Influence of Drying and Storage Conditions on the Volatile Organic Compounds Profile of Spirulina Platensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Strain and Culture Conditions
2.2. Sample Preparation
2.3. HS-SPME
2.4. GC-MS
2.5. Statistical Analysis
3. Results and Discussion
3.1. HS-SPME-GC-MS Results
3.1.1. Maillard-Derived VOCs
3.1.2. Lipid-Derived VOCs
3.1.3. Carotenoid-Derived VOCs
3.1.4. Fermentative-Related VOCs
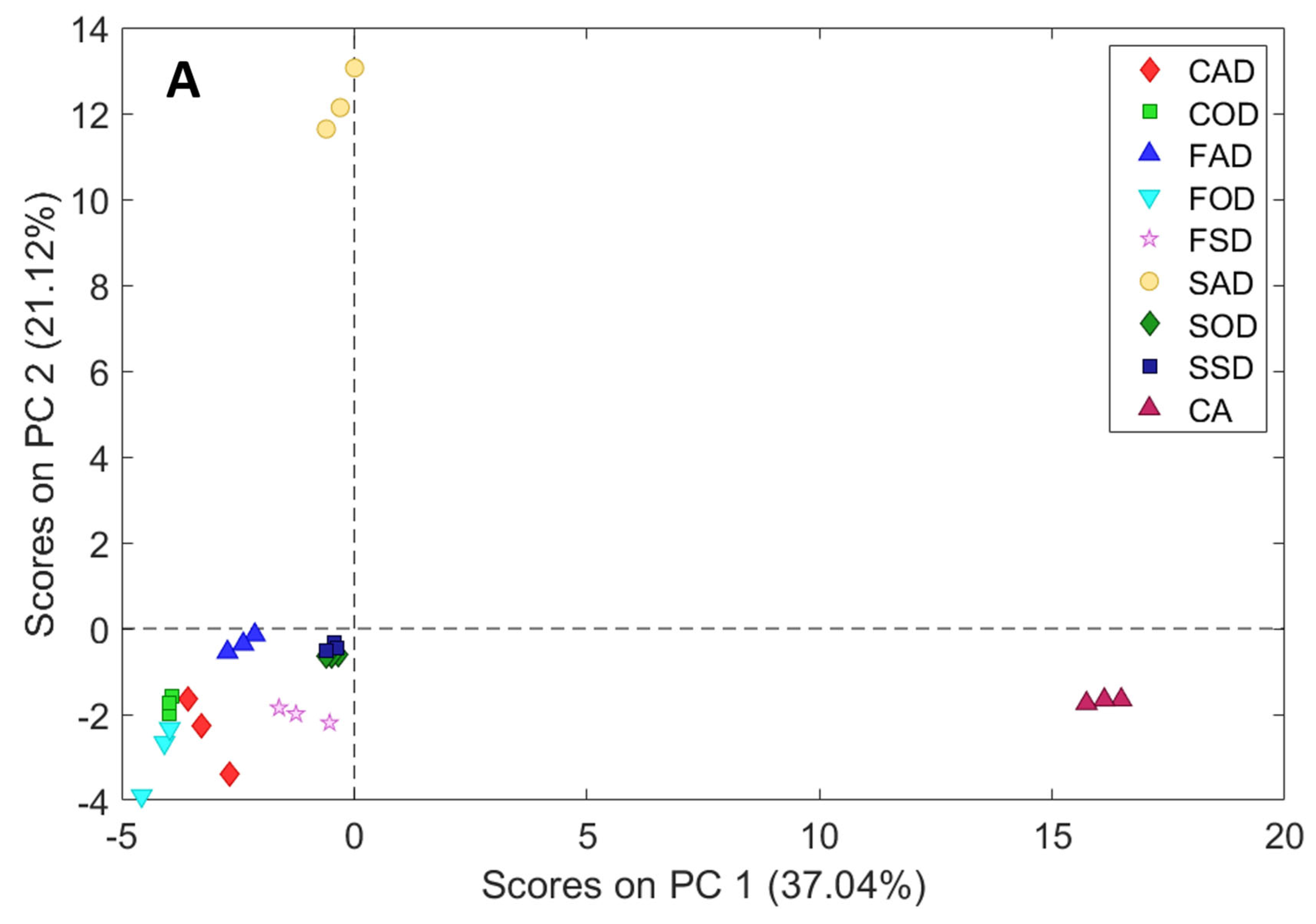
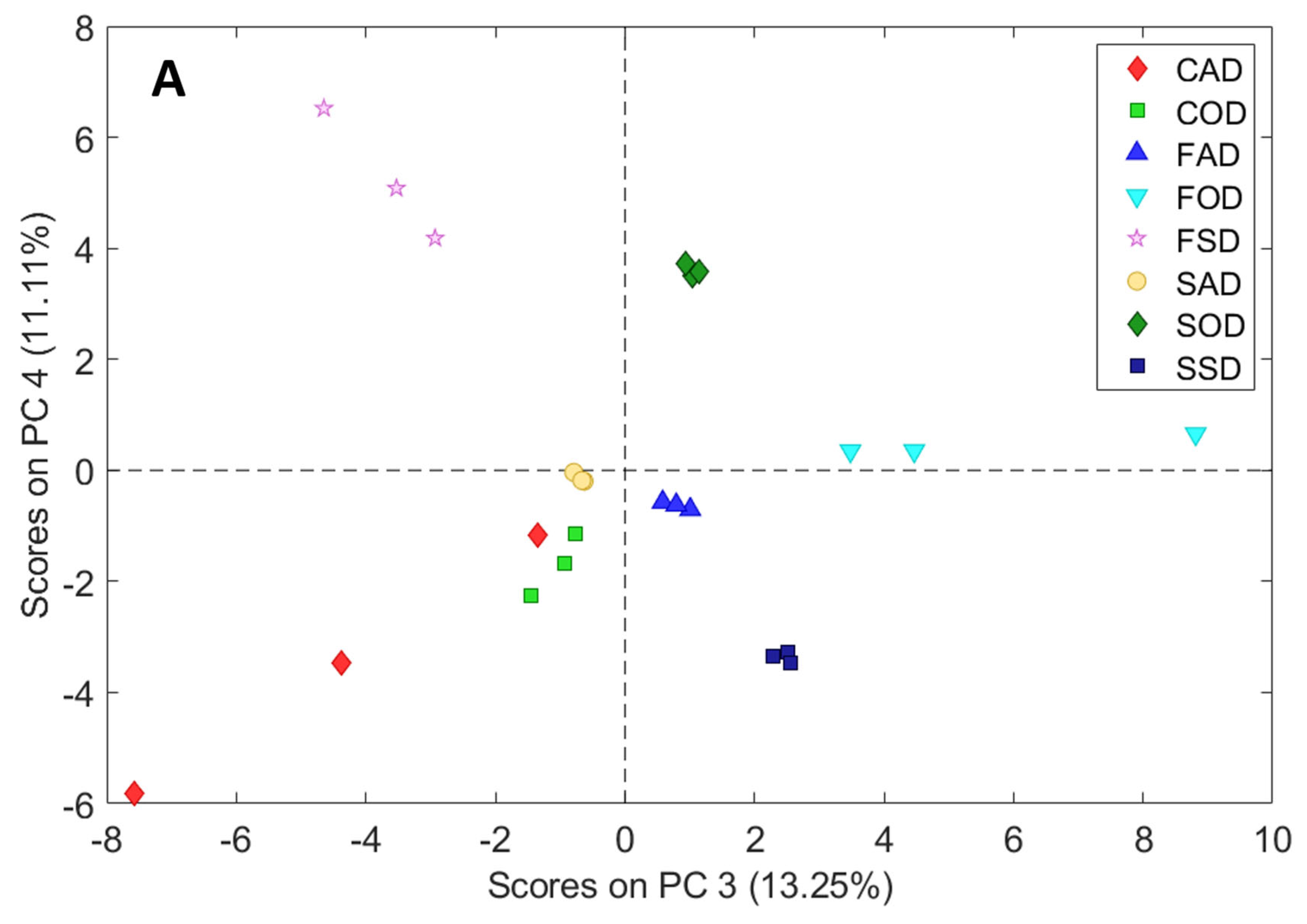
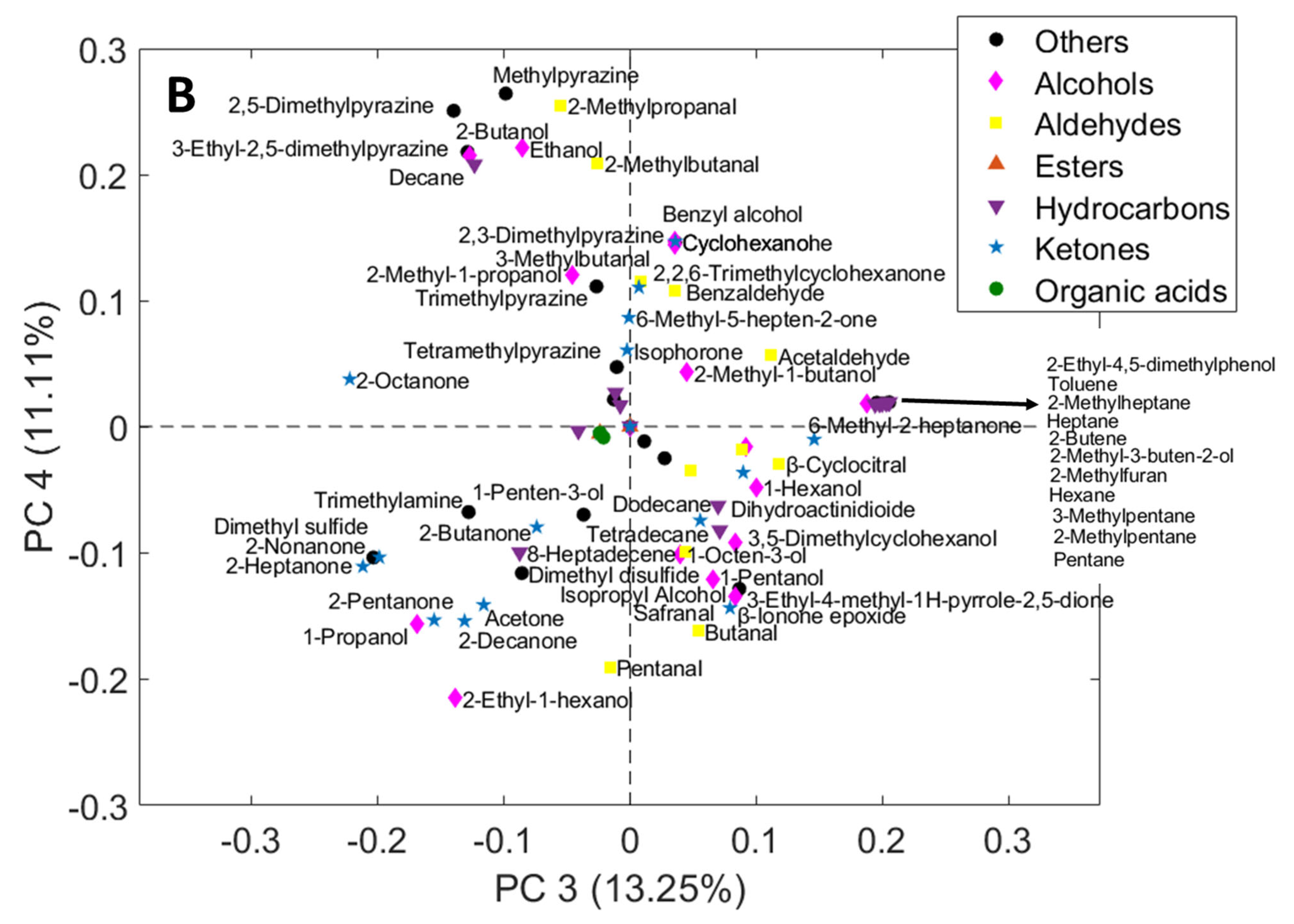
3.2. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Priyanka, S.; Varsha, R.; Verma, R.; Ayenampudi, S.B. Spirulina: A spotlight on its nutraceutical properties and food processing applications. J. Microbiol. Biotechnol. Food Sci. 2023, 12, e4785. [Google Scholar] [CrossRef]
- Lupatini, A.L.; Colla, L.M.; Canan, C.; Colla, E. Potential Application of Microalga Spirulina platensis as a Protein Source. J. Sci. Food Agric. 2017, 97, 724–732. [Google Scholar] [CrossRef] [PubMed]
- Colla, L.M.; Bertolin, T.E.; Costa, J.A.V. Fatty Acids Profile of Spirulina platensis Grown Under Different Temperatures and Nitrogen Concentrations. Z. Naturforschung C 2004, 59, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Kim, H.-J.; Li, M.; Lim, D.; Kim, J.; Kwak, S.-S.; Kang, C.-M.; Ferruzzi, M.; Ahn, M.-J. Two Classes of Pigments, Carotenoids and C-Phycocyanin, in Spirulina Powder and Their Antioxidant Activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The Antioxidant, Immunomodulatory, and Anti-Inflammatory Activities of Spirulina: An Overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef] [PubMed]
- Grosshagauer, S.; Kraemer, K.; Somoza, V. The True Value of Spirulina. J. Agric. Food Chem. 2020, 68, 4109–4115. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-B.; Srisomporn, P.; Hayashi, K.; Tanaka, T.; Sankawa, U.; Hayashi, T. Effects of Structural Modification of Calcium Spirulan, a Sulfated Polysaccharide from Spirulina platensis, on Antiviral Activity. Chem. Pharm. Bull. 2001, 49, 108–110. [Google Scholar] [CrossRef] [PubMed]
