Identification of Bioactive Compounds in Plant Extracts of Greek Flora and Their Antimicrobial and Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Pathogenic Microorganisms
2.3. Extraction Procedure
2.4. Analytical Conditions
2.4.1. High-Performance Liquid Chromatography-Diode Array Detection Analysis (HPLC-DAD)
2.4.2. Μass Spectrometry (MS)
2.5. Evaluation of Antioxidant Activity—DPPH Method
2.6. Determination of Total Phenolic Content
2.7. The Inhibition of DNA Scission Caused by Peroxyl Radical
2.8. In Vitro Evaluation of Antimicrobial Activity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Analysis
3.1.1. HPLC-UV DAD Analysis
3.1.2. Mass Spectrometry Analysis
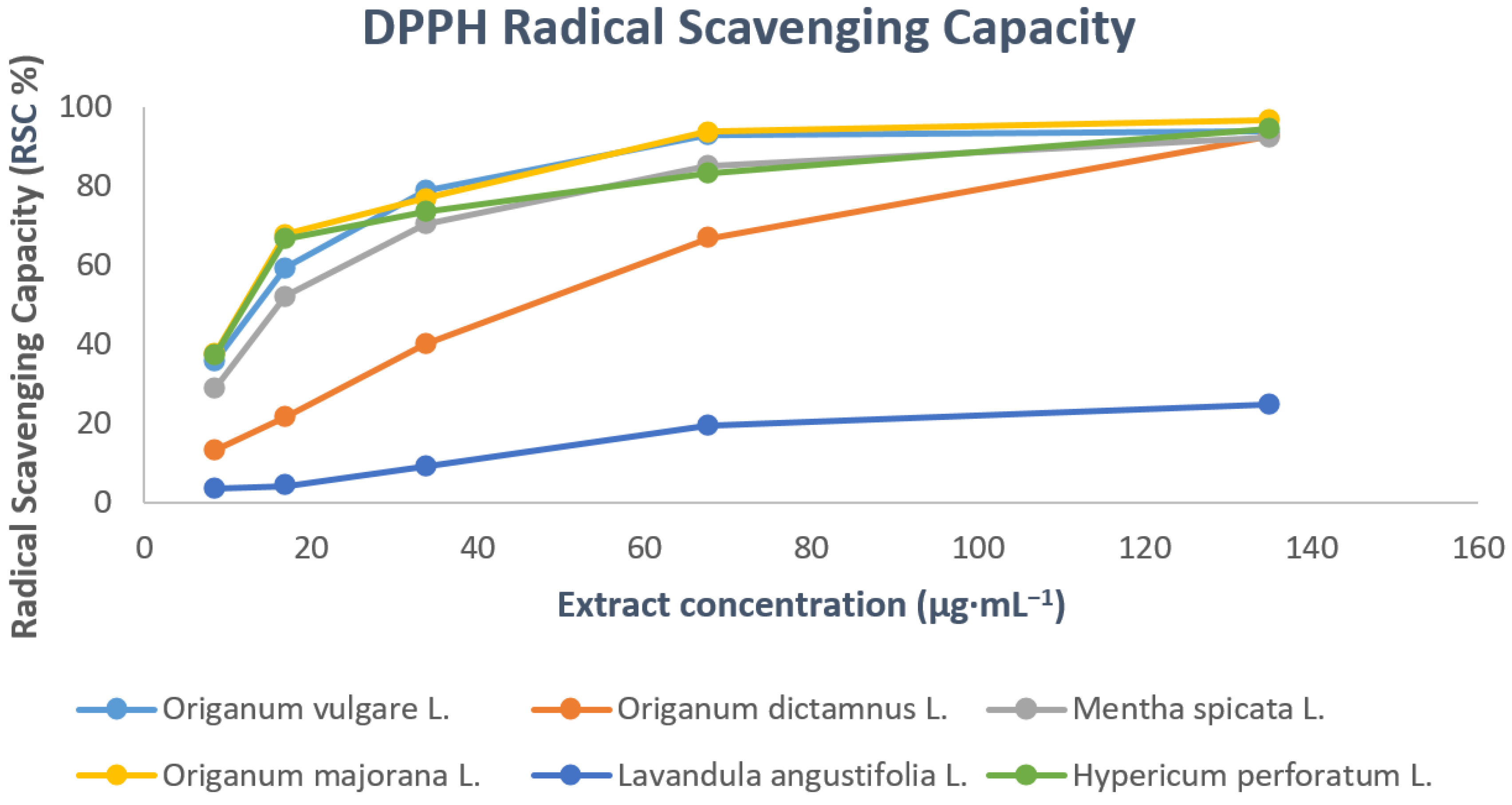
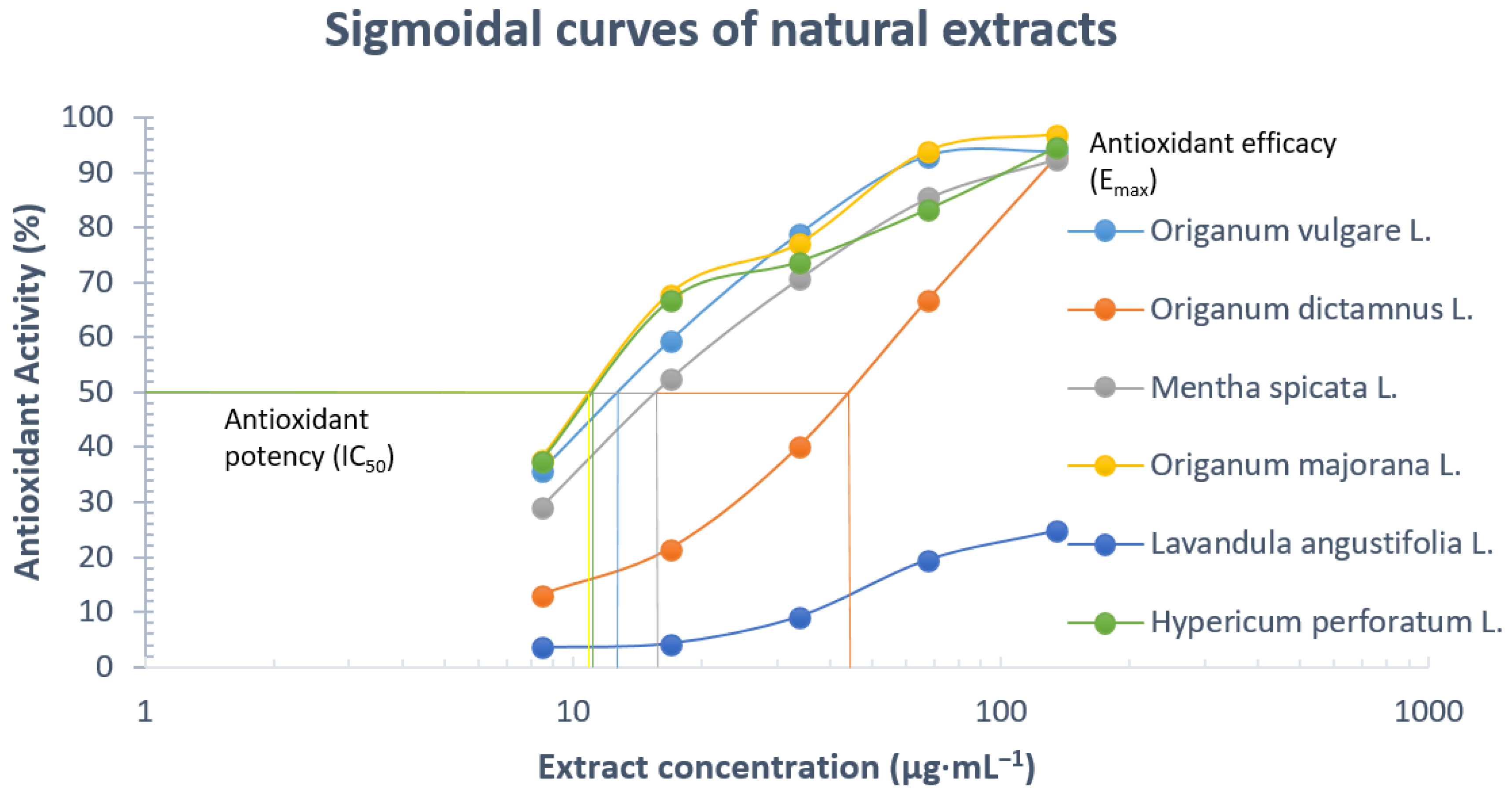
3.2. DPPH Radical Scavenging Activity
3.3. Total Phenolic Content
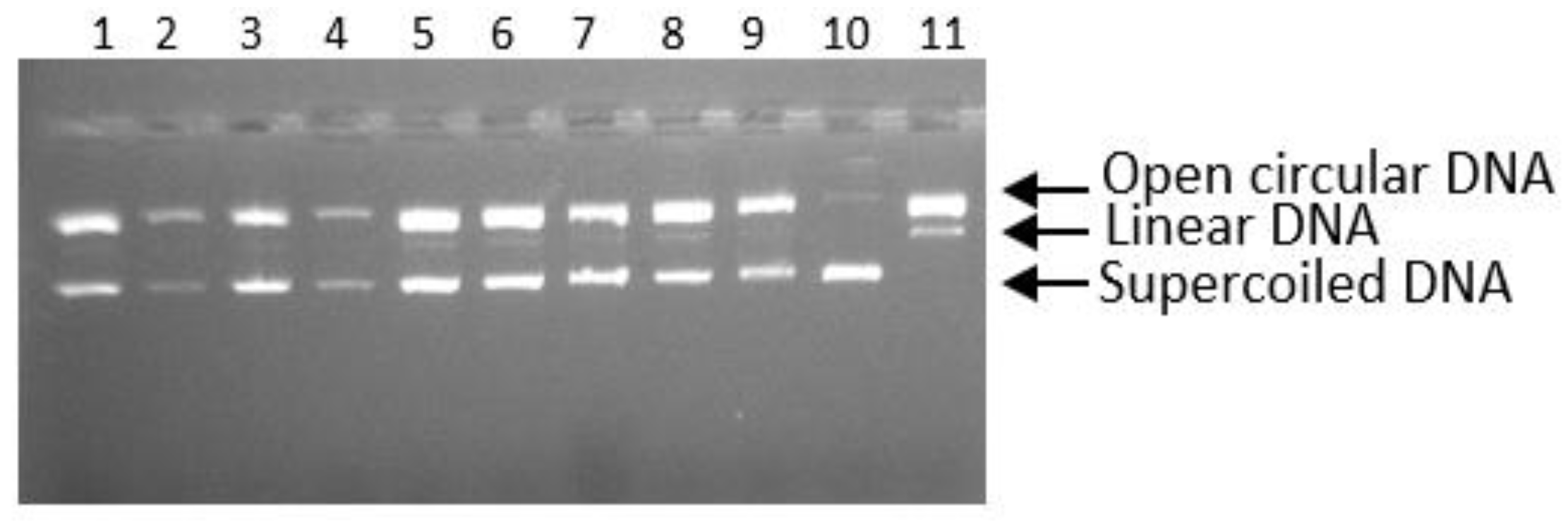
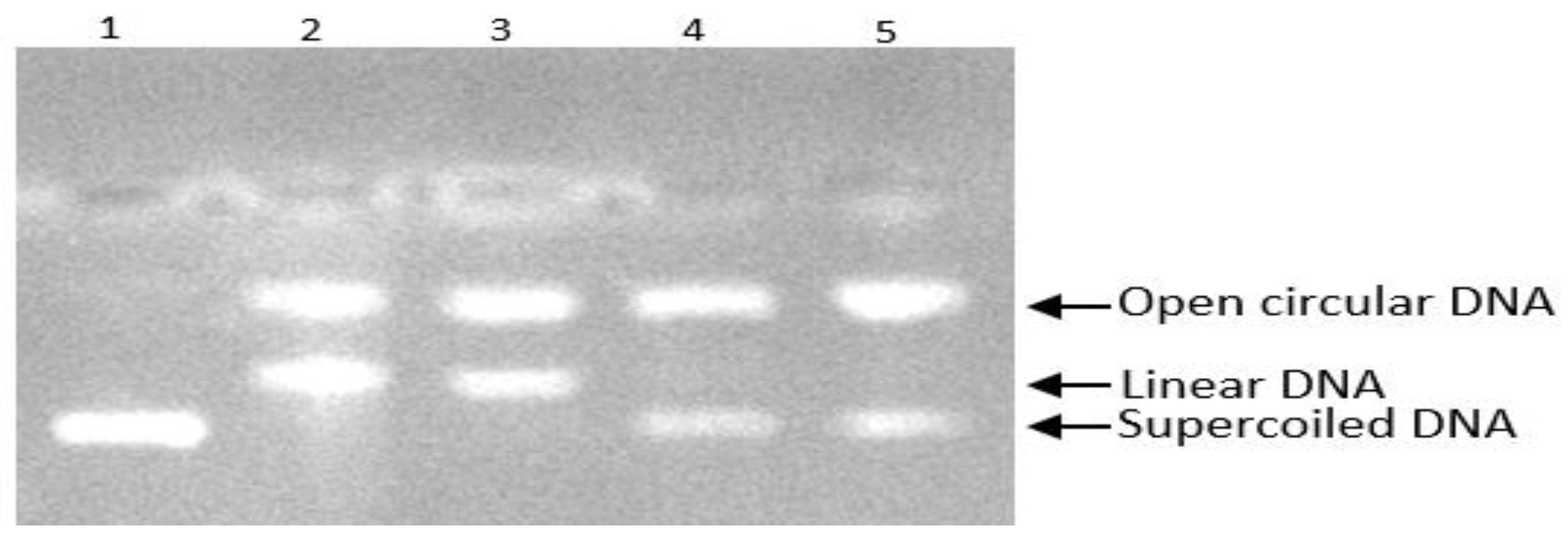
3.4. The Inhibition of DNA Scission Caused by Peroxyl Radical
3.5. In Vitro Evaluation of Antimicrobial Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mathew, B.B.; Tiwari, A.; Jatawa, S.K. Free radicals and antioxidants: A review. J. Pharm. Res. 2011, 4, 4340–4343. [Google Scholar]
- Zeljković, S.Ć.; Topčagić, A.; Požgan, F.; Štefane, B.; Tarkowski, P.; Maksimović, M. Antioxidant activity of natural and modified phenolic extracts from Satureja montana L. Ind. Crops Prod. 2015, 76, 1094–1099. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Behiry, S.I.; Salem, M.Z.; Ali, H.M.; Siddiqui, M.H.; Salem, A.Z. Antifungal, antibacterial, and antioxidant activities of Acacia saligna (Labill.) HL Wendl. flower extract: HPLC analysis of phenolic and flavonoid compounds. Molecules 2019, 24, 700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of natural plant origins: From sources to food industry applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turrini, F.; Donno, D.; Beccaro, G.L.; Pittaluga, A.; Grilli, M.; Zunin, P.; Boggia, R. Bud-derivatives, a novel source of polyphenols and how different extraction processes affect their composition. Foods 2020, 9, 1343. [Google Scholar] [CrossRef]
- Colombo, F.; Restani, P.; Biella, S.; Di Lorenzo, C. Botanicals in functional foods and food supplements: Tradition, efficacy and regulatory aspects. Appl. Sci. 2020, 10, 2387. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Sidira, M.; Skitsa, M.; Tsochantaridis, I.; Pappa, A.; Dimtsoudis, C.; Proestos, C.; Kourkoutas, Y. Assessment of the antimicrobial, antioxidant, and antiproliferative potential of Sideritis raeseri subps. raeseri essential oil. Foods 2020, 9, 860. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Meneses, N.G.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative evaluation of total antioxidant capacities of plant polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef] [Green Version]
- Silva, N.C.C.; Fernandes Júnior, A.J. Biological properties of medicinal plants: A review of their antimicrobial activity. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 402–413. [Google Scholar] [CrossRef]
- Basgedik, B.; Ugur, A.; Sarac, N. Antimicrobial, antioxidant, antimutagenic activities, and phenolic compounds of Iris germanica. Ind. Crops Prod. 2014, 61, 526–530. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef] [PubMed]
