Green Preparation of ZnO Nanoparticles Using Citrus aurantium L. Extract for Dye Adsorption, Antibacterial, and Antioxidant Activities
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of CA and BLC
2.3. Preparation of ZnO NPs, ZnO NPs-B, and ZnO NPs-CK
2.4. Adsorption Assay
2.5. Antibacterial Activity
2.6. Antioxidant Activity
2.6.1. DPPH Assay
2.6.2. ABTS Assay
2.6.3. FRAP Assay
3. Results and Discussion
3.1. Characterizations of ZnO NPs
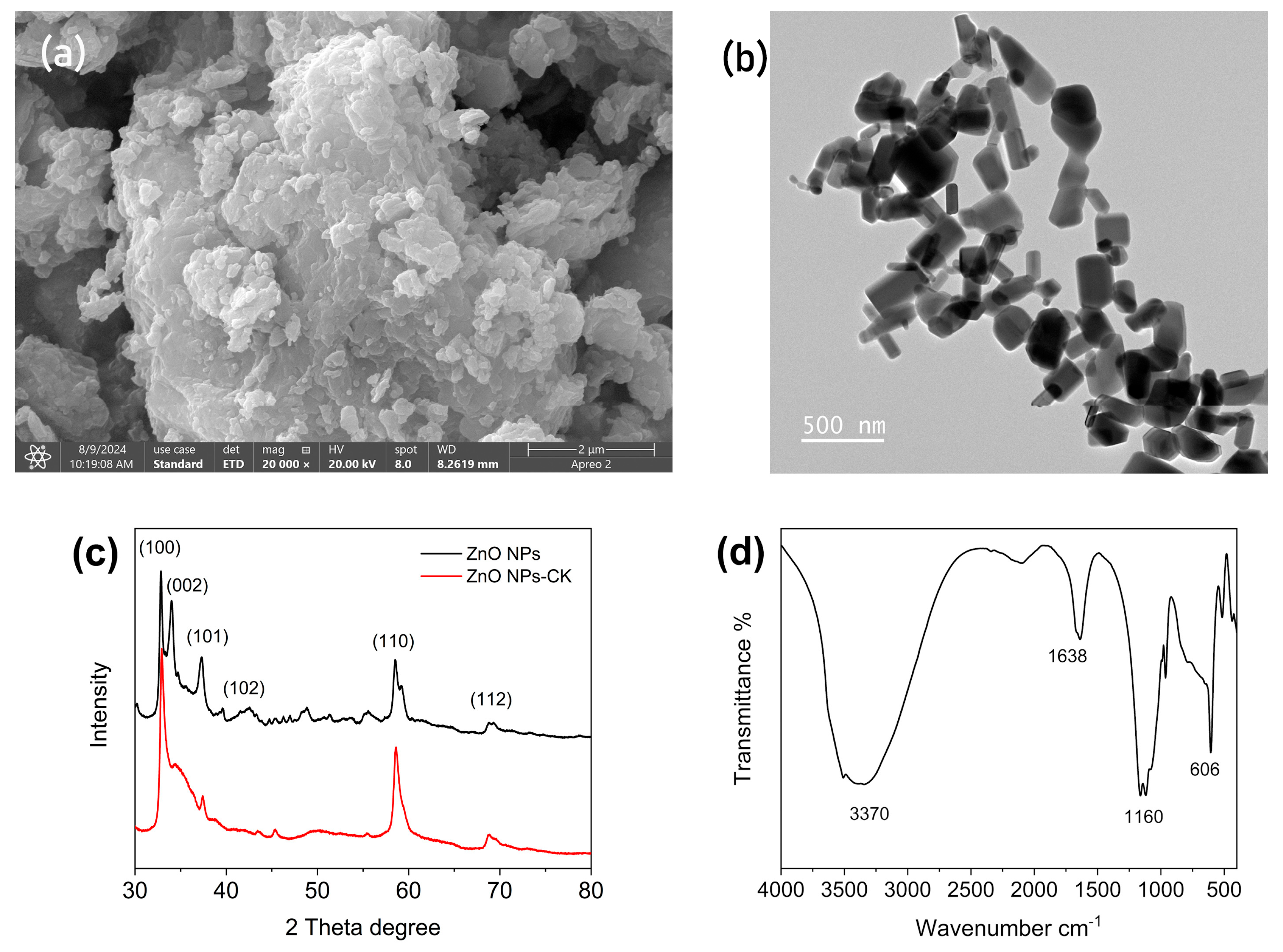
3.1.1. SEM with EDAX
3.1.2. TEM
3.1.3. XRD
3.1.4. IR
3.2. Adsorption of Amaranth Red
3.2.1. The Adsorption Abilities of ZnO NPs
3.2.2. Effect of the Initial Concentration of Amaranth Red
3.2.3. Effect of Adsorbent Amount
3.2.4. Effect of Adsorption Time
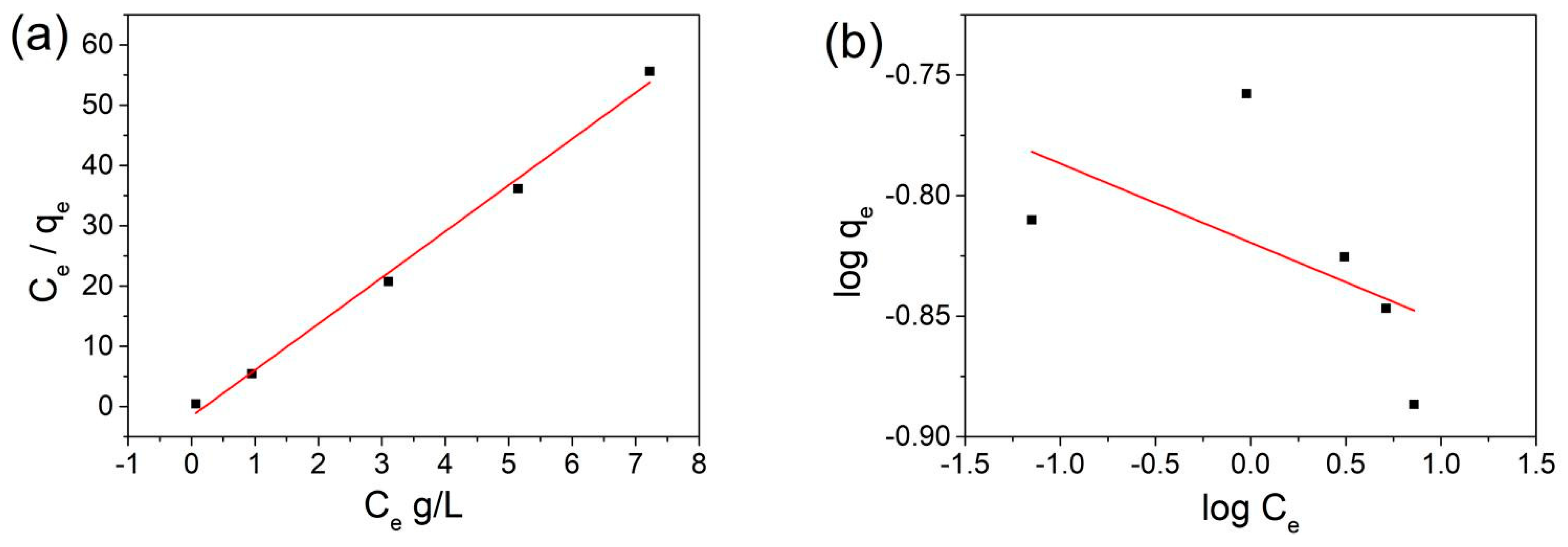
3.3. Adsorption Kinetics Study
3.4. Adsorption Isotherm Study
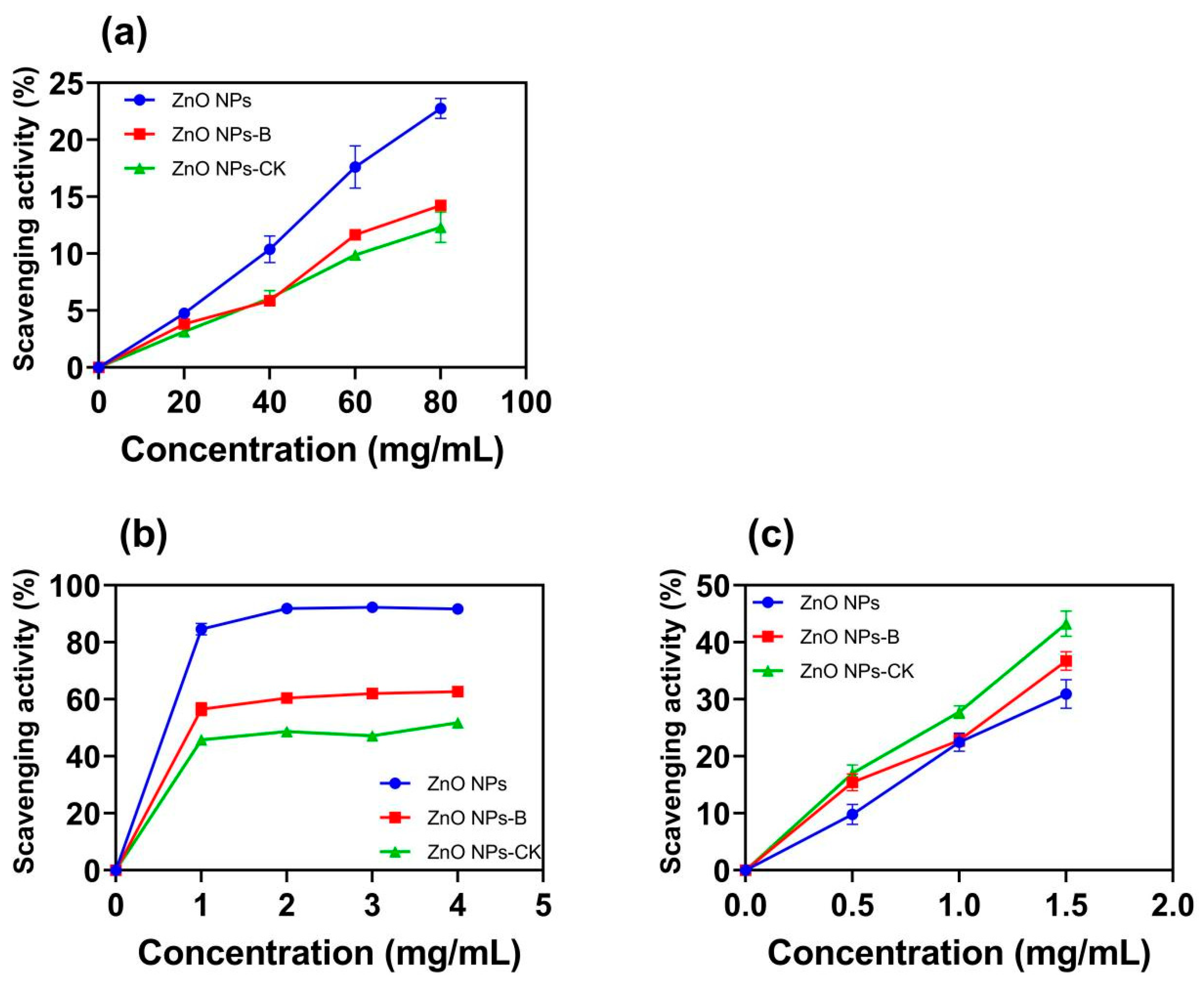
3.5. Antibacterial Activity of ZnO NPs
3.6. Antioxidant Activities of ZnO NPs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Ma, Z.; Wang, G. An Overview of Biological Mechanisms and Strategies for Treating Wastewater from Printing and Dyeing Processes. J. Water Process Eng. 2023, 55, 104242. [Google Scholar] [CrossRef]
- Ceylan, E.; Dindaş, G.B.; Bektaş, N.; Yatmaz, H.C. Modification of Natural Chitosan with Fe and Ce Cations as Photocatalyst Beads: Degradation of Dye Chemicals and Textile Wastewater under UVA Light. J. Environ. Manag. 2022, 310, 114790. [Google Scholar] [CrossRef] [PubMed]
- Tianzhi, W.; Weijie, W.; Hongying, H.; Khu, S.-T. Effect of Coagulation on Bio-Treatment of Textile Wastewater: Quantitative Evaluation and Application. J. Clean. Prod. 2021, 312, 127798. [Google Scholar] [CrossRef]
- Thanh Tu, N.T.; Thien, T.V.; Du, P.D.; Thanh Chau, V.T.; Mau, T.X.; Khieu, D.Q. Adsorptive Removal of Congo Red from Aqueous Solution Using Zeolitic Imidazolate Framework–67. J. Environ. Chem. Eng. 2018, 6, 2269–2280. [Google Scholar] [CrossRef]