- Fithriani, D.; Sinurat, E. Utilization of Spirulina as Functional Food: Phytosterol and Amino Acid Profiles Study. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012028. [Google Scholar] [CrossRef]
- Piñero Estrada, J. Antioxidant Activity of Different Fractions of Spirulina platensis Protean Extract. Il Farm. 2001, 56, 497–500. [Google Scholar] [CrossRef]
- Oliveira, E.G.; Rosa, G.S.; Moraes, M.A.; Pinto, L.A.A. Characterization of Thin Layer Drying of Spirulina platensis Utilizing Perpendicular Air Flow. Bioresour. Technol. 2009, 100, 1297–1303. [Google Scholar] [CrossRef]
- Herrero, M.; Martín-Álvarez, P.J.; Señoráns, F.J.; Cifuentes, A.; Ibáñez, E. Optimization of Accelerated Solvent Extraction of Antioxidants from Spirulina platensis Microalga. Food Chem. 2005, 93, 417–423. [Google Scholar] [CrossRef]
- Ainas, M.; Hasnaoui, S.; Bouarab, R.; Abdi, N.; Drouiche, N.; Mameri, N. Hydrogen Production with the Cyanobacterium Spirulina platensis. Int. J. Hydrogen Energy 2017, 42, 4902–4907. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Freitas, B.C.B.; Rosa, G.M.; Moraes, L.; Morais, M.G.; Mitchell, B.G. Operational and Economic Aspects of Spirulina-Based Biorefinery. Bioresour. Technol. 2019, 292, 121946. [Google Scholar] [CrossRef] [PubMed]
- Parimi, N.S.; Singh, M.; Kastner, J.R.; Das, K.C.; Forsberg, L.S.; Azadi, P. Optimization of Protein Extraction from Spirulina platensis to Generate a Potential Co-Product and a Biofuel Feedstock with Reduced Nitrogen Content. Front. Energy Res. 2015, 3, 30. [Google Scholar] [CrossRef]
- Zhang, F.; Man, Y.B.; Mo, W.Y.; Wong, M.H. Application of Spirulina in Aquaculture: A Review on Wastewater Treatment and Fish Growth. Rev. Aquac. 2020, 12, 582–599. [Google Scholar] [CrossRef]
- Altmann, B.A.; Rosenau, S. Spirulina as Animal Feed: Opportunities and Challenges. Foods 2022, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, I.; Nardone, G.N.; Zanatta, S.; Bertin, W.; Amadio, E. Spirulina for Skin Care: A Bright Blue Future. Cosmetics 2021, 8, 7. [Google Scholar] [CrossRef]
