Analysis of Two Single and Three Double Long-Chain Quaternary Ammonium Compounds via Non-Aqueous Capillary Electrophoresis with Indirect Ultraviolet Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Sample Preparation
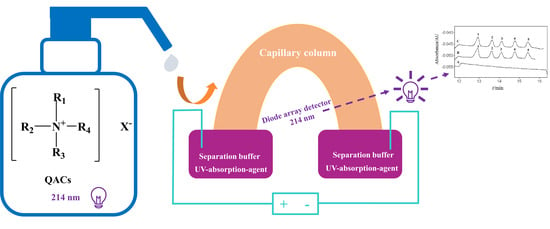
2.3. Instrumental Analysis
2.4. Method Validation
2.5. Application in Real Samples
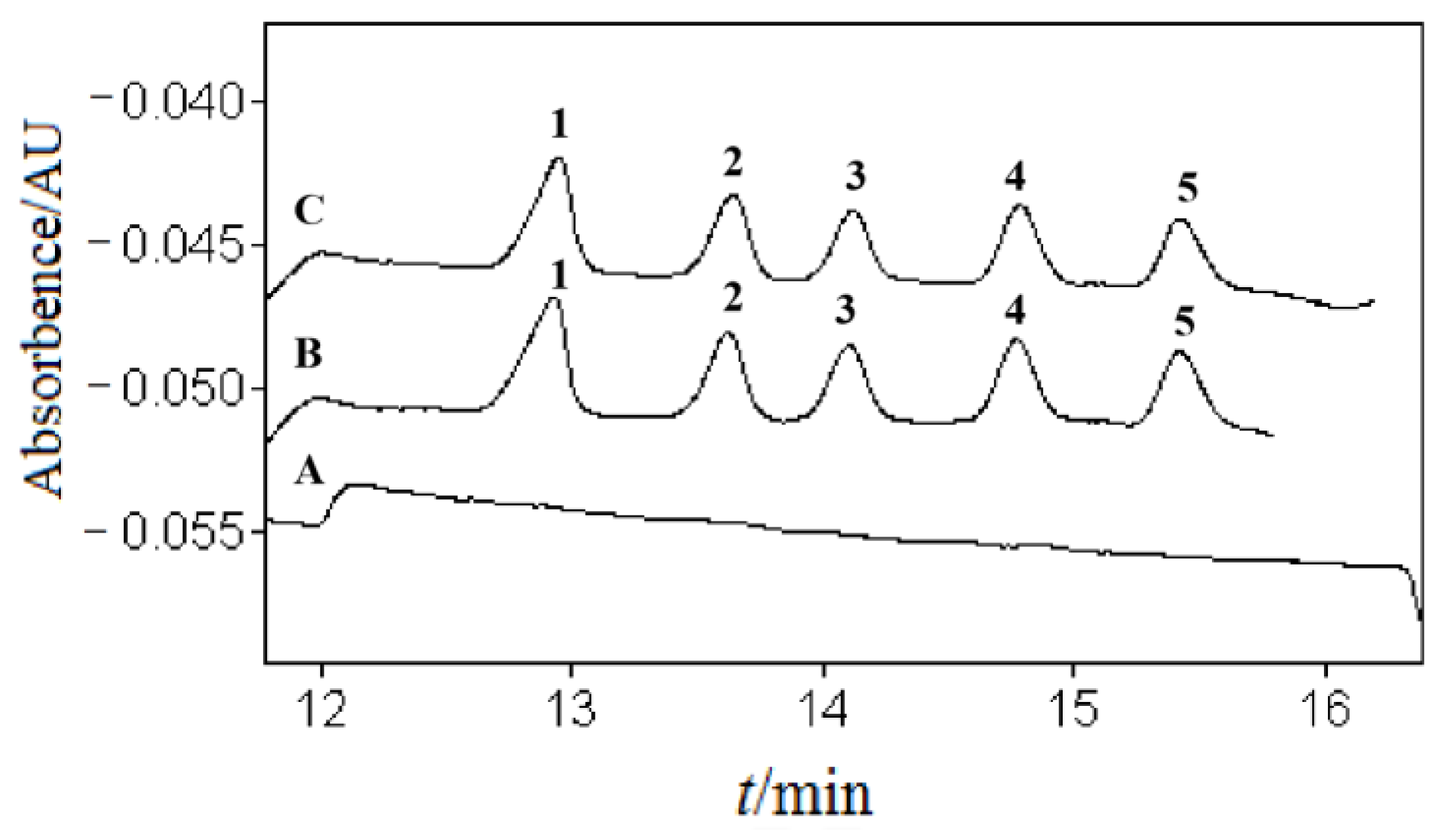
3. Results and Discussion
