Applications of Sample Preparation Techniques in the Analysis of New Psychoactive Substances
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Standards
2.3. Biological Specimens
2.4. Extraction Cartridges
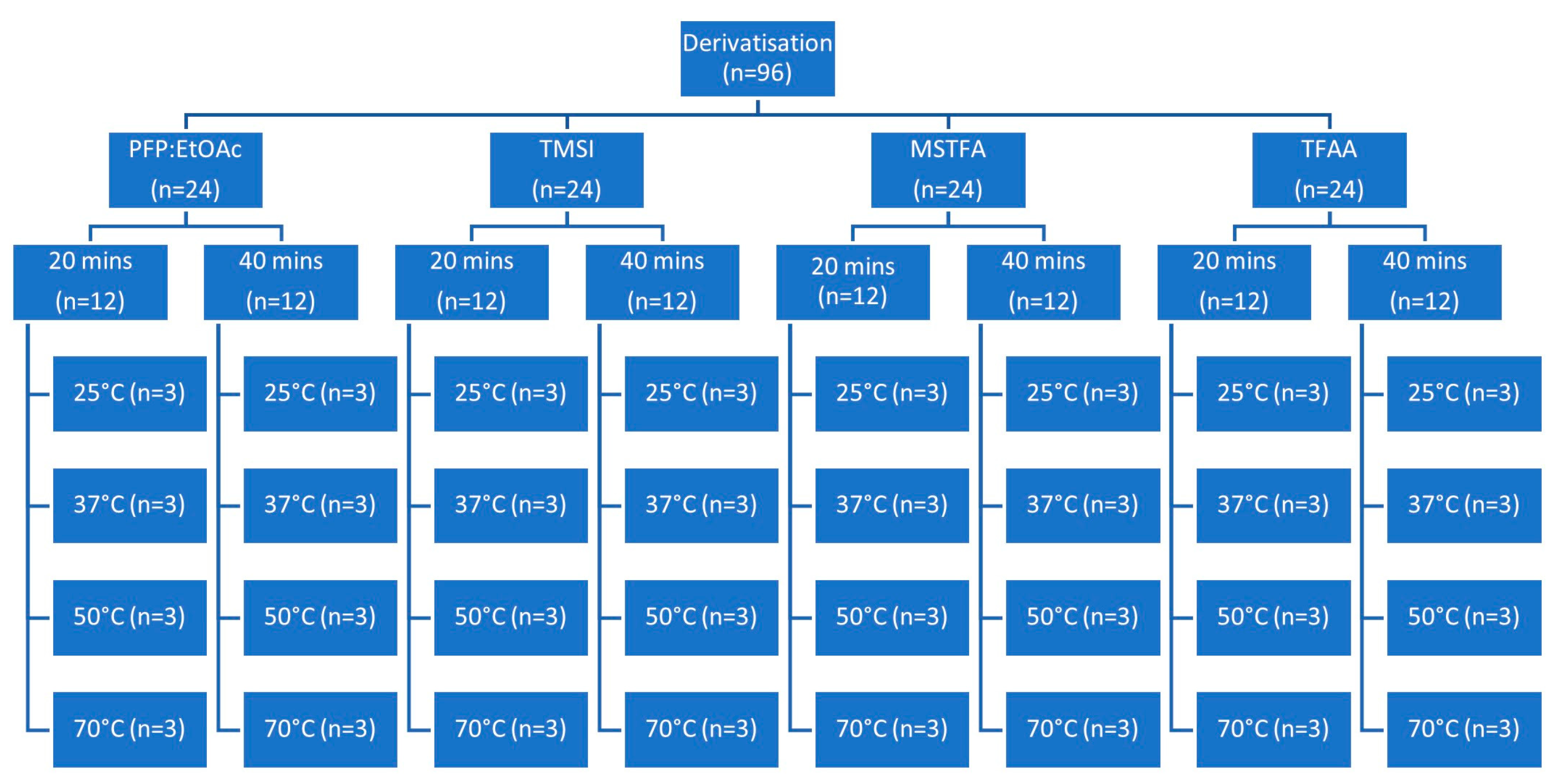
2.5. Derivatization Method Optimization
2.6. Sample Extraction Evaluation
2.6.1. Unextracted Methanolic Sample Preparation
2.6.2. Solid-Phase Extraction Method-ZSDAU020, CSDAU133, XRDAH206, XRPCH50z
2.6.3. Solid-Phase Extraction Method—XCEL I
2.6.4. Solid-Phase Extraction Method—OASIS®
2.6.5. Post Solid-Phase Extraction Method
2.6.6. Supported Liquid Extraction (SLE) Method
2.6.7. Extraction Efficiency Determination for Both SPE and SLE
2.7. Instrumentation
3. Results and Discussion
3.1. Derivatization
3.2. Solid-Phase Extraction
3.3. Supported Liquid Extraction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morais, J.; Evans-Brown, M.; Gallegos, A.; Christie, R.; Jorge, R.; Planchuelo, G.; Sedefov, R. New Psychoactive Substances: 25 Years of Early Warning and Response in Europe. An Update from the EU Early Warning System. Toxicol. Anal. Clin. 2022, 34, S29–S30. [Google Scholar] [CrossRef]
- Kranenburg, R.F.; Stuyver, L.I.; de Ridder, R.; van Beek, A.; Colmsee, E.; van Asten, A.C. Deliberate Evasion of Narcotic Legislation: Trends Visualized in Commercial Mixtures of New Psychoactive Substances Analyzed by GC-Solid Deposition-FTIR. Forensic Chem. 2021, 25, 100346. [Google Scholar] [CrossRef]
- Home Office. New Psychoactive Substances Review: Report of the Expert Panel; Home Office: London, UK, 2014. [Google Scholar]
- What Are NPS? Available online: https://www.unodc.org/LSS/Page/NPS (accessed on 20 July 2024).
- NPS Stimulants and Hallucinogens. Available online: https://www.cfsre.org/nps-discovery/trend-reports/nps-stimulants-and-hallucinogens/report/49?trend_type_id=3 (accessed on 17 March 2023).
- Deaths Related to Drug Poisoning in England and Wales—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/bulletins/deathsrelatedtodrugpoisoninginenglandandwales/2022registrations (accessed on 29 January 2024).
- WEDINOS—Sample Results. Available online: https://www.wedinos.org/sample-results#mylocation (accessed on 17 March 2023).
- Webb, N.E.; Wood, D.M.; Greene, S.L.; Hunter, L.J.; Archer, J.R.; Dines, A.M.; Dargan, P.I. Change in the New Psychoactive Substances Associated with Emergency Department Acute Toxicity Presentations Associated with the Introduction of the UK 2016 Psychoactive Substances Act. Clin. Toxicol. 2019, 57, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, G.; Massariol, A.; Guirguis, A.; Kirton, S.B.; Stair, J.L. NPS Detection in Prison: A Systematic Literature Review of Use, Drug Form, and Analytical Approaches. Drug Test. Anal. 2022, 14, 1350–1367. [Google Scholar] [CrossRef] [PubMed]