- Shao, W.; Ebaid, R.; El-Sheekh, M.; Abomohra, A.; Eladel, H. Pharmaceutical Applications and Consequent Environmental Impacts of Spirulina (Arthrospira): An Overview. Grasas Aceites 2019, 70, 292. [Google Scholar] [CrossRef]
- Ughetti, A.; Roncaglia, F.; Anderlini, B.; D’Eusanio, V.; Russo, A.L.; Forti, L. Integrated Carbonate-Based CO2 Capture—Biofixation through Cyanobacteria. Appl. Sci. 2023, 13, 10779. [Google Scholar] [CrossRef]
- Milovanović, I.; Mišan, A.; Simeunović, J.; Kovač, D.; Jambrec, D.; Mandić, A. Determination of Volatile Organic Compounds in Selected Strains of Cyanobacteria. J. Chem. 2015, 2015, 969542. [Google Scholar] [CrossRef]
- Juneja, A.; Ceballos, R.; Murthy, G. Effects of Environmental Factors and Nutrient Availability on the Biochemical Composition of Algae for Biofuels Production: A Review. Energies 2013, 6, 4607–4638. [Google Scholar] [CrossRef]
- Kumar, M.; Kulshreshtha, J.; Singh, G.P. Growth and Biopigment Accumulation of Cyanobacterium Spirulina platensis at Different Light Intensities and Temperature. Braz. J. Microbiol. 2011, 42, 1128–1135. [Google Scholar] [CrossRef]
- Gordillo, F.J.L.; Jiménez, C.; Figueroa, F.L.; Niell, F.X. Effects of Increased Atmospheric CO2 and N Supply on Photosynthesis, Growth and Cell Composition of the Cyanobacterium Spirulina platensis (Arthrospira). J. Appl. Phycol. 1998, 10, 461–469. [Google Scholar] [CrossRef]
- El-Sheekh, M.; Abomohra, A.E.-F.; Hanelt, D. Optimization of Biomass and Fatty Acid Productivity of Scenedesmus Obliquus as a Promising Microalga for Biodiesel Production. World J. Microbiol. Biotechnol. 2013, 29, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Paraskevopoulou, A.; Kaloudis, T.; Hiskia, A.; Steinhaus, M.; Dimotikali, D.; Triantis, T.M. Volatile Profiling of Spirulina Food Supplements. Foods 2024, 13, 1257. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, C.; Shen, W.; Wang, Z.; Xu, P.; Yu, H. Volatile Compounds Released by Microalgae-Water Phase from Taihu Lake in China. Harmful Algae 2019, 84, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Reese, K.L.; Fisher, C.L.; Lane, P.D.; Jaryenneh, J.D.; Jones, A.D.; Frank, M.; Lane, T.W. Abiotic and Biotic Damage of Microalgae Generate Different Volatile Organic Compounds (VOCs) for Early Diagnosis of Algal Cultures for Biofuel Production. Metabolites 2021, 11, 707. [Google Scholar] [CrossRef]