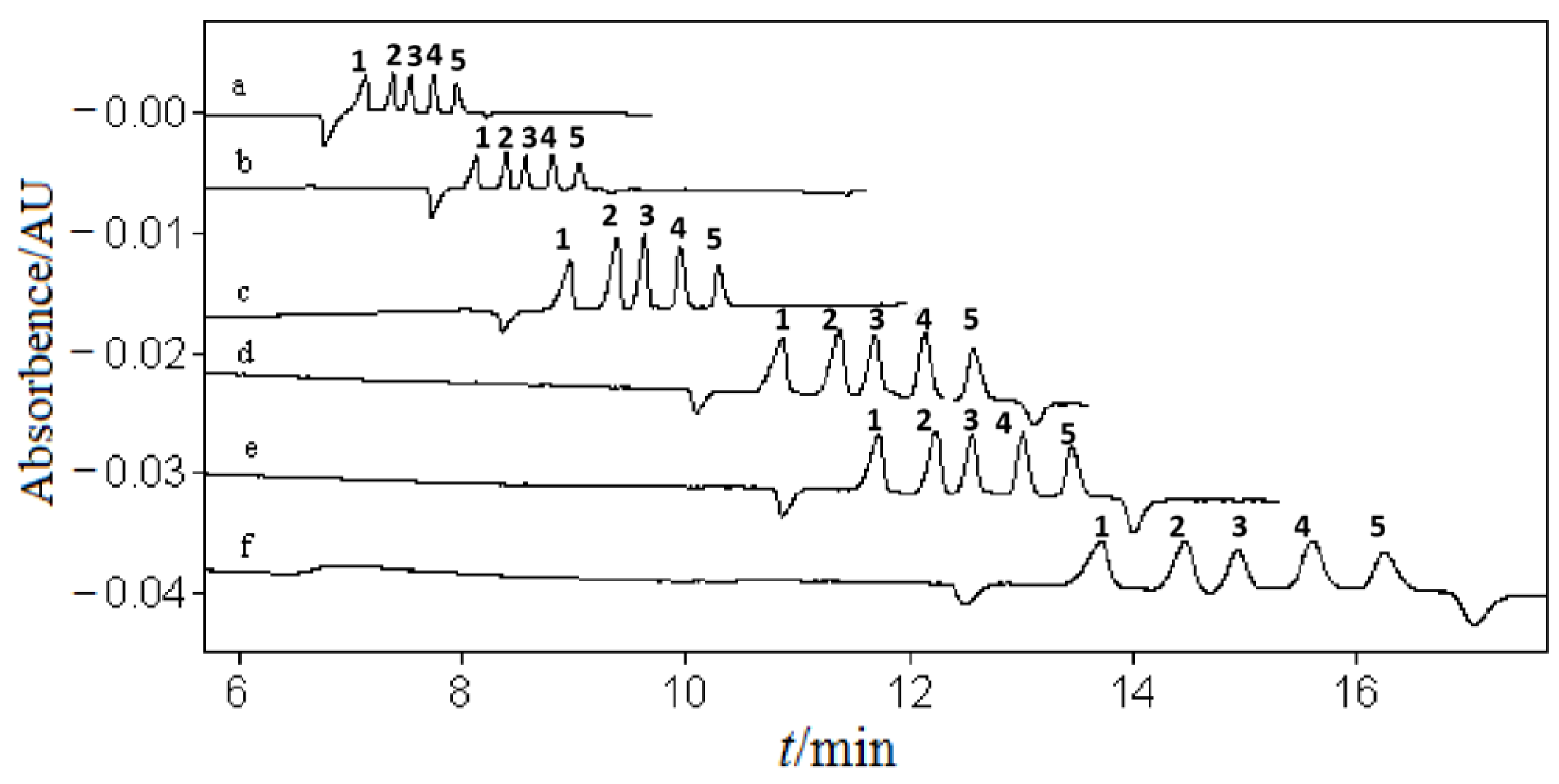
3.1. Optimization of Separation Buffer
3.1.1. Organic Solvent and Its Content
3.1.2. Ultraviolet Absorption Agent and Its Concentration
3.1.3. Conjugated Acid–Base Pair and Their Concentration
3.2. Optimization of Sample Buffer
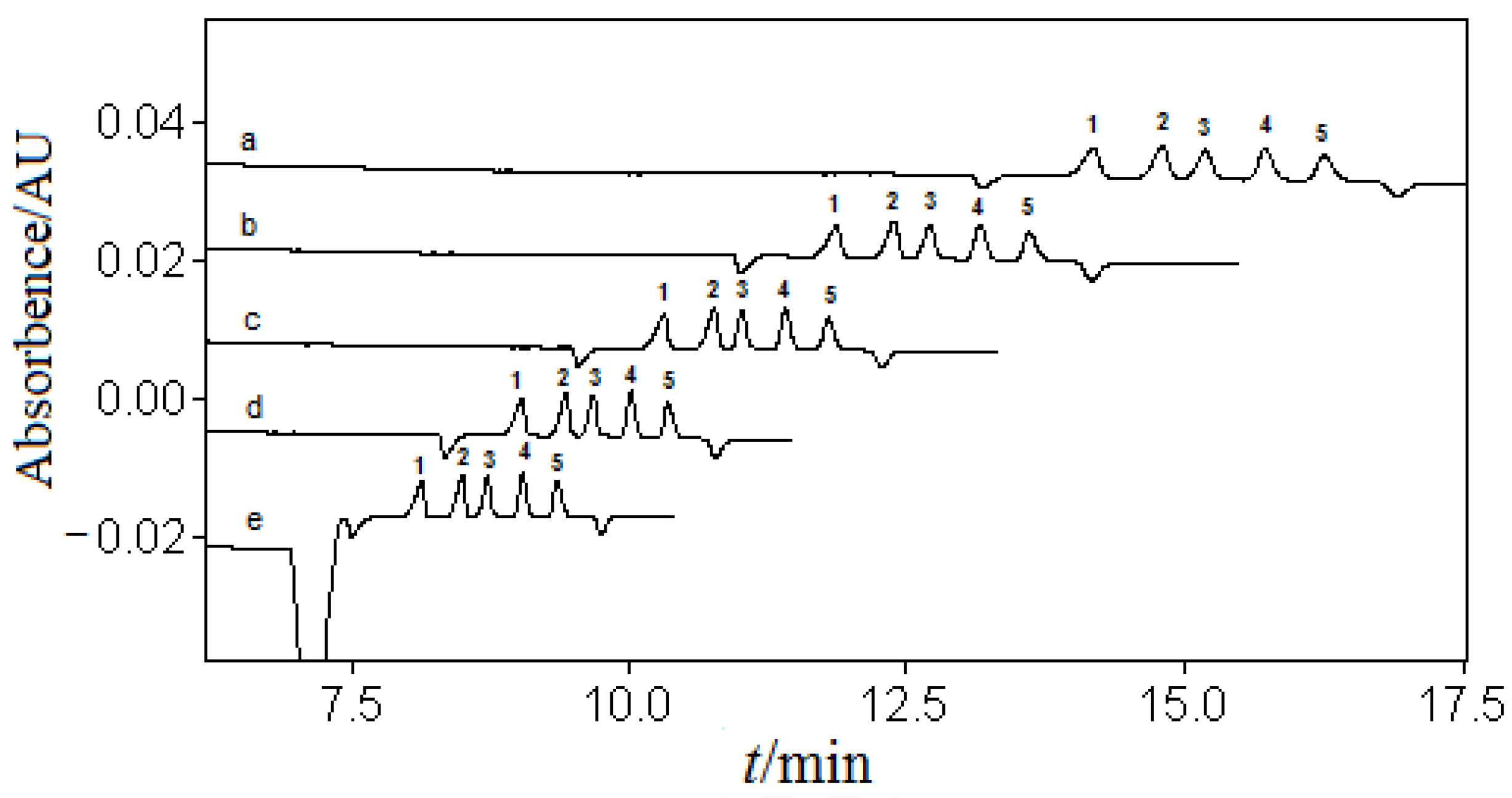
3.2.1. The Choice of the Type and Concentration of Organic or Inorganic Acid