- Analytical Methods Committee. What Causes Most Errors in Chemical Analysis? Anal. Methods 2013, 5, 2914–2915. [Google Scholar] [CrossRef]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New Psychoactive Substances: A Review and Updates. Ther. Adv. Psychopharmacol. 2020, 10, 204512532096719. [Google Scholar] [CrossRef]
- Frinculescu, A.; Shine, T.; Ramsey, J.; Couchman, L.; Frascione, N.; Abbate, V. Analysis of Drugs Seized from Amnesty Bins at Two Major United Kingdom Summer Music Festivals Using Two Portable Gas Chromatography-Mass Spectrometry (GC–MS) Instruments. Drug Test. Anal. 2024. [Google Scholar] [CrossRef]
- Peters, F.T.; Wissenbach, D. Current State-of-the-Art Approaches for Mass Spectrometry in Clinical Toxicology: An Overview. Expert Opin. Drug Metab. Toxicol. 2023, 19, 487–500. [Google Scholar] [CrossRef]
- D’Orazio, A.L.; Mohr, A.L.A.; Chan-Hosokawa, A.; Harper, C.; Huestis, M.A.; Limoges, J.F.; Miles, A.K.; Scarneo, C.E.; Kerrigan, S.; Liddicoat, L.J.; et al. Recommendations for Toxicological Investigation of Drug-Impaired Driving and Motor Vehicle Fatalities—2021 Update. J. Anal. Toxicol. 2021, 45, 529–536. [Google Scholar] [CrossRef]
- Antunes, M.; Sequeira, M.; de Caires Pereira, M.; Caldeira, M.J.; Santos, S.; Franco, J.; Barroso, M.; Gaspar, H. Determination of Selected Cathinones in Blood by Solid-Phase Extraction and GC–MS. J. Anal. Toxicol. 2021, 45, 233–242. [Google Scholar] [CrossRef]
- Mohamed, K.M.; Al-Hazmi, A.H.; Alasiri, A.M.; Ali, M.E.-S. A GC–MS Method for Detection and Quantification of Cathine, Cathinone, Methcathinone and Ephedrine in Oral Fluid. J. Chromatogr. Sci. 2016, 54, 1271–1276. [Google Scholar] [CrossRef]
- Woźniak, M.K.; Banaszkiewicz, L.; Wiergowski, M.; Tomczak, E.; Kata, M.; Szpiech, B.; Namieśnik, J.; Biziuk, M. Development and Validation of a GC–MS/MS Method for the Determination of 11 Amphetamines and 34 Synthetic Cathinones in Whole Blood. Forensic Toxicol. 2020, 38, 42–58. [Google Scholar] [CrossRef]
- Nisbet, L.A.; Wylie, F.M.; Logan, B.K.; Scott, K.S. Gas Chromatography-Mass Spectrometry Method for the Quantitative Identification of 23 New Psychoactive Substances in Blood and Urine. J. Anal. Toxicol. 2019, 43, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Júlio, S.; Ferro, R.A.; Santos, S.; Alexandre, A.; Caldeira, M.J.; Franco, J.; Barroso, M.; Gaspar, H. Synthesis of Emerging Cathinones and Validation of a SPE GC–MS Method for Their Simultaneous Quantification in Blood. Anal Bioanal Chem 2023, 415, 571–589. [Google Scholar] [CrossRef]