- Stramarkou, M.; Papadaki, S.; Kyriakopoulou, K.; Tzovenis, I.; Chronis, M.; Krokida, M. Comparative Analysis of Different Drying Techniques Based on the Qualitative Characteristics of Spirulina platensis Biomass. J. Aquat. Food Prod. Technol. 2021, 30, 498–516. [Google Scholar] [CrossRef]
- Oliveira, E.G.; Duarte, J.H.; Moraes, K.; Crexi, V.T.; Pinto, L.A.A. Optimisation of Spirulina platensis Convective Drying: Evaluation of Phycocyanin Loss and Lipid Oxidation. Int. J. Food Sci. Technol. 2010, 45, 1572–1578. [Google Scholar] [CrossRef]
- Costa, B.R.; Rodrigues, M.C.K.; Rocha, S.F.; Pohndorf, R.S.; Larrosa, A.P.Q.; Pinto, L.A.A. Optimization of Spirulina sp. Drying in Heat Pump: Effects on the Physicochemical Properties and Color Parameters: Spirulina Drying in Heat Pump. J. Food Process. Preserv. 2016, 40, 934–942. [Google Scholar] [CrossRef]
- Silva, J.P.S.; Veloso, C.R.R.; De Souza Barrozo, M.A.; Vieira, L.G.M. Indirect Solar Drying of Spirulina platensis and the Effect of Operating Conditions on Product Quality. Algal Res. 2021, 60, 102521. [Google Scholar] [CrossRef]
- Görs, M.; Schumann, R.; Hepperle, D.; Karsten, U. Quality analysis of commercial Chlorella products used as dietary supplement in human nutrition. J. Appl. Phycol. 2010, 22, 265–276. [Google Scholar] [CrossRef]
- Hosseinizand, H.; Sokhansanj, S.; Lim, C.J. Studying the Drying Mechanism of Microalgae Chlorella Vulgaris and the Optimum Drying Temperature to Preserve Quality Characteristics. Dry. Technol. 2018, 36, 1049–1060. [Google Scholar] [CrossRef]
- Chen, C.-L.; Chang, J.-S.; Lee, D.-J. Dewatering and Drying Methods for Microalgae. Dry. Technol. 2015, 33, 443–454. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, T.; Li, J.; Pan, B.; Hu, Q.; Duan, M.; Zhang, X. Study on the Effect of Spray Drying Process on the Quality of Microalgal Biomass: A Comprehensive Biocomposition Analysis of Spray-Dried S. Acuminatus Biomass. BioEnergy Res. 2022, 15, 320–333. [Google Scholar] [CrossRef]
