, C. Migration of Volatile Compounds from Natural Biomaterials and Their Safety Evaluation as Food Contact Materials. Food Chem. Toxicol. 2020, 142, 111457. [Google Scholar] [CrossRef] [PubMed]
- Asensio, E.; Nieves, S.; Nerín, C. Migration Studies from Food Contact Natural Biomaterials in High Temperature Applications. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Osorio, J.; Aznar, M.; Nerín, C. Identification of Key Odorant Compounds in Starch-Based Polymers Intended for Food Contact Materials. Food Chem. 2019, 285, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Salazar, R.; Domenek, S.; Plessis, C.; Ducruet, V. Quantitative Determination of Volatile Organic Compounds Formed during Polylactide Processing by MHS-SPME. Polym. Degrad. Stab. 2017, 136, 80–88. [Google Scholar] [CrossRef]
- Gavril, G.L.; Wrona, M.; Bertella, A.; Świeca, M.; Râpă, M.; Salafranca, J.; Nerín, C. Influence of Medicinal and Aromatic Plants into Risk Assessment of a New Bioactive Packaging Based on Polylactic Acid (PLA). Food Chem. Toxicol. 2019, 132, 110662. [Google Scholar] [CrossRef]
- Osorio, J.; Aznar, M.; Nerín, C.; Birse, N.; Elliott, C.; Chevallier, O. Ambient Mass Spectrometry as a Tool for a Rapid and Simultaneous Determination of Migrants Coming from a Bamboo-Based Biopolymer Packaging. J. Hazard. Mater. 2020, 398, 122891. [Google Scholar] [CrossRef]
- Ubeda, S.; Aznar, M.; Nerín, C.; Kabir, A. Fabric Phase Sorptive Extraction for Specific Migration Analysis of Oligomers from Biopolymers. Talanta 2021, 233, 122603. [Google Scholar] [CrossRef]
- Wrona, M.; Nerín, C. Analytical Approaches for Analysis of Safety of Modern Food Packaging: A Review. Molecules 2020, 25, 752. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruíz, H.; Eras, J.; Martín-Closas, L.; Pelacho, A.M. Compounds Released from Unused Biodegradable Mulch Materials after Contact with Water. Polym. Degrad. Stab. 2020, 178, 109202. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Mattarozzi, M.; Riboni, N.; Maffini, M.; Scarpella, S.; Bianchi, F.; Careri, M. Reversed-Phase and Weak Anion-Exchange Mixed-Mode Stationary Phase for Fast Separation of Medium-, Long- and Very Long Chain Free Fatty Acids by Ultra-High-Performance Liquid Chromatography-High Resolution Mass Spectrometry. J. Chromatogr. A 2021, 1648, 462209. [Google Scholar] [CrossRef] [PubMed]
- Gómez Ramos, M.J.; Lozano, A.; Fernández-Alba, A.R. High-Resolution Mass Spectrometry with Data Independent Acquisition for the Comprehensive Non-Targeted Analysis of Migrating Chemicals Coming from Multilayer Plastic Packaging Materials Used for Fruit Purée and Juice. Talanta 2019, 191, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Song, X.C.; Canellas, E.; Dreolin, N.; Goshawk, J.; Nerin, C. A Collision Cross Section Database for Extractables and Leachables from Food Contact Materials. J. Agric. Food. Chem. 2022, 70, 4457–4466. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Yusà, V.; Pineda, A.; Coscollà, C. A Fast and Automated Strategy for the Identification and Risk Assessment of Unknown Substances (Ias/Nias) in Plastic Food Contact Materials by Gc-q-Orbitrap Hrms: Recycled Ldpe as a Proof-of-Concept. Toxics 2021, 9, 283. [Google Scholar] [CrossRef]
- Osorio, J.; Aznar, M.; Nerín, C.; Elliott, C.; Chevallier, O. Comparison of LC-ESI, DART, and ASAP for the Analysis of Oligomers Migration from Biopolymer Food Packaging Materials in Food (Simulants). Anal. Bioanal. Chem. 2022, 414, 1335–1345. [Google Scholar] [CrossRef]
- Gies, A.P.; Ellison, S.T.; Chakraborty, A.K.; Kwiecien, N.W.; Hercules, D.M. MALDI-TOF/TOF CID Study of Poly(Butylene Adipate) Fragmentation Reactions. RSC Adv. 2012, 2, 4135–4151. [Google Scholar] [CrossRef]
- Astolfi, M.L.; Marconi, E.; Lorini, L.; Valentino, F.; Silva, F.; Ferreira, B.S.; Canepari, S.; Majone, M. Elemental Concentration and Migratability in Bioplastics Derived from Organic Waste. Chemosphere 2020, 259, 127472. [Google Scholar] [CrossRef]
- ISO 8124-3:2020; Safety of Toys—Part 3: Migration of Certain Elements. British Standards Institution: London, UK, 1994.