- Valerio, F.; Mezzapesa, G.N.; Ghannouchi, A.; Mondelli, D.; Logrieco, A.F.; Perrino, E.V. Characterization and antimicrobial properties of essential oils from four wild taxa of Lamiaceae family growing in Apulia. Agronomy 2021, 11, 1431. [Google Scholar] [CrossRef]
- Raja, R.R. Medicinally potential plants of Labiatae (Lamiaceae) family: An overview. Res. J. Med. Plant 2012, 6, 203–213. [Google Scholar] [CrossRef] [Green Version]
- Carović-StanKo, K.; PeteK, M.; Grdiša, M.; Pintar, J.; Bedeković, D.; Satovic, Z. Medicinal plants of the family Lamiaceae as functional foods—A review. Czech J. Food Sci. 2016, 34, 377–390. [Google Scholar] [CrossRef] [Green Version]
- Cocan, I.; Alexa, E.; Danciu, C.; Radulov, I.; Galuscan, A.; Obistioiu, D.; Morvay, A.A.; Sumalan, R.M.; Poiana, M.A.; Pop, G.; et al. Phytochemical screening and biological activity of Lamiaceae family plant extracts. Exp. Ther. Med. 2018, 15, 1863–1870. [Google Scholar] [CrossRef] [Green Version]
- Ličina, B.Z.; Stefanović, O.D.; Vasić, S.M.; Radojević, I.D.; Dekić, M.S.; Čomić, L.R. Biological activities of the extracts from wild growing Origanum vulgare L. Food Control 2013, 33, 498–504. [Google Scholar] [CrossRef]
- Wurdack, K.J.; Davis, C.C. Malpighiales phylogenetics: Gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. Am. J. Bot. 2009, 96, 1551–1570. [Google Scholar] [CrossRef] [PubMed]
- Saddiqe, Z.; Naeem, I.; Maimoona, A. A review of the antibacterial activity of Hypericum perforatum L. J. Ethnopharmacol. 2010, 131, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Wills, R.B.; Bone, K.; Morgan, M. Herbal products: Active constituents, modes of action and quality control. Nutr. Res. Rev. 2000, 13, 47–77. [Google Scholar] [CrossRef]
- Marrelli, M.; Statti, G.; Conforti, F. Hypericum spp.: An update on the biological activities and metabolic profiles. Mini Rev. Med. Chem. 2020, 20, 66–87. [Google Scholar] [CrossRef] [PubMed]
- Zirak, N.; Shafiee, M.; Soltani, G.; Mirzaei, M.; Sahebkar, A. Hypericum perforatum in the treatment of psychiatric and neurodegenerative disorders: Current evidence and potential mechanisms of action. J. Cell. Physiol. 2019, 234, 8496–8508. [Google Scholar] [CrossRef]
- Galeotti, N. Hypericum perforatum (St John’s wort) beyond depression: A therapeutic perspective for pain conditions. J. Ethnopharmacol. 2017, 200, 136–146. [Google Scholar] [CrossRef]
- Mutlu, B.; Erci, F.; Çakir Koç, R. Production of alginate films containing Hypericum perforatum extract as an antibacterial and antioxidant wound dressing material. J. Bioact. Compat. Polym. 2022, 37, 134–148. [Google Scholar] [CrossRef]
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J. Pharm. Bioallied Sci. 2020, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, J.B.; Veljković, V.B. Extraction of flavonoids from garden (Salvia officinalis L.) and glutinous (Salvia glutinosa L.) sage by ultrasonic and classical maceration. J. Serb. Chem. Soc 2007, 72, 73–80. [Google Scholar] [CrossRef]
- Kouri, G.; Tsimogiannis, D.; Bardouki, H.; Oreopoulou, V. Extraction and analysis of antioxidant components from Origanum dictamnus. Innov. Food Sci. Emerg. Technol. 2007, 8, 155–162. [Google Scholar] [CrossRef]