- Qin, L.; Gao, M.; Zhang, M.; Feng, L.; Liu, Q.; Zhang, G. Application of Encapsulated Algae into MBR for High-Ammonia Nitrogen Wastewater Treatment and Biofouling Control. Water Res. 2020, 187, 116430. [Google Scholar] [CrossRef] [PubMed]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent Advances in the Treatment of Dye-Containing Wastewater from Textile Industries: Overview and Perspectives. Process Saf. Environ. Prot. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Laskar, N.; Kumar, U. Application of Low-Cost, Eco-Friendly Adsorbents for the Removal of Dye Contaminants from Wastewater: Current Developments and Adsorption Technology. Environ. Qual. Manag. 2022, 32, 209–221. [Google Scholar] [CrossRef]
- Teo, S.; Ng, C.; Islam, A.; Abdulkareem-Alsultan, G.; Joseph, C.; Janaun, J.; Taufiq-Yap, Y.; Khandaker, S.; Islam, G.; Znad, H.; et al. Sustainable Toxic Dyes Removal with Advanced Materials for Clean Water Production: A Comprehensive Review. J. Clean. Prod. 2022, 332, 130039. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.; Hussain, M. A State-of-the-Art Review on Wastewater Treatment Techniques: The Effectiveness of Adsorption Method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Baskar, A.V.; Bolan, N.; Hoang, S.A.; Sooriyakumar, P.; Kumar, M.; Singh, L.; Jasemizad, T.; Padhye, L.P.; Singh, G.; Vinu, A.; et al. Recovery, Regeneration and Sustainable Management of Spent Adsorbents from Wastewater Treatment Streams: A Review. Sci. Total Environ. 2022, 822, 153555. [Google Scholar] [CrossRef] [PubMed]
- Selmani, A.; Kovačević, D.; Bohinc, K. Nanoparticles: From Synthesis to Applications and Beyond. Adv. Colloid Interface Sci. 2022, 303, 102640. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Q.; Hayat, Z.; Zhang, D.-D.; Li, M.-Y.; Hu, S.; Wu, Q.; Cao, Y.-F.; Yuan, Y. Zinc Oxide Nanoparticles: Synthesis, Characterization, Modification, and Applications in Food and Agriculture. Processes 2023, 11, 1193. [Google Scholar] [CrossRef]
- Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B. Zinc Oxide Nanoparticles: A Promising Nanomaterial for Biomedical Applications. Drug Discov. Today 2017, 22, 1825–1834. [Google Scholar] [CrossRef]