- Glicksberg, L.; Kerrigan, S. Derivatization. In Principles of Forensic Toxicology; Levine, B.S., Kerrigan, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 163–175. ISBN 978-3-030-42917-1. [Google Scholar]
- Orata, F. Derivatization Reactions and Reagents for Gas Chromatography Analysis. In Advanced Gas Chromatography—Progress in Agricultural, Biomedical and Industrial Applications; IntechOpen: London, UK, 2012; Volume 91, p. 95. [Google Scholar]
- Palamar, J.J.; Salomone, A.; Vincenti, M.; Cleland, C.M. Detection of “Bath Salts” and Other Novel Psychoactive Substances in Hair Samples of Ecstasy/MDMA/“Molly” Users. Drug Alcohol Depend. 2016, 161, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Dresen, S.; Ferreirós, N.; Pütz, M.; Westphal, F.; Zimmermann, R.; Auwärter, V. Monitoring of Herbal Mixtures Potentially Containing Synthetic Cannabinoids as Psychoactive Compounds. J. Mass Spectrom. 2010, 45, 1186–1194. [Google Scholar] [CrossRef]
- Dugues, P.; Martin, M.; Abe, E.; Etting, I.; Edel, Y.; Alvarez, J.C.; Larabi, A. Targeted and Untargeted Screening of New Psychoactive Substances (NPS) and Classical Drugs of Abuse in Paris Using Hair Testing: A 10-Years Study (2012–2021). Toxicol. Anal. Clin. 2022, 34, S80. [Google Scholar] [CrossRef]
- Nisbet, L.A.; Venson, R.; Wylie, F.M.; Scott, K.S. Application of a Urine and Hair Validated LC–MS-MS Method to Determine the Effect of Hair Color on the Incorporation of 25B-NBOMe, 25C-NBOMe and 25I-NBOMe into Hair in the Rat. J. Anal. Toxicol. 2017, 41, 559–565. [Google Scholar] [CrossRef]
- Chou, H.-H.; Hsieh, C.-H.; Chaou, C.-H.; Chen, C.-K.; Yen, T.-H.; Liao, S.-C.; Seak, C.-J.; Chen, H.-Y. Synthetic Cathinone Poisoning from Ingestion of Drug-Laced “Instant Coffee Packets” in Taiwan. Hum. Exp. Toxicol. 2021, 40, 1403–1412. [Google Scholar] [CrossRef]
- Eshleman, A.J.; Wolfrum, K.M.; Reed, J.F.; Kim, S.O.; Johnson, R.A.; Janowsky, A. Neurochemical Pharmacology of Psychoactive Substituted N-Benzylphenethylamines: High Potency Agonists at 5-HT2A Receptors. Biochem. Pharmacol. 2018, 158, 27–34. [Google Scholar] [CrossRef]
- Alsenedi, K.A.; Morrison, C. Comparison of Six Derivatizing Agents for the Determination of Nine Synthetic Cathinones Using Gas Chromatography-Mass Spectrometry. Anal. Methods 2017, 9, 2732–2743. [Google Scholar] [CrossRef]