- Costa, D.C.; Costa, H.S.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 45, 336–354. [Google Scholar] [CrossRef]
- Manousi, N.; Sarakatsianos, I.; Samanidou, V. Extraction techniques of phenolic compounds and other bioactive compounds from medicinal and aromatic plants. In Engineering tools in the Beverage Industry; Woodhead Publishing: Sawston, UK, 2019; Volume 3, pp. 283–314. [Google Scholar] [CrossRef]
- Damiani, T.; Dreolin, N.; Stead, S.; Dall’Asta, C. Critical evaluation of ambient mass spectrometry coupled with chemometrics for the early detection of adulteration scenarios in Origanum vulgare L. Talanta 2021, 227, 122116. [Google Scholar] [CrossRef]
- Nastasijević, B.; Petrović, S.; Leskovac, A. Analysis of Gentiana Lutea Extracts by Atmospheric Solids Analysis Probe Mass Spectrometry. In Specific Methods for Food Safety and Quality; Vinča Institute of Nuclear Sciences: Belgrade, Serbia, 2016. [Google Scholar]
- Alam, M.N.; Bristi, N.J.; Rafiquzzaman, M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi Pharm. J. 2013, 21, 143–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ácsová, A.; Martiniaková, S.; Hojerová, J. Selected methods to determine antioxidant activity of hydrophilic/lipophilic substances. Acta Chim. Slovaca 2019, 12, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Choo, C.; Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. An appraisal of eighteen commonly consumed edible plants as functional food based on their antioxidant and starch hydrolase inhibitory activities. J. Sci. Food Agric. 2015, 95, 2956–2964. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, A.; Shahidi, F. Bioactivities and antiradical properties of millet grains and hulls. J. Agric. Food Chem. 2011, 59, 9563–9571. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.; John, J.A.; Shahidi, F. Polyphenol composition and antioxidant potential of mint leaves. Food Prod. Process. Nutr. 2019, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Madhujith, T.; Amarowicz, R.; Shahidi, F. Phenolic antioxidants in beans and their effects on inhibition of radical-induced DNA damage. J. Am. Oil Chem. Soc. 2004, 81, 691–696. [Google Scholar] [CrossRef]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Proestos, C.; Varzakas, T. Aromatic plants: Antioxidant capacity and polyphenol characterisation. Foods 2017, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, A.; Belwal, T.; Tamta, S.; Bhatt, I.D.; Rawal, R.S. Phenolic compounds, antioxidant capacity and antimutagenic activity in different growth stages of in vitro raised plants of Origanum vulgare L. Mol. Biol. Rep. 2019, 46, 2231–2241. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulou, A.; Karioti, A.; Gousiadou, C.; Lax Vivancos, V.; Kyriazopoulos, P.; Golegou, S.; Skaltsa, H. Depsides and other polar constituents from Origanum dictamnus L. and their in vitro antimicrobial activity in clinical strains. J. Agric. Food Chem. 2010, 58, 6064–6068. [Google Scholar] [CrossRef] [PubMed]
- Aybastıer, Ö.; Şahin, S.; Demir, C. Response surface optimized ultrasonic-assisted extraction of quercetin and isolation of phenolic compounds from Hypericum perforatum L. by column chromatography. Sep. Sci. Technol. 2013, 48, 1665–1674. [Google Scholar] [CrossRef]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Fatiha, B.; Didier, H.; Naima, G.; Khodir, M.; Martin, K.; Léocadie, K.; Caroline, S.; Mohamed, C.; Pierre, D. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae). Ind. Crops Prod. 2015, 74, 722–730. [Google Scholar] [CrossRef]