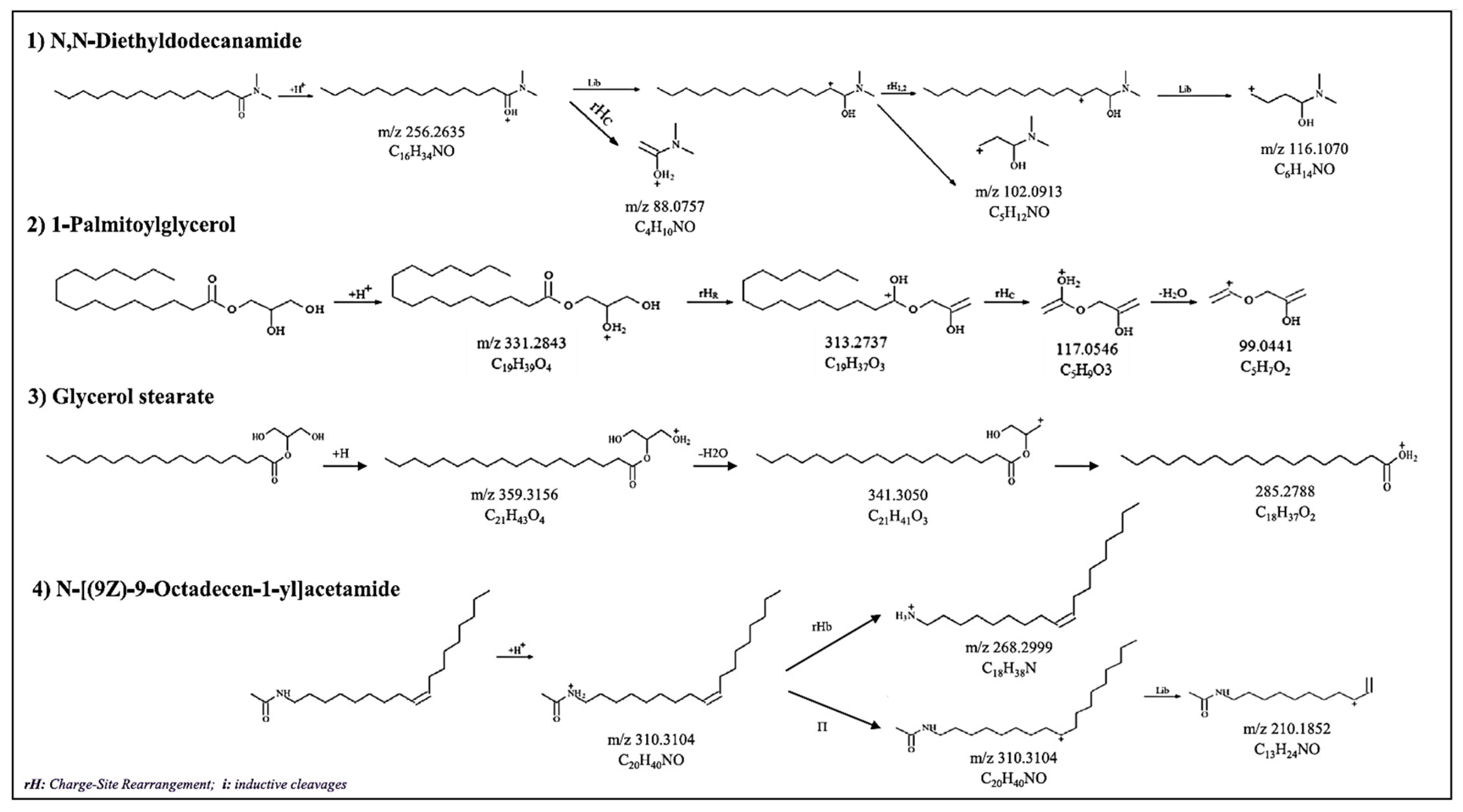
| Bioplastic Material | Extraction/Migration NIAS | Sample Pretreatment | MS Analyzer | Ref. |
|---|---|---|---|---|
| Wheat pulp and wood dishes | Migration test using 3% acetic acid, 10% ethanol, 95% ethanol | HS-SPME | Q | [50] |
| Bamboo, wheat pulp, and palm leaf dishes | Migration using Tenax® | HS-SPME | Q | [51] |
| Starch-based biopolymers | Direct analysis of pellets and films | HS-SPME | Q | [52] |
| Biopolymers based on starch and PLA | Ultrasonication in MeOH/migration using acetic acid 3%, ethanol 10%, ethanol 95%, isooctane, sunflower oil | Direct injection | Q/APGC-QtoF | [39] |
| Biodegradable blend (polyester + 18% PLA) | Dissolution/precipitation/direct analysis/migration using acetic acid 3%, ethanol 10%, ethanol 95% | Direct injection/HS-SPME | Q/APGC-QtoF | [43] |
| PLA-based pellets, film, and retails | Direct analysis of bioplastics | MHS-SPME | Q | [53] |
| PLA-based final product | Ultrasonication in MeOH | Direct injection | QtoF | [40] |
| Biodegradable mulch (PBAT, TPS, PLA, PHB and cereal flour) | Extraction using ultrapure water | Lyophilization-derivatization | Q | [58] |
| Bioplastic Material | Extraction/Migration NIAS | Sample Pretreatment | MS Analyzer | Acquisition Mode | Ref. |
|---|---|---|---|---|---|
| Biodegradable polyester + PLA | Dissolution/precipitation/migration using acetic acid 3%, ethanol 10%, ethanol 95% | Direct injection | QToF/QqQ for quantitation | MSE/SIR (selected ion recording) | [44] |
| PLA and PLA-polyester | Total dissolution/migration using acetic acid 3%, ethanol 10%, ethanol 95% | Direct injection | QToF/IM-QToF | MSE/HDMSE | [45] |
| PLA and starch-based biopolymers | Migration using acetic acid 3%, ethanol 10%, ethanol 95% | Direct injection | QTOF | MSE | [64] |
| 27 biobased plastic material and 16 plant-based materials | Ultrasonication using MeOH | Direct injection | QToF | MSE | [41] |
| PLA-based final product | Migration using water | SPE | QToF | MSE | [26] |
| PBAT + 18% PLA | Migration using acetic acid 3%, pineapple juice | FPSE | QToF/QqQ | MSE/SIR | [56] |
| Bio-PBS and a starch blend | Artificial weathering | SPE | QToF | DIA MS1 scans and MS2 scans of the most intense ion | [49] |
| Bioplastic Material | Extraction/Migration NIAS | GC-MS | LC-MS | Ref. | |
|---|---|---|---|---|---|
| MS Analyzer | MS Analyzer | Acquisition Mode | |||
| Multilayers (containing PLA, PVA, ecovio® EXP 0.5 SL®) | Adhesive dissolution in MeOH/migration using Tenax TA®-MeOH elution | Q | QToF | MSE | [46] |