3.2.2. The Choice of Solvent and Its Content
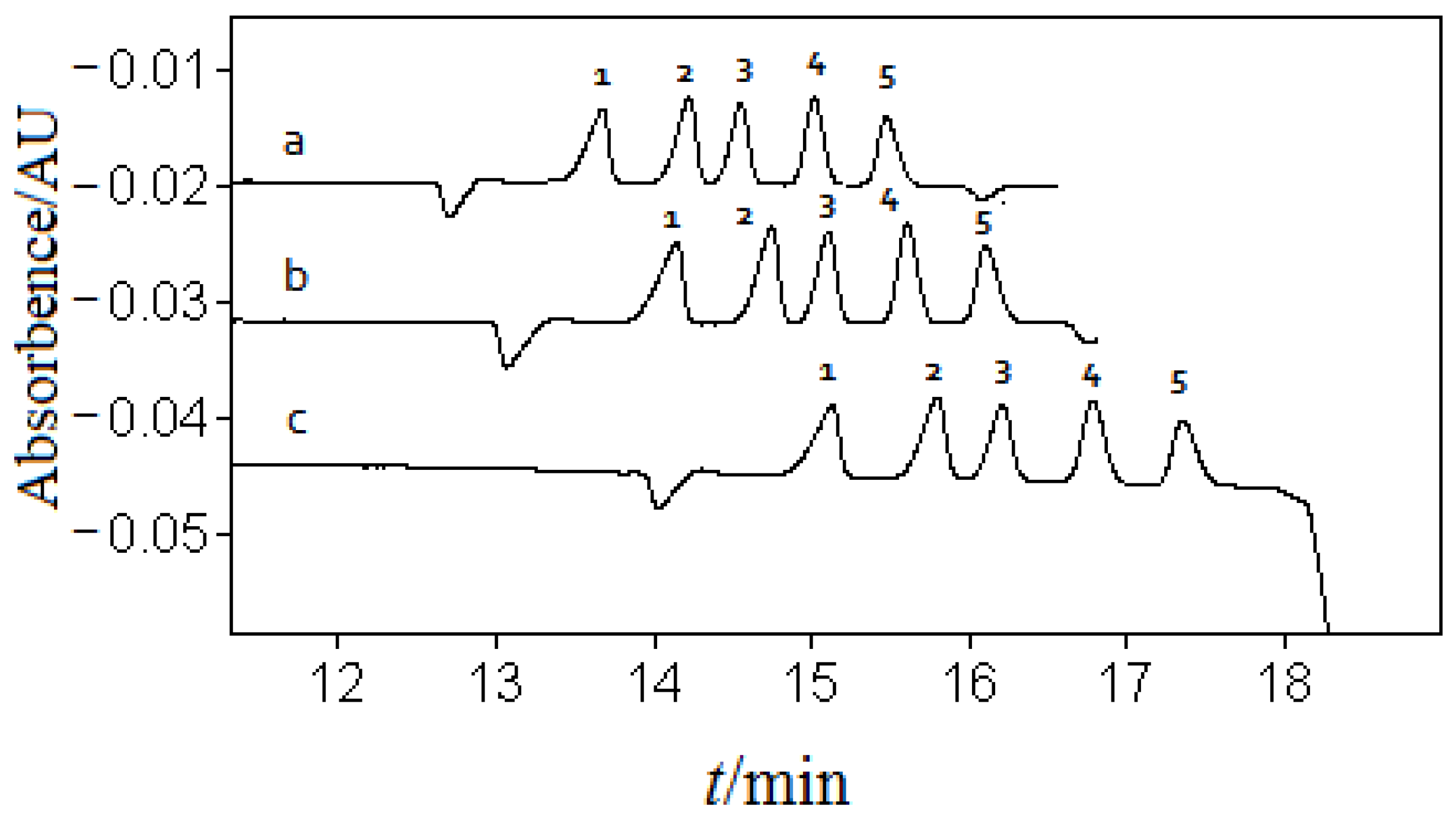
3.3. Optimization of Separation Voltage
3.4. Method Validation
3.5. Application in Real Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hora, P.I.; Pati, S.G.; McNamara, P.J.; Arnold, W.A. Increased use of quaternary amonium compounds during the SARS-CoV-2 pandemic and beyond: Consideration of environmental implications. Environ. Sci. Technol. Lett. 2020, 7, 622–631. [Google Scholar] [CrossRef]
- Tezel, U.; Pavlostathis, S.G. Quaternary ammonium disinfectants: Microbial adaptation, degradation and ecology. Curr. Opin. Biotechnol. 2015, 33, 296–304. [Google Scholar] [CrossRef]
- WS/T 367-2012; Regulation of Disinfection Technique in Healthcare Settings. China Standards Press: Beijing, China, 2012.