- Singh, T.A.; Das, J.; Sil, P.C. Zinc Oxide Nanoparticles: A Comprehensive Review on Its Synthesis, Anticancer and Drug Delivery Applications as Well as Health Risks. Adv. Colloid Interface Sci. 2020, 286, 102317. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Hymavathi, A.; Yaku, G.; Kumar, U. Green Synthesis and Characterization of ZnO Nanoparticles-A Novel Approach Using Carica Papaya Leaf Extract. Mater. Today Proc. 2022, 62, 6854–6856. [Google Scholar] [CrossRef]
- López-Cuenca, S.; Salazar-Peña, R.; Pedroza-Toscano, M. Green Synthesis of Zinc Oxide Nanoparticles: Anticancer and Antibacterial Applications (A Review). Russ. J. Gen. Chem. 2024, 94, 2338–2355. [Google Scholar] [CrossRef]
- Kazemi, S.; Hosseingholian, A.; Gohari, S.D.; Feirahi, F.; Moammeri, F.; Mesbahian, G.; Moghaddam, Z.S.; Ren, Q. Recent Advances in Green Synthesized Nanoparticles: From Production to Application. Mater. Today Sustain. 2023, 24, 100500. [Google Scholar] [CrossRef]
- Haque, M.J.; Bellah, M.M.; Hassan, M.R.; Rahman, S. Synthesis of ZnO Nanoparticles by Two Different Methods & Comparison of Their Structural, Antibacterial, Photocatalytic and Optical Properties. Nano Express 2020, 1, 010007. [Google Scholar] [CrossRef]
- Jadoun, S.; Arif, R.; Jangid, N.; Meena, R. Green Synthesis of Nanoparticles Using Plant Extracts: A Review. Environ. Chem. Lett. 2021, 19, 355–374. [Google Scholar] [CrossRef]
- Yang, X.; Cao, X.; Chen, C.; Liao, L.; Yuan, S.; Huang, S. Green Synthesis of Zinc Oxide Nanoparticles Using Aqueous Extracts of Hibiscus cannabinus L.: Wastewater Purification and Antibacterial Activity. Separations 2023, 10, 466. [Google Scholar] [CrossRef]
- Maksoud, S.; Abdel-Massih, R.M.; Rajha, H.N.; Louka, N.; Chemat, F.; Barba, F.J.; Debs, E. Citrus aurantium L. Active Constituents, Biological Effects and Extraction Methods. An Updated Review. Molecules 2021, 26, 5832. [Google Scholar] [CrossRef]
- Ren, W.; Wang, S.; Zhang, J.; Liu, D. Ethnopharmacology, Chemical Composition and Functions of Citrus aurantium L. J. Food Meas. Charact. 2024, 18, 8843–8864. [Google Scholar] [CrossRef]