- Adaszyńska-Skwirzyńska, M.; Dzięcioł, M. Comparison of phenolic acids and flavonoids contents in various cultivars and parts of common lavender (Lavandula angustifolia) derived from Poland. Nat. Prod. Res. 2017, 31, 2575–2580. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Phenolic compounds in plant foods: Chemistry and health benefits. Prev. Nutr. Food Sci. 2003, 8, 200–218. [Google Scholar] [CrossRef]
- Wang, S.Z.; Fan, X.; Zheng, A.L.; Wang, Y.G.; Dou, Y.Q.; Wei, X.Y.; Zhao, Y.P.; Wang, R.Y.; Zong, Z.M.; Zhao, W. Evaluation of atmospheric solids analysis probe mass spectrometry for the analysis of coal-related model compounds. Fuel 2014, 117, 556–563. [Google Scholar] [CrossRef]
- Petersen, M.; Metzger, J.W. Identification of the reaction products of rosmarinic acid synthase from cell cultures of Coleus blumei by ion spray mass spectrometry and tandem mass spectrometry. Phytochem. Anal. 1993, 4, 131–134. [Google Scholar] [CrossRef]
- Ryan, D.; Robards, K.; Lavee, S. Determination of phenolic compounds in olives by reversed-phase chromatography and mass spectrometry. J. Chromatogr. A 1999, 832, 87–96. [Google Scholar] [CrossRef]
- Rosenberg, E. Characterisation of historical organic dyestuffs by liquid chromatography–mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 33–57. [Google Scholar] [CrossRef] [PubMed]
- Martens, S.M.; Marta, R.A.; Martens, J.K.; McMahon, T.B. Consecutive fragmentation mechanisms of protonated ferulic acid probed by Infrared multiple photon dissociation spectroscopy and electronic structure calculations. J. Am. Soc. Mass Spectrom. 2012, 23, 1697–1706. [Google Scholar] [CrossRef]
- Wang, J.; Yue, Y.D.; Tang, F.; Sun, J. Screening and analysis of the potential bioactive components in rabbit plasma after oral administration of hot-water extracts from leaves of Bambusa textilis McClure. Molecules 2012, 17, 8872–8885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, J.L.; Bispo, V.S.; Filho, A.B.C.; Pinto, I.F.; Dantas, L.S.; Vasconcelos, D.F.; Abreu, F.F.; Melo, D.A.; Matos, I.A.; Freitas, F.P.; et al. Evaluation of chemical constituents and antioxidant activity of coconut water (Cocus nucifera L.) and caffeic acid in cell culture. An. Acad. Bras. Ciências 2013, 85, 1235–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dheer, D.; Singh, D.; Kumar, G.; Karnatak, M.; Chandra, S.; Prakash Verma, V.; Shankar, R. Thymol chemistry: A medicinal toolbox. Curr. Bioact. Compd. 2019, 15, 454–474. [Google Scholar] [CrossRef]
- Fan, Y.; Cao, X.; Zhang, M.; Wei, S.; Zhu, Y.; Ouyang, H.; He, J. Quantitative Comparison and Chemical Profile Analysis of Different Medicinal Parts of Perilla frutescens (L.) Britt. from Different Varieties and Harvest Periods. J. Agric. Food Chem. 2022, 70, 8838–8853. [Google Scholar] [CrossRef]
- AlShaal, S.; Karabet, F.; Daghestani, M. Determination of the antioxidant properties of the Syrian olive leaves extracts and isolation oleuropein by HPLC techniques. Anal. Bioanal. Chem. Res. 2019, 6, 97–110. [Google Scholar] [CrossRef]