- Zuba, D.; Sekuła, K.; Buczek, A. 25C-NBOMe—New Potent Hallucinogenic Substance Identified on the Drug Market. Forensic Sci. Int. 2013, 227, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.R.; Prosser, D.; Maurer, H.H. Studies on the Metabolism and Detectability of the Designer Drug β-Naphyrone in Rat Urine Using GC-MS and LC-HR-MS/MS. Drug Test. Anal. 2013, 5, 259–265. [Google Scholar] [CrossRef]
- Higgins, K.; O’Neill, N.; O’Hara, L.; Jordan, J.; McCann, M.; O’Neill, T.; Clarke, M.; O’Neill, T.; Kelly, G.; Campbell, A. New Psychoactives within Polydrug Use Trajectories—Evidence from a Mixed-method Longitudinal Study. Addiction 2021, 116, 2454–2462. [Google Scholar] [CrossRef] [PubMed]
- Tettey, J.N.; Levissianos, S. The Global Emergence of Nps: An Analysis of a New Drug Trend. In Novel Psychoactive Substances: Policy, Economics and Drug Regulation; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–12. [Google Scholar]
- Namera, A.; Kawamura, M.; Nakamoto, A.; Saito, T.; Nagao, M. Comprehensive Review of the Detection Methods for Synthetic Cannabinoids and Cathinones. Forensic Toxicol. 2015, 33, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.D.; Botch-Jones, S.R.; Flowers, T.; Lewis, C.A. An Evaluation of 25B-, 25C-, 25D-, 25H-, 25I- and 25T2-NBOMe via LC–MS-MS: Method Validation and Analyte Stability. J. Anal. Toxicol. 2014, 38, 479–484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Poole, C.F. New Trends in Solid-Phase Extraction. TrAC Trends Anal. Chem. 2003, 22, 362–373. [Google Scholar] [CrossRef]
- Tabarra, I.; Soares, S.; Rosado, T.; Gonçalves, J.; Luís, Â.; Malaca, S.; Barroso, M.; Keller, T.; Restolho, J.; Gallardo, E. Novel Synthetic Opioids—Toxicological Aspects and Analysis. Forensic Sci. Res. 2019, 4, 111–140. [Google Scholar] [CrossRef]
- Bade, R.; Bijlsma, L.; Sancho, J.V.; Baz-Lomba, J.A.; Castiglioni, S.; Castrignanò, E.; Causanilles, A.; Gracia-Lor, E.; Kasprzyk-Hordern, B.; Kinyua, J. Liquid Chromatography-Tandem Mass Spectrometry Determination of Synthetic Cathinones and Phenethylamines in Influent Wastewater of Eight European Cities. Chemosphere 2017, 168, 1032–1041. [Google Scholar] [CrossRef]
- Chen, P.-P.; Du, P.; Zhou, Z.-L.; Xu, Z.-Q.; Gao, T.-T.; Li, X.-Q. Optimization and Validation of the Analytical Method to Detect New Psychoactive Substances in Wastewater. Huan Jing Ke Xue Huanjing Kexue 2018, 39, 3736–3743. [Google Scholar]
- Kinyua, J.; Covaci, A.; Maho, W.; McCall, A.-K.; Neels, H.; van Nuijs, A.L. Sewage-based Epidemiology in Monitoring the Use of New Psychoactive Substances: Validation and Application of an Analytical Method Using LC-MS/MS. Drug Test. Anal. 2015, 7, 812–818. [Google Scholar] [CrossRef] [PubMed]