- Zahr, S.; Zahr, R.; El Hajj, R.; Khalil, M. Phytochemistry and Biological Activities of Citrus Sinensis and Citrus Limon: An Update. J. Herb. Med. 2023, 41, 100737. [Google Scholar] [CrossRef]
- Suntar, I.; Khan, H.; Patel, S.; Celano, R.; Rastrelli, L. An Overview on Citrus aurantium L.: Its Functions as Food Ingredient and Therapeutic Agent. Oxid. Med. Cell. Longev. 2018, 2018, 7864269. [Google Scholar] [CrossRef]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus Peel Flavonoids as Potential Cancer Prevention Agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef]
- Klimek-Szczykutowicz, M.; Szopa, A.; Ekiert, H. Citrus Limon (Lemon) Phenomenon—A Review of the Chemistry, Pharmacological Properties, Applications in the Modern Pharmaceutical, Food, and Cosmetics Industries, and Biotechnological Studies. Plants 2020, 9, 119. [Google Scholar] [CrossRef]
- Jangra, A.; Singh, J.; Kumar, J.; Rani, K.; Kumar, P.; Kumar, S.; Singh, D.; Kumar, R. Dye Elimination by Surface-Functionalized Magnetite Nanoparticles: Kinetic and Isotherm Studies. Biointerface Res. Appl. Chem. 2022, 13, 325. [Google Scholar] [CrossRef]
- Kandasamy, S.; Velusamy, S.; Thirumoorthy, P.; Periyasamy, M.; Veerasamy, S.; Gopalakrishnan, K.M.; Sathish, U.; Kiramani, V.; Antrini, F.D.G.; Periyasamy, S. Adsorption of Chromium Ions from Aqueous Solutions by Synthesized Nanoparticles. J. Nanomater. 2022, 2022, 6214438. [Google Scholar] [CrossRef]
- Wang, T.; Zhong, X.; Zhang, Z.; Yuan, X.; Zhou, L.; Zheng, Z.; Farhadian, A.; Hu, J. Asphaltene Adsorption of Co3O4 Nanoparticles Modified by SiO2 Film. Appl. Surf. Sci. 2022, 602, 154267. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Menon, S.; Shanmugam, V. Eco-Friendly Synthesis of Zinc Oxide Nanoparticles Using Cinnamomum Tamala Leaf Extract and Its Promising Effect towards the Antibacterial Activity. J. Drug Deliv. Sci. Technol. 2019, 53, 101212. [Google Scholar] [CrossRef]
- Jiménez-Morales, W.A.; del P. Cañizares-Macias, M. Fast FRAP-SIA Method to Determine Antioxidant Capacity. Talanta 2024, 273, 125813. [Google Scholar] [CrossRef] [PubMed]
