Abstract
Enriched lithium isotopes (6Li and 7Li) are essential in the nuclear energy industry, where 6Li is bombarded with neutrons to produce tritium for fusion reactions, while 7Li is used as a core coolant and pH regulator. Separation of 6Li and 7Li by electromigration is a promising method for producing enriched lithium isotopes that fulfill industrial needs. In this work, based on a previously proposed biphasic system electromigration routine, a three-stage system of ‘LiCl aqueous solution (anolyte)|B12C4-[EMIm][NTf2] organic solution|NH4Cl aqueous solution (catholyte)’ was constructed and the rules of lithium isotope separation and lithium-ion migration investigated. It was shown that the isotope enrichment effect of the catholyte was greatly affected by the experimental conditions, while that of the organic solution was less affected. As the B12C4 concentration increased, enhancement of 7Li enrichment in the catholyte and 6Li enrichment in the organic solution was observed, and α(C/O) and α(O/A) reached 0.975 and 1.018 at B12C4 of 0.5 mol/L. With the increase in current, migration time, and LiCl concentration, the isotope that was enriched in the catholyte trended from 7Li to 6Li (about 6 mA, 12 h or LiCl of 5 mol/L). Taking lithium-ion transport efficiency and lithium isotope separation effect into consideration together, a current of at least 6 mA, duration of at least 12 h, LiCl concentration of at least 1 mol/L and B12C4 concentration of 0.2 mol/L are suggested for the electromigration process. The work provides an important reference for system construction and experimental design of a biphasic electromigration separation method, which is expected to be an industrial alternative because of its environmental protection and high efficiency.
1. Introduction
Lithium naturally consists of two stable isotopes, 6Li and 7Li, of which the natural abundances are 7.5% and 92.5%, respectively [1]. Both 6Li and 7Li are essential materials in the nuclear power industry. 6Li is bombarded with neutrons to produce tritium for fusion reactions, which are used as nuclear fuel in fusion reactors; for this purpose, 6Li must be enriched to an abundance of at least 30% [2]. Moreover, due to its low neutron capture cross-section, highly enriched 7Li, with an abundance of over 99.99%, is used as a core coolant in thorium-based molten salt reactors in order to adjust the reaction rate on the one hand and to ensure the safety of the reactor, on the other hand, to improve the efficiency of heat exchange. Highly enriched 7LiOH with an abundance of over 99.9% is commonly used as a pH regulator in pressurized water reactors to maintain a neutral or weakly alkaline water environment for the steady operation of the reactor [3,4]. Obviously, lithium isotopes with natural abundance cannot meet the application needs of the nuclear industry and must be separated and enriched.
The separation of 6Li and 7Li is extremely difficult due to their very similar chemical properties. Lewis and MacDonald [5] first started research on lithium isotope separation in the 1930s by exchanging 6Li and 7Li between lithium amalgam and lithium salt solution. In the following decades, this lithium amalgam method was extensively investigated and applied in industry [6,7,8,9,10,11]. Since then, more lithium separation methods have been developed, including electrochemical separation [12,13,14,15,16,17], solvent extraction [18,19,20,21,22,23], ion exchange technique [24,25,26,27,28,29], laser separation technique [30,31,32,33,34,35] and so on. Among these, the electrochemical separation method and solvent extraction method have attracted more attention, and there is a lot of interest in system construction. For instance, Cui et al. [22,36,37] proposed a strategy for separating lithium isotopes by forming stable complex [Li(B15C5/B12C4)(H2O)][FeCl4] and developed a series of functionalized ionic liquids of grafted crown ethers for lithium isotope separation. Yan et al. [23,38] focused on the research and development of functionalized separation membranes for grafted crown ether and found that 6Li was enriched in the membrane phase in most cases, with the separation factor being up to 1.049.
The lithium amalgam method is the only separation method that is industrially applied at present. However, this method can generate great environmental hazards and needs to be replaced by more eco-friendly and high-efficiency methods such as the Electromigration method. Since the beginning of this century, the separation of isotopes by electromigration has attracted much attention, and a variety of lithium-ion (Li+) migration systems have been developed. For example, Takami et al. [15] used an electrolytic cell with graphite as the anode, LiCoO2 as the cathode, and LiClO4-ethylene carbonate as the electrolyte to separate lithium isotopes and found that 6Li was enriched in the cathode and 7Li was enriched in the electrolyte, with a separation factor of 1.02–1.03. In another example, Zhang et al. [39,40] used electrophoresis and electromigration methods to separate lithium isotopes in carbonic propylene and ionic organic liquid solutions and obtained separation factors of 1.03 and 1.05, respectively. Results of theoretical simulations show that large differences in the diffusion factors of 6Li and 7Li would lead to high separation factors.