| Drug Name | CAS No | Chemical Class | Chemical Structure | |
|---|---|---|---|---|
| 25D-NBOME | 1539266-35-7 | Phenethylamine | 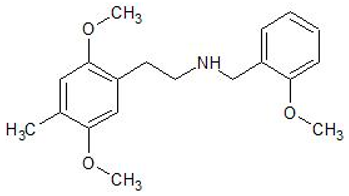 | |
| 25E-NBOMe | 1539266-39-1 | Phenethylamine |  | |
| 25H-NBOME | 1566571-52-5 | Phenethylamine |  | |
| 25I-NBOMe | 1043868-97-8 | Phenethylamine | 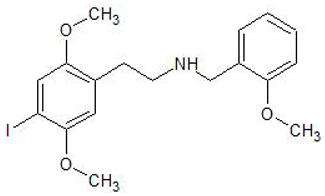 | |
| 25N-NBOMe | 1566571-65-0 | Phenethylamine | 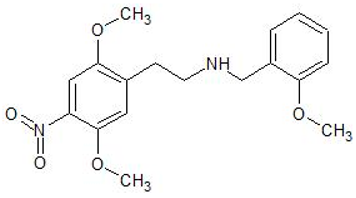 | |
| 25P-NBOMe | 1539266-43-7 | Phenethylamine |  | |
| 3-MEO-PCE | 1797121-52-8 | Arylcyclohexylamine | 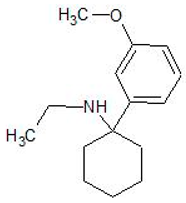 | |
| 5-APB | 286834-80-8 | Phenethylamine |  | |
| 6-APB | 286834-84-2 | Phenethylamine | 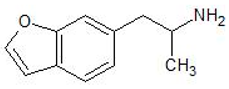 | |
| Benzedrone | 1797979-43-1 | Phenethylamine | 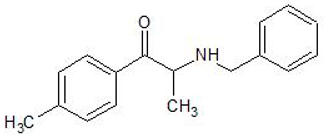 | |
| Butylone | 17762-90-2 | Phenethylamine | 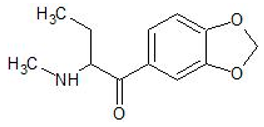 | |
| Ethylone | 1454266-19-3 | Phenethylamine |  | |
| Flephedrone | 7589-35-7 | Phenethylamine | 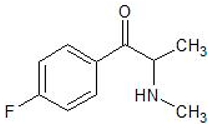 | |
| MDPV | 2748590-34-1 | Phenethylamine |  | |
| Mephedrone | 1189726-22-4 | Phenethylamine | 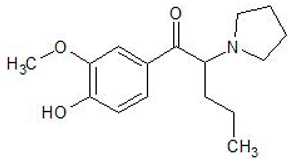 | |
| Mescaline-NBOMe | 1354632-01-1 | Phenethylamine |  | |
| Methiopropamine | 7464-94-0 | Thiophene analogue of methamphetamine |  | |
| Methoxetamine | 1239908-48-5 | Arylcyclohexylamine |  | |
| Naphyrone | 850352-11-3 | Phenethylamine |  | |
| N-Methyl-N-(trimethylsilyl)trifluoroacetamide | 24589-78-4 | Silylating reagent | 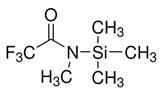 | |
| Pentafluoropropionic anhydride | 356-42-3 | Acylation reagent | 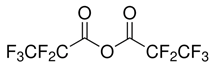 | |
| Trifluoroacetic anhydride | 407-25-0 | Acylation reagents |  | |
| N-Trimethylsilylimidazole | 18156-74-6 | Silylating reagent | 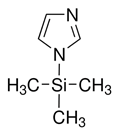 | |
| Cartridge Type | Sorbent Type | Particle Size | Cartridge Size |
|---|---|---|---|
| ZSDAU020 | Octyl + benzyl sulfonic acid | 200 mg | 10 mL |
| CSDAU133 | Octyl + benzyl sulfonic acid | 130 mg | 3 mL |
| XRDAH206 | H.F. C8 + benzyl sulfonic acid | 200 mg | 6 mL |
| XRPCH50z | H.F. propylsulfonic acid | 500 mg | 10 mL |
| XCEL 1 | Proprietary | 130 mg | 3 mL |
| Oasis | Oasis MCX | 150 mg | 6 mL |
| SLE ISOLUTE® | Diatomaceous Earth | - | 1 mL |
| DRUG NAME | PFPA:EtOAc | MSTFA:Toluene | TFAA:EtOAc | TMSI:Pyridine |
|---|---|---|---|---|