- Molole, G.J.; Gure, A.; Abdissa, N. Determination of total phenolic content and antioxidant activity of Commiphora mollis (Oliv.) Engl. resin. BMC Chem. 2022, 16, 48. [Google Scholar] [CrossRef]
- Benslama, A.; Daci, S.; Nabti, L.Z.; Bendif, H.; Harrar, A. Assessment of polyphenols contents, antibacterial and antioxidant activities of Origanum majorana extracts. Eur. J. Biol. Res. 2021, 11, 509–518. [Google Scholar] [CrossRef]
- Spiridon, I.; Colceru, S.; Anghel, N.; Teaca, C.A.; Bodirlau, R.; Armatu, A. Antioxidant capacity and total phenolic contents of oregano (Origanum vulgare), lavender (Lavandula angustifolia) and lemon balm (Melissa officinalis) from Romania. Nat. Prod. Res. 2011, 25, 1657–1661. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Lytoudi, K.; Mavromelanidou, O.K.; Zoumpoulakis, P.; Sinanoglou, V.J. Antioxidant capacity of selected plant extracts and their essential oils. Antioxidants 2013, 2, 11–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.E.; Oladeinde, F.O.; Kinyua, A.M.; Michelin, R.; Makinde, J.M.; Jaiyesimi, A.A.; Mbiti, W.N.; Kamau, G.N.; Kofi-Tsekpo, W.M.; Pramanik, S.; et al. Comparative assessment of total phenolic content in selected medicinal plants. Niger. J. Nat. Prod. Med. 2008, 12, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food Sci. Technol. 2017, 54, 219–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graßmann, J. Terpenoids as plant antioxidants. Vitam. Horm. 2005, 72, 505–535. [Google Scholar] [CrossRef]
- Sekeroglu, N.; Urlu, E.; Kulak, M.; Gezici, S.; Dang, R. Variation in total polyphenolic contents, DNA protective potential and antioxidant capacity from aqueous and ethanol extracts in different plant parts of Hypericum perforatum L. Indian J. Pharm. Educ. Res. 2017, 51, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kalpoutzakis, E.; Chatzimitakos, T.; Athanasiadis, V.; Mitakou, S.; Aligiannis, N.; Bozinou, E.; Gortzi, O.; Skaltsounis, L.A.; Lalas, S.I. Determination of the Total Phenolics Content and Antioxidant Activity of Extracts from Parts of Plants from the Greek Island of Crete. Plants 2023, 12, 1092. [Google Scholar] [CrossRef]
- Burri, S.C.; Ekholm, A.; Håkansson, Å.; Tornberg, E.; Rumpunen, K. Antioxidant capacity and major phenol compounds of horticultural plant materials not usually used. J. Funct. Foods 2017, 38, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant, anti-inflammatory and DNA scission inhibitory activities of phenolic compounds in selected onion and potato varieties. J. Funct. Foods 2013, 5, 930–939. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breimer, L.H. Molecular mechanisms of oxygen radical carcinogenesis and mutagenesis: The role of DNA base damage. Mol. Carcinog. 1990, 3, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Kitts, D.D. Antioxidant, prooxidant, and cytotoxic activities of solvent-fractionated dandelion (Taraxacum officinale) flower extracts in vitro. J. Agric. Food Chem. 2003, 51, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Chishti, S.; Kaloo, Z.A.; Sultan, P. Medicinal importance of genus Origanum: A review. J. Pharmacogn. Phytother 2013, 5, 170–177. [Google Scholar]