- Mohapatra, S.; Yutao, L.; Goh, S.G.; Ng, C.; Luhua, Y.; Tran, N.H.; Gin, K.Y. Quaternary ammonium compounds of emerging concern: Classification, occurrence, fate, toxicity and antimicrobial resistance. J. Hazard. Mater. 2023, 445, 130393. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, S.; Zhang, X.; Zeng, S.; Xu, Y.; Nie, W.; Zhou, Y.; Xu, T.; Chen, P. Quaternary ammonium salts: Insights into synthesis and new directions in antibacterial applications. Bioconjugate Chem. 2023, 34, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Liu, S. Antibacterial surface design—Contact kill. Prog. Surf. Sci. 2016, 91, 136–153. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary ammonium compounds: An antimicrobial mainstay and platform for innovation to address bacterial resistance. Acs Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Niu, B.; Yi, J.; Deng, Z.; Song, J.; Chen, Q.; Baynes, R.E. Toxicity and metal corrosion of glutaraldehyde-didecyldimethylammonium bromide as a disinfectant agent. Biomed. Res. Int. 2018, 2018, 9814209. [Google Scholar] [CrossRef]
- Jantafong, T.; Ruenphet, S.; Punyadarsaniya, D.; Takehara, K. The study of effect of didecyl dimethyl ammonium bromide on bacterial and viral decontamination for biosecurity in the animal farm. Vet. World 2018, 11, 706–711. [Google Scholar] [CrossRef]
- Fait, M.E.; Bakas, L.; Garrote, G.L.; Morcelle, S.R.; Saparrat, M.C.N. Cationic surfactants as antifungal agents. Appl. Microbiol. Biotechnol. 2019, 103, 97–112. [Google Scholar] [CrossRef]
- Gerba, C.P. Quaternary ammonium biocides: Efficacy in application. Appl. Environ. Microb. 2015, 81, 464–469. [Google Scholar] [CrossRef]
- GB 38850-2020; State Administration for Market Regulation; Standardization Administration of China. List for Materials and Restricted Substances in Disinfectant. China Standards Press: Beijing, China, 2020.
- GB/T 26369-2020; State Administration for Market Regulation; Standardization Administration of China. Hygienic Requirement for Quaternary Ammonium Disinfectant. China Standards Press: Beijing, China, 2020.
- Price, R.; Wan, P. Determination of quaternary ammonium compounds by potentiometric titration with an ionic surfactant electrode: Single-laboratory validation. J. AOAC Int. 2010, 93, 1542–1552. [Google Scholar] [CrossRef]
- Smolinska, M.; Ostapiv, R.; Yurkevych, M.; Poliuzhyn, L.; Korobova, O.; Kotsiumbas, I.; Tesliar, H.; Danielson, N.D. Determination of benzalkonium chloride in a disinfectant by UV spectrophotometry and gas and high-performance liquid chromatography: Validation, comparison of characteristics, and economic feasibility. Int. J. Anal. Chem. 2022, 2022, 2932634. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.J.; Lin, Y.K.; Yang, J.Q. Determination of 7 kinds of quaternary ammonium salt in disinfectants by HPLC. Chin. J. Anal. Instrum. 2021, 4, 49–53. [Google Scholar] [CrossRef]
- Li, Z.; Lakuleswaran, M.; Kannan, K. LC-MS/MS methods for the determination of 30 quaternary ammonium compounds including benzalkonium and paraquat in human serum and urine. J. Chromatogr. B 2023, 1214, 123562. [Google Scholar] [CrossRef]
- GB/T 39873-2021; State Administration for Market Regulation; Standardization Administration of China. Determination of Quaternary Ammonium Salt in Disinfectant-Liquid Chromatography-Tandem Mass Spectrometry. China Standards Press: Beijing, China, 2021.