| 25D-NBOME | 178, 121, 461 | 166, 150, 121 | 178, 165, 135 | 150, 149, 121 |
| 25E-NBOMe | 192, 475, 121 | 180, 150, 121 | 192, 179, 121 | 167, 149, 121 |
| 25H-NBOME | 164, 121, 447 | 150, 121, 91 | 164, 151, 121 | 150, 121, 91 |
| 25I-NBOMe | 121, 573, 185 | 150, 121, 91 | 290, 277, 121 | 150, 121, 91 |
| 25N-NBOMe | 209, 166, 121 | 167, 150, 121 | 209, 166, 121 | 150, 121, 91 |
| 25P-NBOMe | 206, 193, 489 | 194, 150, 121 | 206, 198, 193 | 194, 150, 121 |
| 3-MEO-PCE | 190, 233, 176 | 233, 190, 176 | 190, 191, 176 | 190, 233, 72 |
| 5-APB | 131, 158,190 | 132,131,77 | 158, 140, 131 | Undetected |
| 6-APB | 131, 158, 190 | 132,131,77 | 158, 140, 131 | Undetected |
| Benzedrone | 91, 119, 148 | 135, 134, 91 | 230, 148, 119 | Undetected |
| Butylone | 149, 218, 121 | 149, 121,72 | 168, 149, 121 | Undetected |
| Ethylone | 218, 190, 367 | 149,121,72 | 168, 149, 121 | Undetected |
| Flephedrone | 123, 204, 160 | Undetected | 154, 123, 110 | Undetected |
| MDPV | 126, 149, 110 | 126, 149, 110 | 126, 149, 110 | 126, 149, 110 |
| Mephedrone | 204, 160, 323 | 119, 91, 58 | 154, 119, 110 | Undetected |
| Mescaline-NBOMe | 194, 181, 477 | 247, 73,149 | 194, 181, 121 | 182, 150, 121 |
| MPA | 124, 204, 160 | Undetected | 154, 124, 110 | Undetected |
| MXE | 190, 219, 134 | 219, 190, 176 | 219, 218, 190 | 219, 190, 176 |
| Naphyrone | 126, 155, 127 | 126, 155, 127 | 126, 155, 127 | 126, 149, 127 |
| Analyte | PFPA:EtOAc | MSTFA:Toluene | TFAA:EtOAc | TMSI:Pyridine | Statistically Significant Factor | ||||
|---|---|---|---|---|---|---|---|---|---|
| Incubation Temperature (°C) | Incubation Time (min) | Incubation Temperature (°C) | Incubation Time (min) | Incubation Temperature (°C) | Incubation Time (min) | Incubation Temperature (°C) | Incubation Time (min) | ||
| 25D-NBOME | 37 | 20 | 50 | 20 | 50 | 40 | 37 | 20 | Agent (p = 0.000) |
| 25E-NBOMe | 37 | 20 | 50 | 20 | 50 | 40 | 37 | 20 | Agent (p = 0.000) |
| 25H-NBOME | 24 | 40 | 50 | 20 | 50 | 40 | 37 | 20 | Agent (p = 0.000) |
| 25I-NBOMe | 37 | 20 | 50 | 20 | 50 | 40 | 37 | 20 | Agent (p = 0.000) Time (p = 0.002) |
| 25N-NBOMe | 70 | 20 | 50 | 20 | 50 | 40 | 37 | 20 | Agent (p = 0.000) |
| 25P-NBOMe | 37 | 40 | 50 | 20 | 50 | 40 | 37 | 20 | Agent (p = 0.000) |
| 3-MEO-PCE | 24 | 40 | 24 | 40 | 37 | 40 | 37 | 20 | Agent (p = 0.000) |
| 5-APB | 24 | 40 | 24 | 40 | 24 | 40 | Not Detected | Agent (p = 0.000) | |
| 6-APB | 24 | 40 | 24 | 40 | 24 | 40 | Not Detected | Agent (p = 0.000) | |
| Benzedrone | 70 | 20 | 24 | 40 | 37 | 40 | Not Detected | Agent (p = 0.000) | |
| Butylone | 24 | 40 | 24 | 40 | 37 | 40 | Not Detected | Agent (p = 0.000) | |