- Okmen, G.; Balpınar, N. The biological activities of Hypericum perforatum L. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 213–218. [Google Scholar] [CrossRef] [Green Version]
- Akram, M.; Riaz, M.; Wadood, A.W.C.; Hazrat, A.; Mukhtiar, M.; Ahmad Zakki, S.; Daniyal, M.; Shariati, M.A.; Said Khan, F.; Zainab, R. Medicinal plants with anti-mutagenic potential. Biotechnol. Biotechnol. Equip. 2020, 34, 309–318. [Google Scholar] [CrossRef] [Green Version]
- Sen, A.; Batra, A. Evaluation of antimicrobial activity of different solvent extracts of medicinal plant: Melia azedarach L. Int. J. Curr. Pharm. Res. 2012, 4, 67–73. [Google Scholar]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The minimum inhibitory concentration of antibiotics: Methods, interpretation, clinical relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Salem, M.Z.; Mohamed, A.A.; Ali, H.M.; Al Farraj, D.A. Characterization of phytoconstituents from alcoholic extracts of four woody species and their potential uses for management of six Fusarium oxysporum isolates identified from some plant hosts. Plants 2021, 10, 1325. [Google Scholar] [CrossRef] [PubMed]
- Gonelimali, F.D.; Lin, J.; Miao, W.; Xuan, J.; Charles, F.; Chen, M.; Hatab, S.R. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Front. Microbiol. 2018, 9, 1639. [Google Scholar] [CrossRef]
- Pezzani, R.; Vitalini, S.; Iriti, M. Bioactivities of Origanum vulgare L.: An update. Phytochem. Rev. 2017, 16, 1253–1268. [Google Scholar] [CrossRef]
- Duletić-Laušević, S.; Aradski, A.A.; Kolarević, S.; Vuković-Gačić, B.; Oalđe, M.; Živković, J.; Šavikin, K.; Marin, P.D. Antineurodegenerative, antioxidant and antibacterial activities and phenolic components of Origanum majorana L.(Lamiaceae) extracts. J. Appl. Bot. Food Qual 2018, 91, 126–134. [Google Scholar] [CrossRef]
- Patocka, J. The chemistry, pharmacology, and toxicology of the biologically active constituents of the herb Hypericum perforatum L. J. Appl. Biomed. 2003, 1, 61–70. [Google Scholar] [CrossRef] [Green Version]
- de Assis Alves, T.; Pinheiro, P.F.; Praça-Fontes, M.M.; Andrade-Vieira, L.F.; Corrêa, K.B.; de Assis Alves, T.; da Cruz, F.A.; Júnior, V.L.; Ferreira, A.; Soares, T.C.B. Toxicity of thymol, carvacrol and their respective phenoxyacetic acids in Lactuca sativa and Sorghum bicolor. Ind. Crops Prod. 2018, 114, 59–67. [Google Scholar] [CrossRef]
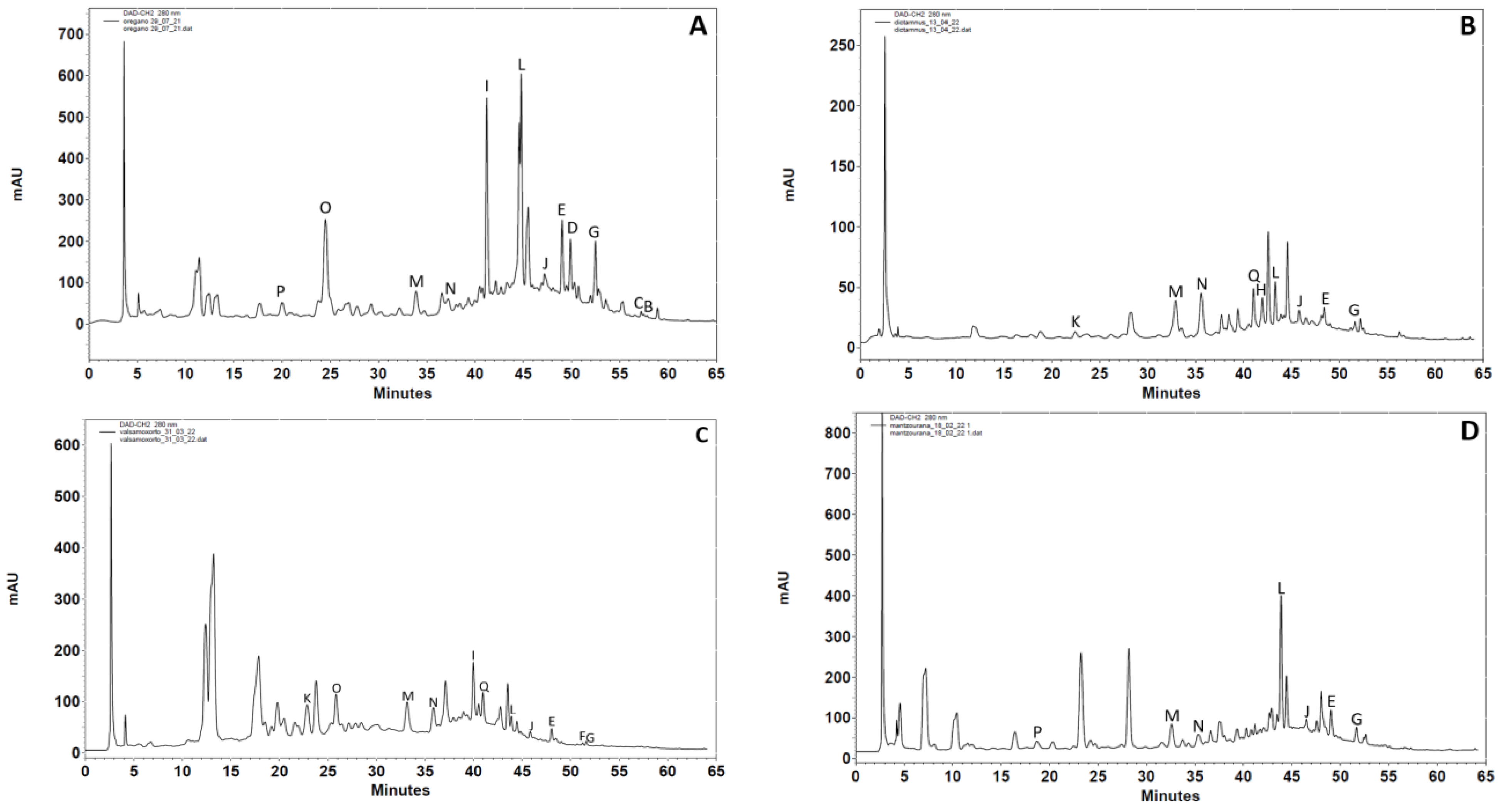


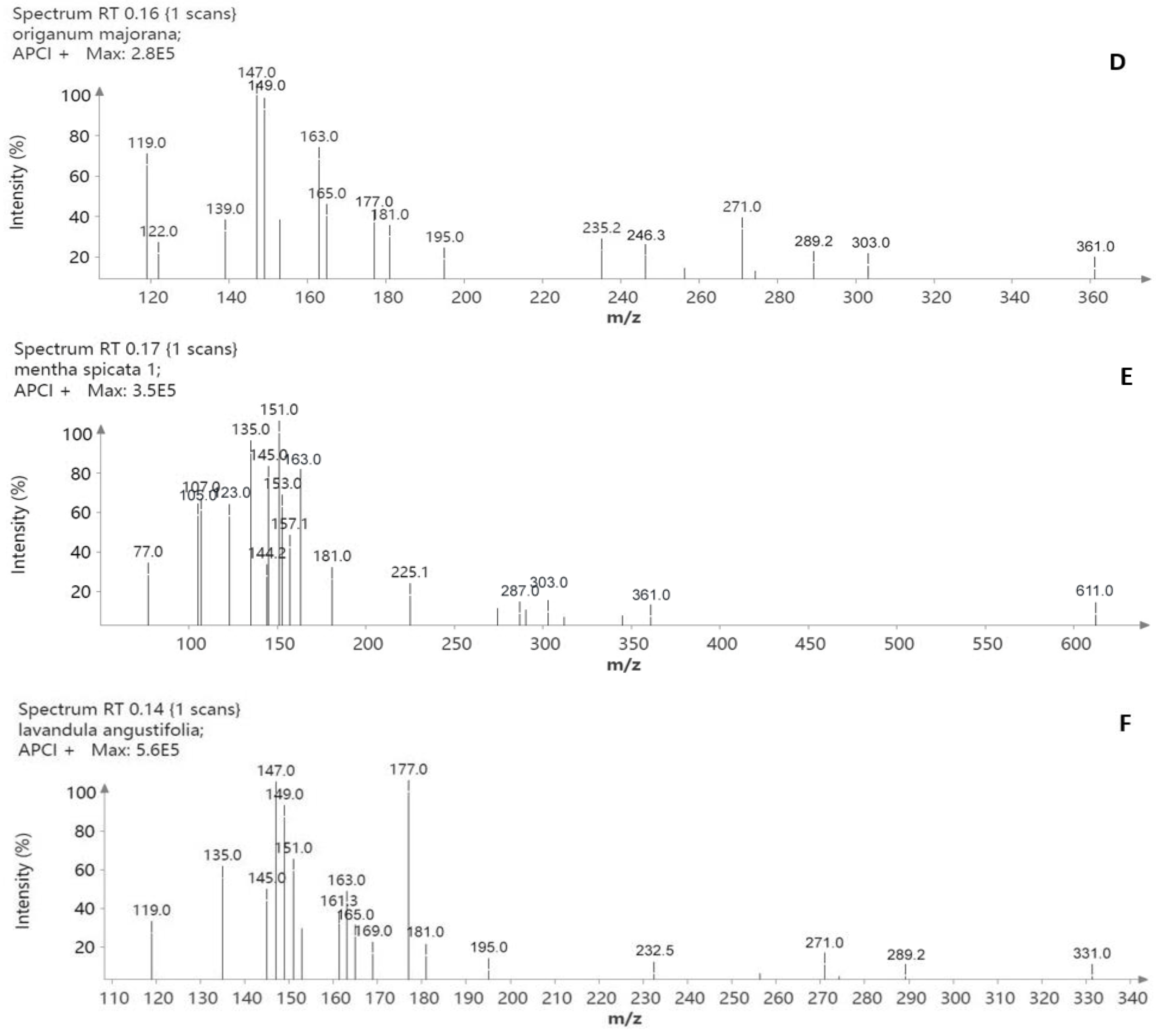

| Standard Compounds | Origanum vulgare L. | Origanum dictamnus L. | Hypericum perforatum L. | Origanum majorana L. | Mentha spicata L. | Lavandula angustifolia L. |
|---|---|---|---|---|---|---|
| Concentration (μg·mL−1) | ||||||
| (A) Naringenin | - | - | - | - | - | - |
| (B) Thymol | 0.27 ± 0.10 | - | - | - | 0.61 ± 0.02 | - |
| (C) Carvacrol | 9.17 ± 0.74 | - | - | - | - | - |
| (D) Luteolin | 25.97 ± 0.36 | - | - | - | 19.89 ± 0.25 | - |
| (E) Quercetin | 40.01 ± 0.92 | 10.73 ± 0.13 | 10.14 ± 0.74 | 14.52 ± 0.59 | 46.03 ± 0.13 | - |
| (F) Kaempferol | - | - | 3.62 ± 0.46 | - | 7.42 ± 0.45 | - |
| (G) Apigenin | 33.32 ± 0.85 | 2.45 ± 0.05 | 1.90 ± 0.96 | 4.30 ± 0.11 | - | 2.17 ± 0.13 |
| (H) Apigenin-7-glucoside | - | 9.04 ± 0.03 | - | - | - | - |
| (I) Rutin | 249.18 ± 1.2 | - | 57.03 ± 0.32 | - | 40.58 ± 0.09 | - |