- Kiran Kumar, A.B.V.; Saila, E.S.; Narang, P.; Aishwarya, M.; Raina, R.; Gautam, M.; Shankar, E.G. Biofunctionalization and Biological Synthesis of the ZnO Nanoparticles: The Effect of Raphanus Sativus (White Radish) Root Extract on Antimicrobial Activity against MDR Strain for Wound Healing Applications. Inorg. Chem. Commun. 2019, 100, 101–106. [Google Scholar] [CrossRef]
- Khan, M.M.; Saadah, N.H.; Khan, M.E.; Harunsani, M.H.; Tan, A.L.; Cho, M.H. Potentials of Costus Woodsonii Leaf Extract in Producing Narrow Band Gap ZnO Nanoparticles. Mater. Sci. Semicond. Process. 2019, 91, 194–200. [Google Scholar] [CrossRef]
- Alalawy, A.I.; Saleh, F.M.; Saeedi, N.H.; Panneerselvam, C.; Sakran, M.I.; Khasim, S.; Parveen, H.; Mohammedsaleh, Z.M.; Mukhtar, S.; Faridi, U.; et al. Green Synthesis of Bb-ZnO NPs Using Barleria Buxifolia Leaf Extract: Investigations into Their Physicochemical, Biological and Anti-Cancer Properties. Polyhedron 2025, 267, 117366. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, H.; Kumar, P.; Rani, G.; Maken, S. Syzygium Cumini Leaf Extract Mediated Green Synthesis of ZnO Nanoparticles: A Sustained Release for Anticancer, Antimicrobial, Antioxidant, and Anti-Corrosive Applications. J. Mol. Struct. 2025, 1325, 141017. [Google Scholar] [CrossRef]
- Sharma, A.; Nagraik, R.; Venkidasamy, B.; Khan, A.; Dulta, K.; Kumar Chauhan, P.; Kumar, D.; Shin, D.-S. In Vitro Antidiabetic, Antioxidant, Antimicrobial, and Cytotoxic Activity of Murraya Koenigii Leaf Extract Intercedes ZnO Nanoparticles. Luminescence 2023, 38, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.I.; Villegas, V.A.; Sicairos, S.P.; Guevara, E.H.; Brito Perea, M.D.; Sánchez, B.L. Synthesis and Characterization of Zinc Peroxide Nanoparticles for the Photodegradation of Nitrobenzene Assisted by UV-Light. Catalysts 2020, 10, 1041. [Google Scholar] [CrossRef]
- Açıkgöz, M.; Rahmanberdyyev, N.; Başkan, G. Olive Leaf Extract-Assisted Preparation of Nanoferrite for Adsorptive Removal of Cationic Dye. Mater. Chem. Phys. 2024, 323, 129662. [Google Scholar] [CrossRef]