| Ethylone | 24 | 40 | 24 | 40 | 37 | 40 | Not Detected | Agent (p = 0.000) | |
| Flephedrone | 70 | 20 | Not Detected | 37 | 40 | Not Detected | Agent (p = 0.000) | ||
| MDPV | 24 | 40 | 24 | 40 | 37 | 40 | Agent (p = 0.000) | 20 | Agent (p = 0.000) |
| Mephedrone | 70 | 20 | 24 | 40 | 37 | 40 | Agent (p = 0.000) | Agent (p = 0.000) | |
| Mescaline-NBOMe | 37 | 40 | 50 | 20 | 50 | 40 | Agent (p = 0.000) | 20 | Agent (p = 0.000) |
| MPA | 70 | 20 | Not Detected | 37 | 40 | Not Detected | Agent (p = 0.000) | ||
| MXE | 24 | 40 | 24 | 40 | 37 | 40 | 37 | 20 | Agent (p = 0.000) |
| Naphyrone | 70 | 20 | 24 | 40 | 37 | 40 | 37 | 20 | Agent (p = 0.000) |
| Analyte | Blood | Urine | Plasma | Serum | ||||
|---|---|---|---|---|---|---|---|---|
| Cartridge Type | % Extraction Efficiency | Cartridge Type | % Extraction Efficiency | Cartridge Type | % Extraction Efficiency | Cartridge Type | % Extraction Efficiency | |
| 25D-NBOME | CSDAU | 102 | XRDAH 206 | 109 | ZSDAU | 117 | ZSDAU | 111 |
| 25E-NBOMe | CSDAU | 110 | CSDAU | 110 | ZSDAU | 111 | ZSDAU | 108 |
| 25H-NBOME | CSDAU | 99 | CSDAU | 81 | CSDAU | 93 | CSDAU | 81 |
| 25I-NBOMe | CSDAU | 90 | ZSDAU | 90 | CSDAU | 98 | ZSDAU | 118 |
| 25N-NBOMe | ZSDAU | 81 | ZSDAU | 98 | ZSDAU | 105 | ZSDAU | 118 |
| 25P-NBOMe | XRDAH 206 | 69 | ZSDAU | 76 | CSDAU | 70 | CSDAU | 95 |
| 3-MEO-PCE | CSDAU | 58 | XCEL 1 | 96 | XRDAH 206 | 60 | ZSDAU | 50 |
| 5-APB | CSDAU | 49 | XCEL 1 | 95 | CSDAU | 63 | CSDAU | 71 |
| 6-APB | CSDAU | 65 | XCEL 1 | 94 | CSDAU | 98 | CSDAU | 51 |
| Benzedrone | CSDAU | 104 | OASIS® | 114 | CSDAU | 105 | XRDAH 206 | 32 |
| Butylone | XCEL 1 | 80 | OASIS® | 119 | XCEL 1 | 101 | XCEL 1 | 87 |
| Ethylone | ZSDAU | 115 | OASIS® | 87 | XCEL 1 | 79 | XCEL 1 | 79 |
| Flephedrone | CSDAU | 65 | CSDAU | 81 | CSDAU | 67 | CSDAU | 63 |
| MDPV | CSDAU | 106 | XCEL 1 | 105 | XCEL 1 | 109 | XCEL 1 | 100 |
| Mephedrone | CSDAU | 112 | CSDAU | 101 | CSDAU | 88 | CSDAU | 106 |
| Mescaline-NBOMe | CSDAU | 71 | CSDAU | 77 | CSDAU | 91 | ZSDAU | 63 |
| MPA | CSDAU | 90 | XCEL 1 | 80 | CSDAU | 49 | CSDAU | 88 |
| MXE | CSDAU | 57 | XCEL 1 | 70 | CSDAU | 100 | CSDAU | 60 |
| Naphyrone | CSDAU | 74 | CSDAU | 116 | CSDAU | 105 | CSDAU | 66 |
| Average Extraction Efficiency | - | 84 | - | 95 | - | 90 | - | 81 |
| Blood % Extraction Efficiency | Urine % Extraction Efficiency | Plasma % Extraction Efficiency | Serum % Extraction Efficiency | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | SLE | SPE | % Difference | SLE | SPE | % Difference | SLE | SPE | % Difference | SLE | SPE | % Difference |