- Orset, S.; Leach, G.C.; Morais, R.; Young, A.J. Spray-Drying of the Microalga Dunaliella Salina: Effects on β-Carotene Content and Isomer Composition. J. Agric. Food Chem. 1999, 47, 4782–4790. [Google Scholar] [CrossRef]
- Güroy, B.; Karadal, O.; Mantoğlu, S.; Irmak Cebeci, O. Effects of Different Drying Methods on C-Phycocyanin Content of Spirulina platensis Powder. Ege J. Fish. Aquat. Sci. 2017, 34, 129–132. [Google Scholar] [CrossRef]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Impact of Different Storage Methods on Bioactive Compounds in Arthrospira platensis Biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef]
- Kumar, K.R.; Mahadevaswamy, M.; Venkataraman, L.V. Storage Quality of Powdered Cyanobacterium? Spirulina platensis. Z. Lebensm.-Unters.-Forsch. 1995, 201, 289–292. [Google Scholar] [CrossRef]
- Sammlung von Algenkulturen Göttingen 02 Spirulina Medium 2008. Available online: http://Sagdb.Uni-Goettingen.de/Culture_media/02%20Spirulina%20Medium.Pdf (accessed on 11 February 2024).
- Wold, S.; Esbensen, K.; Geladi, P. Principal Component Analysis. Chemom. Intell. Lab. Syst. 1987, 2, 37–52. [Google Scholar] [CrossRef]
- Moran, L.; Bou, G.; Aldai, N.; Ciardi, M.; Morillas-España, A.; Sánchez-Zurano, A.; Barron, L.J.R.; Lafarga, T. Characterisation of the Volatile Profile of Microalgae and Cyanobacteria Using Solid-Phase Microextraction Followed by Gas Chromatography Coupled to Mass Spectrometry. Sci. Rep. 2022, 12, 3661. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, T.; Schieberle, P. Formation of Aroma-Active Strecker-Aldehydes by a Direct Oxidative Degradation of Amadori Compounds. J. Agric. Food Chem. 2000, 48, 4301–4305. [Google Scholar] [CrossRef]
- Rainer Cremer, D. The Reaction Kinetics for the Formation of Strecker Aldehydes in Low Moisture Model Systems and in Plant Powders. Food Chem. 2000, 71, 37–43. [Google Scholar] [CrossRef]