- Mozafarjalali, M.; Hamidian, A.H.; Sayadi, M.H. Microplastics as Carriers of Iron and Copper Nanoparticles in Aqueous Solution. Chemosphere 2023, 324, 138332. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-Y.; Zeng, W.-Y. Adsorption of Cu(II) by Poly-γ-Glutamate/Apatite Nanoparticles. Polymers 2021, 13, 962. [Google Scholar] [CrossRef]
- Tahir, H.; Anwer, M.; Khan, S.; Saad, M. Enhancement of Adsorption and Photocatalytic Activity of MgO Nanoparticles for the Treatment of Textile Dye Using Ultrasound Assisted Process by Response Surface Methodology. Desalination Water Treat. 2024, 319, 100429. [Google Scholar] [CrossRef]
- Alam, S.; Ullah, R.; ur Rahman, N.; Ilyas, M.; Ullah, S.; Zahoor, M.; Umar, M.N.; Ullah, R.; Ali, E.A. Synthesis of Metallic Nanoparticles and Its Application in Adsorption of Metanil Yellow and Rhodamine B from Aqueous Solutions. Desalination Water Treat. 2023, 313, 186–195. [Google Scholar] [CrossRef]
- Zhang, N.; Reguyal, F.; Sarmah, A.K. Effect of Iron Nanoparticles on Chromium Adsorption in Aqueous Solution Using Magnetic Biochar: A Site Energy Distribution Analysis. Environ. Pollut. 2024, 346, 123593. [Google Scholar] [CrossRef]
- Cheperli, A.; Mokaber-Esfahani, M.; Taleghani, A.; Bahalkeh, F. Biosynthesis, Characterization and Antimicrobial Activities of Zinc Oxide Nanoparticles from Leaf and Seed Extracts of Malva Neglecta Wallr. Inorg. Nano-Met. Chem. 2024. [Google Scholar] [CrossRef]
- Elbrolesy, A.; Abdou, Y.; Elhussiny, F.A.; Morsy, R. Novel Green Synthesis of UV-Sunscreen ZnO Nanoparticles Using Solanum Lycopersicum Fruit Extract and Evaluation of Their Antibacterial and Anticancer Activity. J. Inorg. Organomet. Polym. Mater. 2023, 33, 3750–3759. [Google Scholar] [CrossRef]
- Zhao, C.-Y.; Tan, S.-X.; Xiao, X.-Y.; Qiu, X.-S.; Pan, J.-Q.; Tang, Z.-X. Effects of Dietary Zinc Oxide Nanoparticles on Growth Performance and Antioxidative Status in Broilers. Biol. Trace Elem. Res. 2014, 160, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fiévet, F. Toxicological Impact Studies Based on Escherichia Coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef] [PubMed]