| 25D-NBOMe | 71 | 102 | −31 | 108 | 63 | 45 | 97 | 59 | 38 | 120 | 85 | 35 |
| 25E-NBOMe | 82 | 110 | −28 | 75 | 110 | −35 | 22 | 65 | −43 | 43 | 56 | −13 |
| 25H-NBOMe | 77 | 99 | −22 | 49 | 81 | −32 | 41 | 93 | −52 | 40 | 81 | −41 |
| 25I-NBOMe | 81 | 90 | −9 | 48 | 83 | −35 | 34 | 98 | −64 | 33 | 78 | −45 |
| 25N-NBOMe | 105 | 56 | 49 | 65 | 76 | −11 | 52 | 67 | −15 | 55 | 54 | 1 |
| 25P-NBOMe | 74 | 51 | 23 | 61 | 74 | −13 | 43 | 70 | −27 | 32 | 95 | −63 |
| 3-MEO-PCE | 88 | 58 | 30 | 108 | 86 | 22 | 77 | 50 | 27 | 100 | 36 | 64 |
| 5-APB | 75 | 49 | 26 | 59 | 74 | −15 | 64 | 63 | 1 | 70 | 71 | −1 |
| 6-APB | 61 | 65 | −4 | 69 | 93 | −24 | 56 | 98 | −42 | 34 | 51 | −17 |
| Benzedrone | 32 | 104 | −72 | 71 | 88 | −17 | 72 | 105 | −33 | 70 | 22 | 48 |
| Butylone | 61 | 65 | −4 | 72 | 83 | −11 | 69 | 65 | 4 | 69 | 29 | 40 |
| Ethylone | 90 | 70 | 20 | 73 | 79 | −6 | 69 | 74 | −5 | 70 | 31 | 39 |
| Flephedrone | 79 | 65 | 14 | 73 | 81 | −8 | 74 | 67 | 7 | 69 | 63 | 6 |
| MDPV | 117 | 106 | 11 | 75 | 74 | 1 | 64 | 75 | −11 | 68 | 75 | −7 |
| Mephedrone | 77 | 112 | −35 | 73 | 101 | −28 | 71 | 88 | −17 | 71 | 106 | −35 |
| Mescaline-NBOMe | 78 | 71 | 7 | 72 | 77 | −5 | 66 | 91 | −25 | 67 | 63 | 4 |
| Methiopropamine | 43 | 90 | −47 | 73 | 55 | 18 | 63 | 49 | 14 | 63 | 88 | −25 |
| Methoxetamine | 87 | 57 | 30 | 73 | 53 | 20 | 66 | 100 | −34 | 66 | 60 | 6 |
| Naphyrone | 72 | 74 | −2 | 94 | 116 | −22 | 104 | 105 | −1 | 87 | 66 | 21 |
| Average | 76 | 79 | −2 | 73 | 81 | −8 | 63 | 78 | −15 | 65 | 64 | 1 |
| Statistical Difference (p) | 0.729 | 0.113 | 0.029 | 0.911 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisbet, L.A.; Wylie, F.M.; Scott, K.S. Applications of Sample Preparation Techniques in the Analysis of New Psychoactive Substances. Separations 2024, 11, 258. https://doi.org/10.3390/separations11090258
Nisbet LA, Wylie FM, Scott KS. Applications of Sample Preparation Techniques in the Analysis of New Psychoactive Substances. Separations. 2024; 11(9):258. https://doi.org/10.3390/separations11090258
Chicago/Turabian StyleNisbet, Lorna A., Fiona M. Wylie, and Karen S. Scott. 2024. "Applications of Sample Preparation Techniques in the Analysis of New Psychoactive Substances" Separations 11, no. 9: 258. https://doi.org/10.3390/separations11090258
APA StyleNisbet, L. A., Wylie, F. M., & Scott, K. S. (2024). Applications of Sample Preparation Techniques in the Analysis of New Psychoactive Substances. Separations, 11(9), 258. https://doi.org/10.3390/separations11090258