However, in these single-phase systems, it is difficult to obtain a stable separation effect. To solve this problem, in our previous work, a biphasic system strategy of aqueous–organic solution was proposed, and ‘Organic solution containing Li+ (anolyte)| Ammonium chloride (NH4Cl) solution (catholyte)’ [41] and ‘Lithium salt solution (anolyte)|Ionic liquids (ILs)—Crown ether organic solution|NH4Cl solution (catholyte)’ [42] were developed. It is interesting that there is a reversal of the 7Li or 6Li enrichment effect in the catholyte with voltage increase [41], and the migration of Li+ is affected by the synergistic effect of the electric field, diffusion and chelation [42]. In addition, Wang et al. [43,44,45,46] confirmed that crown ether has multiple effects on Li+ migration and isotope separation and a separation factor of 1.40 or even 1.67 can be obtained under appropriate conditions.
In this method, there is no production of hazardous compounds (such as mercury), and the difficulty of handling a large number of feeds is avoided (compared to solvent extraction); this has the advantage of environmental protection and efficiency. Moreover, compared with the single-phase system, in the biphasic system, the separation of lithium isotopes does not rely on the kinetic process, but occurs at the interface of the two phases (thermodynamics process), which greatly improves the durability of the separation effect.
Furthermore, lithium isotope separation systems have also received much attention. For example, Zhang et al. [47,48,49] constructed the bromobenzene 15-crown-5/ionic liquids system to separate lithium isotopes via extraction of Li+. By using MD simulation and DFT calculation, they elucidated the mechanism of Li+ extraction and lithium isotope separation and provided new insights into the interphase transport mechanism of Li+. Ju et al. [50] studied the effect of the ratio of ionic liquid and diluent in an organic solution on the separation factor. Mao et al. [51,52] developed the 4-NO2-B15C5/[BMIm][NTf2] system and proposed a new strategy for the study of water content in Li-ion–crown ether complexes. Zhou [53] and Zhao [54] et al. explored the effects of the cavity size of crown ether on Li+ transport and lithium isotope separation during extraction and electromigration, respectively.
In order to expand the types of crown ethers that can be used for electromigration separation of lithium isotopes in the ‘biphasic system’, benzo-12-crown-4-ether (B12C4) is adopted as a phase transfer catalyst, and the rules of lithium isotope separation are further studied. The effects of electric field intensity, migration time, the concentration of lithium salt and the concentration of crown ether on Li+ transport and lithium isotope separation in ‘LiCl aqueous solution|B12C4-[EMIm][NTf2] organic solution|NH4Cl aqueous solution’ system are investigated.
2. Materials and Methods