- Mohorič, U.; Beutner, A.; Krickl, S.; Touraud, D.; Kunz, W.; Matysik, F. Surfactant-free microemulsion electrokinetic chromatography (SF-MEEKC) with UV and MS detection-a novel approach for the separation and ESI-MS detection of neutral compounds. Anal. Bioanal. Chem. 2016, 408, 8681–8689. [Google Scholar] [CrossRef] [PubMed]
- Konop, M.; Rybka, M.; Waraksa, E.; Laskowska, A.K.; Nowiński, A.; Grzywacz, T.; Karwowski, W.J.; Drapała, A.; Kłodzińska, E.M. Electrophoretic determination of trimethylamine (TMA) in biological samples as a novel potential biomarker of cardiovascular diseases methodological approach. Int. J. Environ. Res. Public Health 2021, 18, 12318. [Google Scholar] [CrossRef]
- Buglione, L.; See, H.H.; Hauser, P.C. Study on the effects of electrolytes and solvents in the determination of quaternary ammonium ions by nonaqueous capillary electrophoresis with contactless conductivity detection. Electrophoresis 2013, 34, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, A.P.; Nishida, S.; Calixto, L.A.; Da Silva, H.D.T.; Moraes, M.L.L. Evaluation of the stability of polyamines in human serum at different storage temperatures using capillary electrophoresis with indirect ultraviolet detection. Biomed. Chromatogr. 2023, 37, e5601. [Google Scholar] [CrossRef]
- Weiss, C.S.; Hazlett, J.S.; Datta, M.H.; Danzer, M.H. Determination of quaternary ammonium compounds by capillary electrophoresis using direct and indirect UV detection. J. Chroamtogr. A 1992, 608, 325–332. [Google Scholar] [CrossRef]
- Shamsi, S.A.; Danielson, N.D. Individual and simultaneous class separations of cationic and anionic surfactants using capillary electrophoresis with indirect photometric detection. Anal. Chem. 1995, 67, 4210–4216. [Google Scholar] [CrossRef]
- Heinig, K.; Vogt, C.; Werner, G. Determination of cationic surfactants by capillary electrophoresis with indirect photometric detection. J. Chromatogr. A 1997, 781, 17–22. [Google Scholar] [CrossRef]
- Liu, H.; Ding, W. Determination of homologues of quaternary ammonium surfactants by capillary electrophoresis using indirect UV detection. J. Chromatogr. A 2004, 1025, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Koike, R.; Kitagawa, F.; Otsuka, K. Simultaneous determination of amphoteric surfactants in detergents by capillary electrophoresis with indirect UV detection. J. Chromatogr. A 2007, 1139, 136–142. [Google Scholar] [CrossRef]
- Moreira, R.C.; Lopes, M.S.; Medeiros Junior, I.; Coltro, W.K.T. High performance separation of quaternary amines using microchip non-aqueous electrophoresis coupled with contactless conductivity detection. J. Chromatogr. A 2017, 1499, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Walbroehl, Y.; Jorgenson, J.W. On-column UV absorption detector for open tubular capillary zone electrophoresis. J. Chromatogr. A 1984, 315, 135–143. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Frolov, N.A.; Egorova, K.S.; Seitkalieva, M.M.; Ananikov, V.P. Quaternary ammonium compounds (QACs) and ionic liquids (ILs) as biocides: From simple antiseptics to tunable antimicrobials. Int. J. Mol. Sci. 2021, 22, 6793. [Google Scholar] [CrossRef]