- Payne, A.C.; Mazzer, A.; Clarkson, G.J.J.; Taylor, G. Antioxidant Assays—Consistent Findings from FRAP and ORAC Reveal a Negative Impact of Organic Cultivation on Antioxidant Potential in Spinach but Not Watercress or Rocket Leaves. Food Sci. Nutr. 2013, 1, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Donmez, S.; Keyvan, E. Green Synthesis of Zinc Oxide Nanoparticles Using Grape Seed Extract and Evaluation of Their Antibacterial and Antioxidant Activities. Inorg. Nano-Met. Chem. 2023, 54, 1137–1144. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Jha, S.; Tripathi, S.K.; Awasthi, R.R.; Bhardwaj, A.K.; Singh, A.K.; Dikshit, A. Antioxidant Response of Calendula officinalis L. Assisted Synthesized Zinc Oxide Nanoparticles. Mater. Res. Express 2024, 11, 085005. [Google Scholar] [CrossRef]



| Element | Zn NPs (At%) | Zn NPs-CK (At%) |
|---|---|---|
| N | 0.25 | 0 |
| O | 75.54 | 60.43 |
| Zn | 24.21 | 39.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Liu, L.; Chen, C.; Liao, L.; Huang, S. Green Preparation of ZnO Nanoparticles Using Citrus aurantium L. Extract for Dye Adsorption, Antibacterial, and Antioxidant Activities. Separations 2025, 12, 18. https://doi.org/10.3390/separations12020018
Yang X, Liu L, Chen C, Liao L, Huang S. Green Preparation of ZnO Nanoparticles Using Citrus aurantium L. Extract for Dye Adsorption, Antibacterial, and Antioxidant Activities. Separations. 2025; 12(2):18. https://doi.org/10.3390/separations12020018
Chicago/Turabian StyleYang, Xitao, Liangliang Liu, Chenxiao Chen, Liping Liao, and Siqi Huang. 2025. "Green Preparation of ZnO Nanoparticles Using Citrus aurantium L. Extract for Dye Adsorption, Antibacterial, and Antioxidant Activities" Separations 12, no. 2: 18. https://doi.org/10.3390/separations12020018
APA StyleYang, X., Liu, L., Chen, C., Liao, L., & Huang, S. (2025). Green Preparation of ZnO Nanoparticles Using Citrus aurantium L. Extract for Dye Adsorption, Antibacterial, and Antioxidant Activities. Separations, 12(2), 18. https://doi.org/10.3390/separations12020018