The reagents used in the experiments are listed in Table 1. Information on the instruments used in the experiments is shown in Table 2.

Table 1.
Reagents used in the investigation.

Table 2.
Instruments used in the investigation.
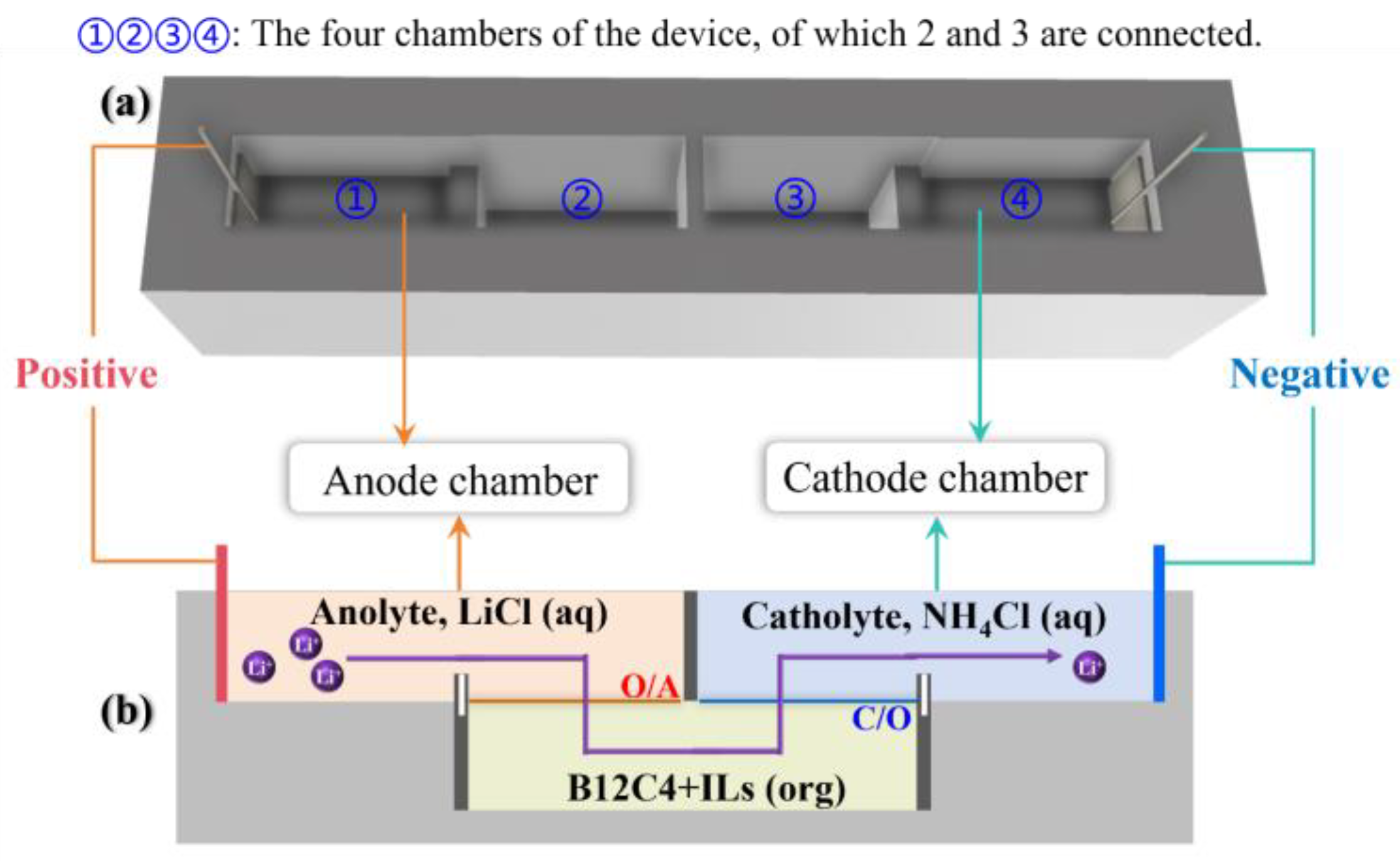
LiCl solution (6 mL, anolyte) is injected into the anode chamber (chamber ① shown in Figure 1a) and connected to the positive electrode of the DC power supply. NH4Cl solution (6 mL, catholyte) is injected into the cathode chamber (chamber ② shown in Figure 1a) and connected to the negative electrode of the DC power supply. The electrodes are made of platinum wire. The organic solution (6 mL) consists of [EMIm][NTf2] (30% by volume) and B12C4 dissolved in anisole (70% by volume).
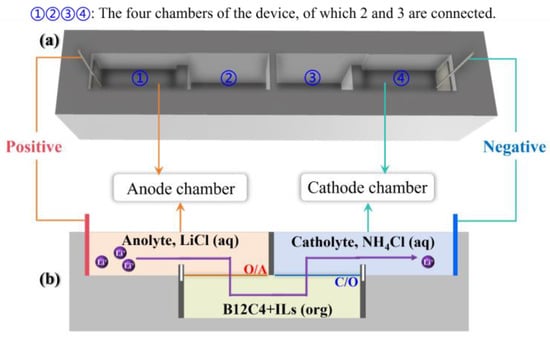
Figure 1.
System diagram: experimental device sketch (a); the biphasic system and Li+ flow (b).
The device can be divided into four parts: the lower half of chambers ② and ③ are connected, while the upper half is separated by a fixed plate. Before the solution is injected, ① and ②, ③ and ④ are separated by two movable plates. First, the organic solution is injected into the lower half of ② and ③, then the catholyte is injected into ④ and the upper half of ③ at the same time, and finally, the anolyte is injected into ① and the upper half of ② at the same time. After carefully removing the two movable plates, an anolyte–organic solution interface (O/A) is formed in ② (Figure 1b), and an organic solution–catholyte interface (C/O) is formed in ③ (Figure 1b).
As shown in Figure 1, the Li+ ions transfer into organic solution (O) from the anolyte (A) through the O/A interface and then migrate into the catholyte (C) through the C/O interface [55]. The whole system consists of three sections: anolyte, an organic solution consisting of crown ether and an ionic liquid, catholyte. The rules of Li+ transport and lithium isotope separation in the B12C4 system were explored under various experimental conditions with current (0–10 mA), migration time (1–24 h), B12C4 concentration (0–0.5 mol/L) and LiCl concentration (0.01–10 mol/L) being taken into account.
The Li+ concentration was measured by ICP-OES at a wavelength of 670.781 nm and a standard sample range of 0–20 mg/L. The organic solution sample was extracted three times with hydrochloric acid (1.000 mol/L) to transfer lithium ions in the organic solution to the hydrochloric acid for analysis. The concentration of all samples was diluted to within 0–20 mg/L.
The Δ7Li is calculated from the test results of MC-ICP-MS according to Equation (1).
where 7Li/6Li represents the ratio of 7Li content to 6Li content in the samples, the subscript ‘Sp’ represents the experimental sample, and ‘St’ represents the lithium isotope standard sample (L-SVEC lithium carbonate [56], 7Li/6Li = 12.1025 ± 0.0016, National Institute of Standards & Technology, USA).