| Bamboo-based biopolymer | Migration using acetic acid 3%, ethanol 10%, ethanol 95% | Q | QToF | MSE | [55] |
| Multilayer biodegradable polymer (40% polyester + 60% PLA) | Migration using cold and hot tea | Q | IM-QToF | HDMSE | [31] |
| Bioactive packaging based on PLA | Migration using 3% acetic acid acid, 10% ethanol, 95% ethanol | Q | QToF | MSE | [54] |
| Monolayer film with PLA, polylimonene PL, and ZnO NPs | Migration using 3% acetic acid acid, 10% ethanol | Q-Orbitrap | Q-Orbitrap | AIF | [35] |
| Bioplastic Material | Extraction/Migration NIAS | MS Ionization Technique | Sample Injection | Mass Analyzer | Ref. |
|---|---|---|---|---|---|
| PBAT and PBS resins | Soxhlet extraction (ethanol/THF/acetone)/dissolution in CHCl3, hexafluoroisopropanol/alcoholysis using MeOH | MALDI | Direct injection | FTICR | [42] |
| PBA | Direct analysis | MALDI | Dried droplet method | ToF/ToF | [65] |
| Bamboo-based biopolymer | Migration using acetic acid 3%, ethanol 10%, ethanol 95% | DART | Pipette-spotted onto quick strip | Q | [55] |
| PLA and starch-based biopolymers | Migration using acetic acid 3%, ethanol 10%, ethanol 95% | DART/ASAP | Pipette-spotted onto quick strip/dipping capillary/direct injection | Q/QToF | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riboni, N.; Bianchi, F.; Cavazza, A.; Piergiovanni, M.; Mattarozzi, M.; Careri, M. Mass Spectrometry-Based Techniques for the Detection of Non-Intentionally Added Substances in Bioplastics. Separations 2023, 10, 222. https://doi.org/10.3390/separations10040222
Riboni N, Bianchi F, Cavazza A, Piergiovanni M, Mattarozzi M, Careri M. Mass Spectrometry-Based Techniques for the Detection of Non-Intentionally Added Substances in Bioplastics. Separations. 2023; 10(4):222. https://doi.org/10.3390/separations10040222
Chicago/Turabian StyleRiboni, Nicolò, Federica Bianchi, Antonella Cavazza, Maurizio Piergiovanni, Monica Mattarozzi, and Maria Careri. 2023. "Mass Spectrometry-Based Techniques for the Detection of Non-Intentionally Added Substances in Bioplastics" Separations 10, no. 4: 222. https://doi.org/10.3390/separations10040222
APA StyleRiboni, N., Bianchi, F., Cavazza, A., Piergiovanni, M., Mattarozzi, M., & Careri, M. (2023). Mass Spectrometry-Based Techniques for the Detection of Non-Intentionally Added Substances in Bioplastics. Separations, 10(4), 222. https://doi.org/10.3390/separations10040222