| (J) Eriodictyol | 2.14 ± 0.44 | 4.45 ± 0.11 | 5.09 ± 0.62 | 1.72 ± 0.22 | - | 35.09 ± 1.2 |
| (K) Vanillic acid | - | 4.22 ± 0.31 | 18.68 ± 0.91 | - | - | 8.50 ± 0.71 |
| (L) Rosmarinic acid | 23.80 ± 1.2 | 15.32 ± 0.34 | 5.63 ± 0.81 | 46.63 ± 0.45 | 126.38 ± 0.23 | - |
| (M) p-coumaric acid | 10.67 ± 0.81 | 5.35 ± 0.16 | 6.06 ± 0.87 | 8.58 ± 0.56 | - | 25.29 ± 0.33 |
| (N) Ferulic acid | 2.29 ± 0.17 | 13.99 ± 0.22 | 7.55 ± 0.88 | 16.47 ± 0.86 | - | 24.18 ± 0.97 |
| (O) Caffeic acid | 79.33 ± 0.95 | - | 20.99 ± 1.1 | - | 19.54 ± 0.56 | 60.56 ± 0.82 |
| (P) Hydroxybenzoic acid | 36.23 ± 0.25 | - | - | 20.26 ± 0.34 | - | - |
| (Q) Benzoic acid | - | 30.79 ± 0.73 | 45.72 ± 1.06 | - | 16.41 ± 0.18 | - |
| Herbal Extract | Concentration (μg·mL−1) | Radical Scavenging Capacity (RSC%) |
|---|---|---|
| Origanum vulgare L. | 8.44 | 35.76 ± 0.14 |
| 16.88 | 59.41 ± 0.14 | |
| 33.75 | 78.82 ± 0.14 | |
| 67.50 | 93.05 ± 0.15 | |
| 135.00 | 93.88 ± 0.16 | |
| Origanum dictamnus L. | 8.44 | 13.17 ± 0.09 |
| 16.88 | 21.53 ± 0.14 | |
| 33.75 | 40.11 ± 0.23 | |
| 67.50 | 66.82 ± 0.28 | |
| 135.00 | 92.82 ± 0.3 | |
| Mentha spicata L. | 8.44 | 28.94 ± 0.06 |
| 16.88 | 52.35 ± 0.19 | |
| 33.75 | 70.59 ± 0.23 | |
| 67.50 | 85.29 ± 0.24 | |
| 135.00 | 92.47 ± 0.26 | |
| Origanum majorana L. | 8.44 | 37.76 ± 0.32 |
| 16.88 | 67.90 ± 0.34 | |
| 33.75 | 77.00 ± 0.35 | |
| 67.50 | 93.88 ± 0.38 | |
| 135.00 | 96.94 ± 0.39 | |
| Lavandula angustifolia L. | 8.44 | 3.50 ± 0.08 |
| 16.88 | 4.20 ± 0.12 | |
| 33.75 | 9.10 ± 0.23 | |
| 67.50 | 19.40 ± 0.33 | |
| 135.00 | 24.70 ± 0.41 | |
| Hypericum perforatum L. | 8.44 | 37.41 ± 0.06 |
| 16.88 | 66.70 ± 0.11 | |
| 33.75 | 73.76 ± 0.13 | |
| 67.50 | 83.29 ± 0.13 | |
| 135.00 | 94.58 ± 0.15 |
| Herbal Extract | Concentration (μg·mL−1) | Radical Scavenging Capacity (RSC%) | ||||
|---|---|---|---|---|---|---|
| 0 min | 15 min | 30 min | 45 min | 60 min | ||
| Origanum vulgare L. | 8.44 | 2.41 | 14.70 | 25.41 | 35.76 | 34.76 |
| 16.88 | 13.76 | 25.65 | 57.53 | 59.41 | 58.64 | |
| 33.75 | 38.47 | 43.41 | 74.12 | 78.82 | 68.01 | |
| 67.50 | 55.29 | 69.53 | 78.94 | 93.05 | 89.12 | |
| 135.00 | 74.11 | 76.94 | 90.24 | 93.88 | 92.53 | |
| Origanum dictamnus L. | 8.44 | 11.53 | 12.71 | 12.94 | 13.17 | 13.41 |
| 16.88 | 18.47 | 19.18 | 20.00 | 21.53 | 24.00 | |
| 33.75 | 30.59 | 31.76 | 36.47 | 40.11 | 46.12 | |
| 67.50 | 47.29 | 61.76 | 64.70 | 66.82 | 78.71 | |
| 135.00 | 77.00 | 84.11 | 88.82 | 92.82 | 92.47 | |
| Mentha spicata L. | 8.44 | 13.65 | 17.41 | 27.88 | 28.94 | 28.94 |
| 16.88 | 41.41 | 44.00 | 48.94 | 52.35 | 51.76 | |
| 33.75 | 58.71 | 64.82 | 67.88 | 70.59 | 70.24 | |
| 67.50 | 71.18 | 71.76 | 78.71 | 85.29 | 85.06 | |
| 135.00 | 77.41 | 85.76 | 91.41 | 92.47 | 91.88 | |
| Origanum majorana L. | 8.44 | 26.59 | 31.01 | 37.65 | 37.76 | 37.76 |
| 16.88 | 62.24 | 64.71 | 68.59 | 67.90 | 66.71 | |
| 33.75 | 72.71 | 79.29 | 78.71 | 77.00 | 76.94 | |
| 67.50 | 81.65 | 88.71 | 92.82 | 93.88 | 93.88 | |
| 135.00 | 88.47 | 92.47 | 94.71 | 96.94 | 96.47 | |
| Lavandula angustifolia L. | 8.44 | 1.19 | 1.19 | 2.12 | 3.50 | 3.53 |
| 16.88 | 1.19 | 1.76 | 2.47 | 4.20 | 3.53 | |
| 33.75 | 2.47 | 5.53 | 11.76 | 9.10 | 8.24 | |
| 67.50 | 3.50 | 8.23 | 16.12 | 19.40 | 18.35 | |
| 135.00 | 5.89 | 11.76 | 17.65 | 24.70 | 23.53 | |