- Mottram, D.S. The Maillard Reaction: Source of Flavour in Thermally Processed Foods. In Flavours and Fragrances; Berger, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 269–283. ISBN 978-3-540-49338-9. [Google Scholar]
- Ould Bellahcen, T.; Cherki, M.; Sánchez, J.A.C.; Cherif, A.; El Amrani, A. Chemical Composition and Antibacterial Activity of the Essential Oil of Spirulina platensis from Morocco. J. Essent. Oil Bear. Plants 2019, 22, 1265–1276. [Google Scholar] [CrossRef]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-State Fermentation of Arthrospira Platensis to Implement New Food Products: Evaluation of Stabilization Treatments and Bacterial Growth on the Volatile Fraction. Foods 2020, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Barden, L.; Decker, E.A. Lipid Oxidation in Low-Moisture Food: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2467–2482. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Rai, P.K.; Jeon, Y.J.; Kim, K.-H.; Kwon, E.E. The Role of Algae and Cyanobacteria in the Production and Release of Odorants in Water. Environ. Pollut. 2017, 227, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Van Durme, J.; Goiris, K.; De Winne, A.; De Cooman, L.; Muylaert, K. Evaluation of the Volatile Composition and Sensory Properties of Five Species of Microalgae. J. Agric. Food Chem. 2013, 61, 10881–10890. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Ibdah, M.; Meir, A.; Yosef, E.; Zamir, D.; Tadmor, Y. Not Just Colors—Carotenoid Degradation as a Link between Pigmentation and Aroma in Tomato and Watermelon Fruit. Trends Food Sci. Technol. 2005, 16, 407–415. [Google Scholar] [CrossRef]
- Jing, G.; Li, T.; Qu, H.; Yun, Z.; Jia, Y.; Zheng, X.; Jiang, Y. Carotenoids and Volatile Profiles of Yellow- and Red-Fleshed Papaya Fruit in Relation to the Expression of Carotenoid Cleavage Dioxygenase Genes. Postharvest Biol. Technol. 2015, 109, 114–119. [Google Scholar] [CrossRef]
- Paparella, A.; Shaltiel-Harpaza, L.; Ibdah, M. β-Ionone: Its Occurrence and Biological Function and Metabolic Engineering. Plants 2021, 10, 754. [Google Scholar] [CrossRef] [PubMed]