- Peres, R.G.; Moraes, E.P.; Micke, G.A.; Tonin, F.G.; Tavares, M.F.M.; Rodriguez-Amaya, D.B. Rapid method for the determination of organic acids in wine by capillary electrophoresis with indirect UV detection. Food Control 2009, 20, 548–552. [Google Scholar] [CrossRef]
- Do Nascimento, M.P.; Marinho, M.V.; Sousa, R.A.D.; Leal De Oliveira, M.A. Mixture design of an electrolyte system for the simultaneous separation of Cl−, SO42−, NO3−, NO2−, and HCO3− in shrimp-farming water by CZE-UV. Anal. Methods 2023, 15, 311–321. [Google Scholar] [CrossRef]
- Guo, H.; Lu, K.; Zhang, J. Generation and influencing factors of electroosmotic flow in capillary electrophoresis based on chromatographic function. Chin. Cent. South Pharm. 2019, 17, 84–87. [Google Scholar]
- Jiang, R.K.; Ding, X.J. Simultaneous determination of triclosan, triclocarban and p-chloro-m-xylenol in disinfectang, personal care products and oiltment bu non-aquaeous capillary electrophoresis. Chin. J. Chromatogr. 2023, 41, 168–177. [Google Scholar] [CrossRef]
- Lin, C.; Lin, W.; Chiou, W. Capillary zone electrophoretic separation of alkylbenzyl quaternary ammonium compounds: Effect of organic modifier. J. Chromatogr. A 1996, 722, 345–352. [Google Scholar] [CrossRef]
- Liu, L.; You, W.; Zheng, L.; Chen, F.; Jia, Z. Determination of peimine and peiminine in Bulbus Fritillariae Thunbergii by capillary electrophoresis by indirect UV detection using N-(1-naphthyl)ethylenediamine dihydrochloride as probe. Electrophoresis 2012, 33, 2152–2158. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Araia, M.; Leck, C.; Emmer, Å. Characterization of exopolysaccharides in marine colloids by capillary electrophoresis with indirect UV detection. Anal. Chim. Acta 2010, 662, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Wang, L.; Liu, W.; Chen, D.D.Y. Quantitative nonaqueous capillary electrophoresis–mass spectrometry method for determining active ingredients in plant extracts. Anal. Chem. 2017, 89, 1411–1415. [Google Scholar] [CrossRef]
| Chemical Name | Molecular Formula | CAS Number | Chemical Structure |
|---|---|---|---|
| Dodecyl trimethyl ammonium bromide | C15H34BrN | 1119-94-4 | 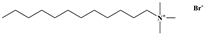 |
| Tetradecyl trimethyl ammonium bromide | C17H38BrN | 1119-97-7 |  |
| Dioctyl dimethyl ammonium chloride | C18H40ClN | 5538-94-3 |  |
| Octyldecyl dimethyl ammonium chloride | C20H44ClN | 32426-11-2 |  |
| Didecyl dimethyl ammonium bromide | C22H48BrN | 2390-68-3 | 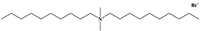 |
| Analytes | Linearity Range (mg/L) | Equation of Linear Regression | Correlation Coefficient |
|---|---|---|---|
| DTAB | 5–100 | y = 71.124x − 58.861 | 0.9994 |
| TTAB | 5–100 | y = 44.845x − 7.752 | 0.9995 |
| DDAC | 5–100 | y = 36.408x − 7.911 | 0.9996 |
| ODAC | 5–100 | y = 41.05x − 32.093 | 0.9991 |
| DDAB | 5–100 | y = 36.25x − 24.121 | 0.9998 |