| Hypericum perforatum L. | 8.44 | 26.47 | 28.24 | 36.00 | 37.41 | 36.47 |
| 16.88 | 37.53 | 48.71 | 64.47 | 66.70 | 55.18 | |
| 33.75 | 49.65 | 58.12 | 69.53 | 73.76 | 67.88 | |
| 67.50 | 69.76 | 76.47 | 84.12 | 83.29 | 83.06 | |
| 135.00 | 84.12 | 88.47 | 93.29 | 94.58 | 94.11 | |
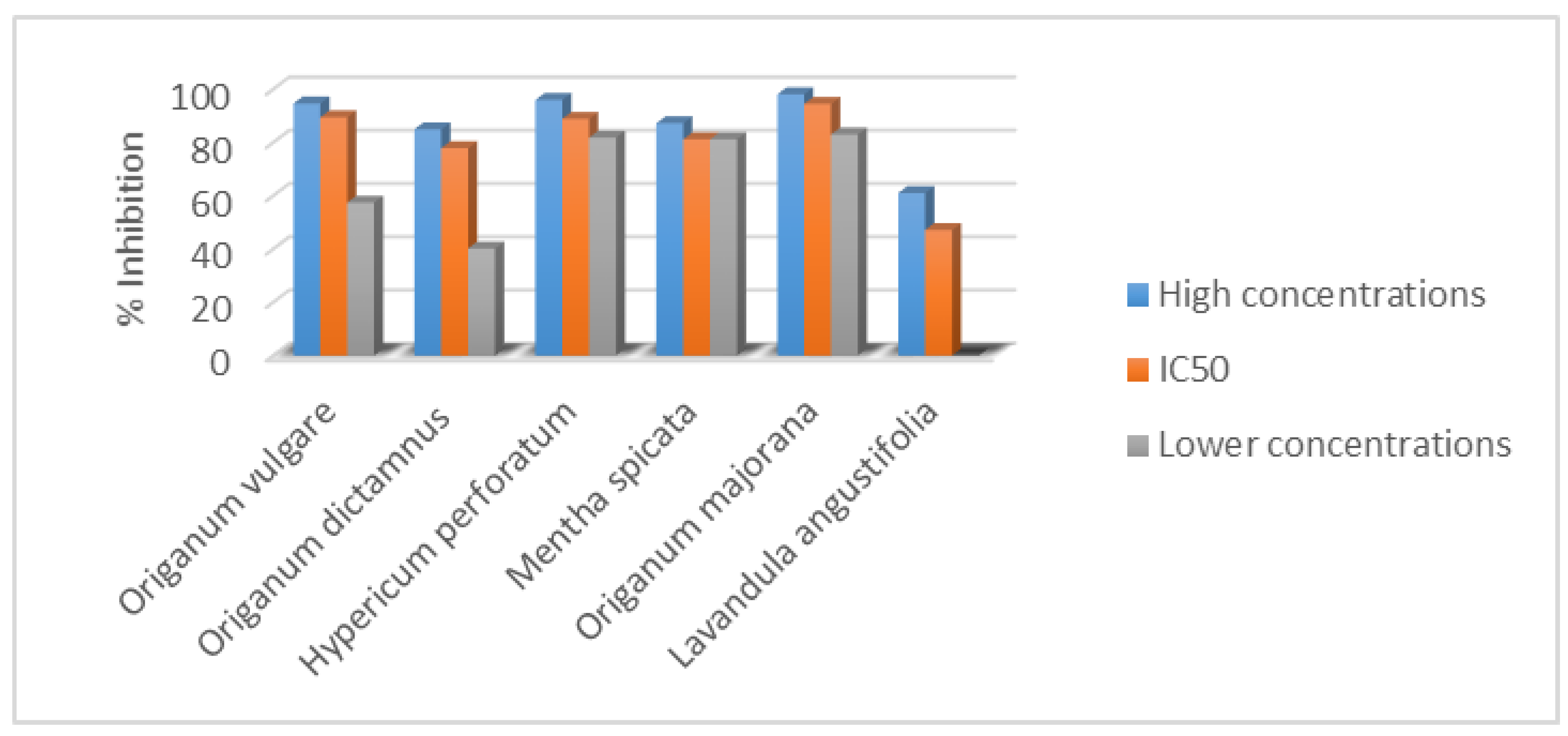
| Extract | % Inhibition |
|---|---|
| Origanum vulgare L. (135 μg·mL−1) | 94.60 ± 1.51 |
| Origanum vulgare L. (IC50 = 12.10 μg·mL−1) | 89.42 ± 0.85 |
| Origanum vulgare L. (2 μg·mL−1) | 57.40 ± 1.23 |
| Origanum dictamnus L. (135 μg·mL−1) | 84.91 ± 1.82 |
| Origanum dictamnus L. (IC50 = 37.50 μg·mL−1) | 77.83 ± 1.97 |
| Origanum dictamnus L. (2 μg·mL−1) | 40.16 ± 0.91 |
| Hypericum perforatum L. (135 μg·mL−1) | 95.95 ± 0.74 |
| Hypericum perforatum L. (IC50 = 11.00 μg·mL−1) | 88.83 ± 1.66 |
| Hypericum perforatum L. (2 μg·mL−1) | 81.80 ± 1.14 |
| Mentha spicata L. (135 μg·mL−1) | 87.20 ± 0.35 |
| Mentha spicata L. (IC50 = 16.93 μg·mL−1) | 81.15 ± 1.21 |
| Mentha spicata L. (2 μg·mL−1) | 80.73 ± 0.83 |
| Origanum majorana L. (135 μg·mL−1) | 98.05 ± 0.75 |
| Origanum majorana L. (IC50 = 10.31 μg·mL−1) | 94.42 ± 1.52 |
| Origanum majorana L. (2 μg·mL−1) | 82.98 ± 1.33 |
| Lavandula angustifolia L. (135 μg·mL−1) | - |
| Lavandula angustifolia L. (IC50 = 3200 μg·mL−1) | 47.08 ± 0.93 |
| Lavandula angustifolia L. (5000 μg·mL−1) | 61.00 ± 1.42 |
| MIC (Minimum Inhibitory Concentration) μg·mL−1 | ||||||
|---|---|---|---|---|---|---|
| Aqueοus Νatural Extracts | Staphylococcus aureus ATCC 25923 | Enterococcus faecalis ATCC 29212 | Listeria monocytogenes ATCC 35152 | Salmonella enterica ATCC 14028 | Escherichia coli ATCC 25922 | Klebsiella pneumoniae ATCC 13883 |
| Origanum vulgare L. | 135 | 300 | 135 | 300 | 500 | 135 |
| Origanum dictamnus L. | 135 | nd | 80 | 40 | nd | nd |
| Hypericum perforatum L. | 34 | 34 | 30 | 400 | 135 | 650 |
| Origanum majorana L. | 1 | 30 | 5 | 135 | 67.5 | 135 |
| Mentha spicata L. | 135 | 500 | 5 | 135 | 135 | 60 |
| Lavandula angustifolia L. | nd | nd | nd | nd | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsakni, A.; Chatzilazarou, A.; Tsakali, E.; Tsantes, A.G.; Van Impe, J.; Houhoula, D. Identification of Bioactive Compounds in Plant Extracts of Greek Flora and Their Antimicrobial and Antioxidant Activity. Separations 2023, 10, 373. https://doi.org/10.3390/separations10070373
Tsakni A, Chatzilazarou A, Tsakali E, Tsantes AG, Van Impe J, Houhoula D. Identification of Bioactive Compounds in Plant Extracts of Greek Flora and Their Antimicrobial and Antioxidant Activity. Separations. 2023; 10(7):373. https://doi.org/10.3390/separations10070373
Chicago/Turabian StyleTsakni, Aliki, Archontoula Chatzilazarou, Efstathia Tsakali, Andreas G. Tsantes, Jan Van Impe, and Dimitra Houhoula. 2023. "Identification of Bioactive Compounds in Plant Extracts of Greek Flora and Their Antimicrobial and Antioxidant Activity" Separations 10, no. 7: 373. https://doi.org/10.3390/separations10070373
APA StyleTsakni, A., Chatzilazarou, A., Tsakali, E., Tsantes, A. G., Van Impe, J., & Houhoula, D. (2023). Identification of Bioactive Compounds in Plant Extracts of Greek Flora and Their Antimicrobial and Antioxidant Activity. Separations, 10(7), 373. https://doi.org/10.3390/separations10070373