- Höckelmann, C.; Jüttner, F. Off-Flavours in Water: Hydroxyketones Andβ-Ionone Derivatives as New Odour Compounds of Freshwater Cyanobacteria. Flavour Fragr. J. 2005, 20, 387–394. [Google Scholar] [CrossRef]
- Yamamoto, M.; Baldermann, S.; Yoshikawa, K.; Fujita, A.; Mase, N.; Watanabe, N. Determination of Volatile Compounds in Four Commercial Samples of Japanese Green Algae Using Solid Phase Microextraction Gas Chromatography Mass Spectrometry. Sci. World J. 2014, 2014, 289780. [Google Scholar] [CrossRef] [PubMed]
- Fujise, D.; Tsuji, K.; Fukushima, N.; Kawai, K.; Harada, K. Analytical Aspects of Cyanobacterial Volatile Organic Compounds for Investigation of Their Production Behavior. J. Chromatogr. A 2010, 1217, 6122–6125. [Google Scholar] [CrossRef] [PubMed]
- Bandow, N.; Gartiser, S.; Ilvonen, O.; Schoknecht, U. Evaluation of the Impact of Construction Products on the Environment by Leaching of Possibly Hazardous Substances. Environ. Sci. Eur. 2018, 30, 14. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ughetti, A.; D’Eusanio, V.; Strani, L.; Russo, A.L.; Roncaglia, F. Influence of Drying and Storage Conditions on the Volatile Organic Compounds Profile of Spirulina Platensis. Separations 2024, 11, 180. https://doi.org/10.3390/separations11060180
Ughetti A, D’Eusanio V, Strani L, Russo AL, Roncaglia F. Influence of Drying and Storage Conditions on the Volatile Organic Compounds Profile of Spirulina Platensis. Separations. 2024; 11(6):180. https://doi.org/10.3390/separations11060180
Chicago/Turabian StyleUghetti, Alberto, Veronica D’Eusanio, Lorenzo Strani, Andrea Luca Russo, and Fabrizio Roncaglia. 2024. "Influence of Drying and Storage Conditions on the Volatile Organic Compounds Profile of Spirulina Platensis" Separations 11, no. 6: 180. https://doi.org/10.3390/separations11060180
APA StyleUghetti, A., D’Eusanio, V., Strani, L., Russo, A. L., & Roncaglia, F. (2024). Influence of Drying and Storage Conditions on the Volatile Organic Compounds Profile of Spirulina Platensis. Separations, 11(6), 180. https://doi.org/10.3390/separations11060180