| Analytes | Spiked Levels (mg/L) | Recoveries (%) | Intra-day RSD (%) | Inter-day RSD (%) |
|---|---|---|---|---|
| DTAB | 20 | 106.4 | 3.8 | 5.1 |
| 40 | 109.3 | 0.6 | 1.4 | |
| 60 | 93.2 | 2.9 | 4.7 | |
| TTAB | 20 | 109.7 | 3.7 | 7.2 |
| 40 | 113.7 | 0.5 | 4.4 | |
| 60 | 95.6 | 2.4 | 3.3 | |
| DDAC | 20 | 110.4 | 2.8 | 5.1 |
| 40 | 110.7 | 0.4 | 1.9 | |
| 60 | 92.3 | 2.8 | 4.3 | |
| ODAC | 20 | 114.7 | 3.6 | 7.4 |
| 40 | 112.6 | 0.5 | 2.2 | |
| 60 | 94.1 | 2.7 | 3.8 | |
| DDAB | 20 | 112.5 | 2.5 | 4.5 |
| 40 | 108.6 | 0.4 | 0.9 | |
| 60 | 95.2 | 2.9 | 5.2 |
| Sample | Brand | Sample Type | Average Concentrations (g/L) | ||||
|---|---|---|---|---|---|---|---|
| DTAB | TTAB | DDAC | ODAC | DDAB | |||
| 1 | Jian Zhisu | Disinfectant laundry detergents | / | / | 14.5 | 30.7 | 25.2 |
| 2 | Lv San | Disinfectant laundry detergents | / | / | / | / | 26.8 |
| 3 | Shu Bitai | Hand sanitizers | / | / | / | / | 1.4 |
| 4 | An Lijiu | Skin cleaners | / | / | / | 1.0 | 1.1 |
| 5 | Neosanitiz | Skin cleaners | / | / | / | / | 0.49 |
| 6 | Luo Wa | Disinfectants | / | / | 5.3 | 11.0 | 8.0 |
| 7 | Yi Kang | Disinfectants | / | / | 0.8 | 1.6 | 1.9 |
| 8 | Xi Debao | Disinfectants | / | / | / | / | 30.0 |
| 9 | Jing Guan | Disinfectants | / | / | / | / | 0.9 |
| 10 | Mo Runlai | Disinfectants | / | / | / | / | 1.5 |
| 11 | Jie Lijia | Disinfectants | 0.2 | 0.1 | 0.5 | 0.8 | 2.5 |
| 12 | Jian Zhisu | Disinfectants | / | / | 0.01 | 0.03 | 0.02 |
| 13 | Xin Pu | Disinfectants | / | / | / | / | 0.8 |
| 14 | Li Erkang | Disinfectants | / | / | 0.1 | 0.3 | 0.1 |
| 15 | An Jie | Disinfectants | / | / | / | / | 0.7 |
| 16 | Yi Kanghuoxing | Disinfectants | / | / | 3.9 | 5.3 | 3.2 |
| 17 | Xiao Boshi | Disinfectant wet wipes | 0.1 | 0.1 | 0.7 | 0.9 | 1.1 |
| Detection rate (%) | 11.8 | 11.8 | 47.1 | 52.9 | 100.0 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, K.; Jiang, R.; Wang, P.; Zhang, J.; Shao, B.; Ding, X. Analysis of Two Single and Three Double Long-Chain Quaternary Ammonium Compounds via Non-Aqueous Capillary Electrophoresis with Indirect Ultraviolet Detection. Separations 2023, 10, 387. https://doi.org/10.3390/separations10070387
Yao K, Jiang R, Wang P, Zhang J, Shao B, Ding X. Analysis of Two Single and Three Double Long-Chain Quaternary Ammonium Compounds via Non-Aqueous Capillary Electrophoresis with Indirect Ultraviolet Detection. Separations. 2023; 10(7):387. https://doi.org/10.3390/separations10070387
Chicago/Turabian StyleYao, Kai, Ruoke Jiang, Ping Wang, Jing Zhang, Bing Shao, and Xiaojing Ding. 2023. "Analysis of Two Single and Three Double Long-Chain Quaternary Ammonium Compounds via Non-Aqueous Capillary Electrophoresis with Indirect Ultraviolet Detection" Separations 10, no. 7: 387. https://doi.org/10.3390/separations10070387
APA StyleYao, K., Jiang, R., Wang, P., Zhang, J., Shao, B., & Ding, X. (2023). Analysis of Two Single and Three Double Long-Chain Quaternary Ammonium Compounds via Non-Aqueous Capillary Electrophoresis with Indirect Ultraviolet Detection. Separations, 10(7), 387. https://doi.org/10.3390/separations10070387