The separation factor α is calculated by the Δ7Li using Equation (2)
where 6Li/7Li represents the ratio of 6Li content to 7Li content in the samples, subscripts ‘A’ and ‘B’ represent the two related phases. For example, α(O/A) refers to the separation factor between organic solution and anolyte. The larger the |α-1|, the better the separation effect between the two phases, and α = 1 indicates no separation effect.
Furthermore, Δ7Li was defined to represent the enrichment effect in a particular phase compared to another (Equation (3)), where subscripts ‘A’ and ‘B’ represent the two phases. For example, Δ7Li(O/A) < 0 or Δ7Li(O/A) > 0 indicates 6Li enrichment or 7Li enrichment in organic solution compared to the anolyte, respectively, and the greater the |Δ7Li|, the better the enrichment effect.
3. Results and Discussion
3.1. Driving Effect of Electric Field
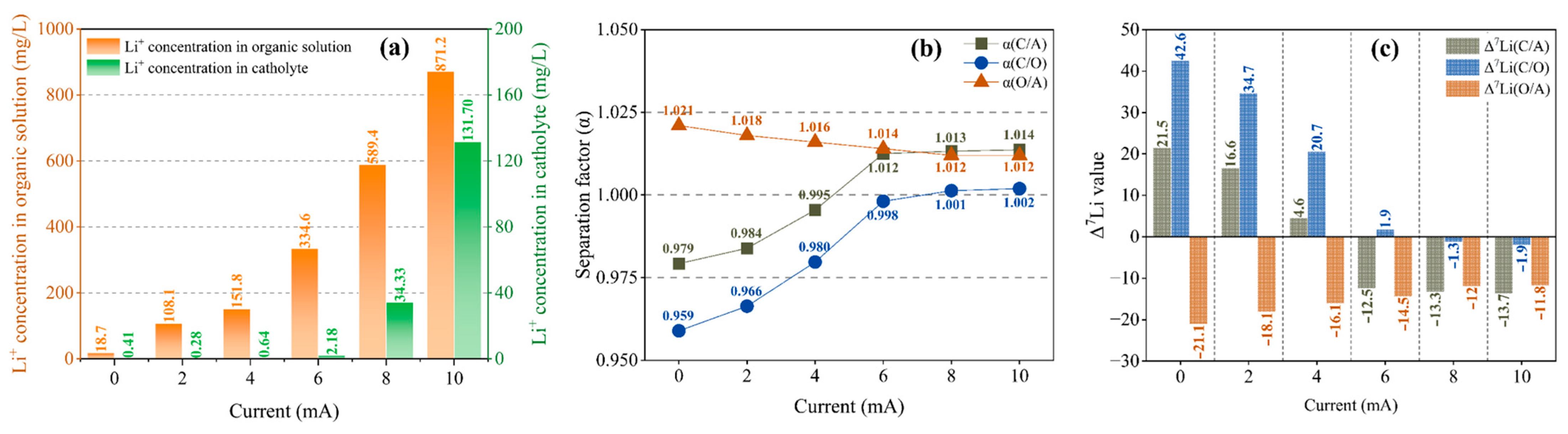
Figure 2a shows that the Li+ concentration in the organic solution increases uniformly with the increase in the current, and the Li+ concentration in the catholyte is extremely low in 0–6 mA, with small fluctuations. Only when the current reaches 8 mA does the Li+ concentration increase significantly, i.e., 2.18 mg/L, 34.33 mg/L and 131.70 mg/L at 6 mA, 8 mA and 10 mA, respectively. The decisive factor for the rate of Li+ migration into the catholyte is the dissociation of chelated Li+ in the organic solution. The above phenomenon shows that when the current reaches over 8 mA, the chelated Li+ in the organic solution begins to dissociate in large quantities, while Li+ migrates into the catholyte significantly, as shown in Figure 2a.
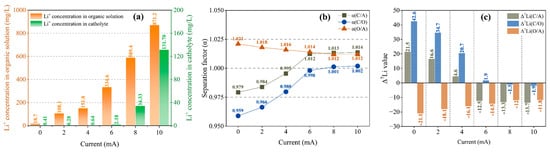
Figure 2.
Li+ concentration in an organic solution and a catholyte (a); separation factor (b); and Δ7Li (c) at different currents at a migration time of 12 h and B12C4 and LiCl concentrations of 0.2 mol/L and 1 mol/L.
For the separation effect between anolyte and organic solution, α(O/A) is always greater than 1 but shows a slowly decreasing trend as the current increases (Figure 2b), which indicates a weakening trend of the separation effect. Figure 2c shows that the Δ7Li(O/A) value is always negative, indicating that the organic solution is always enriched with 6Li compared to the anolyte. However, with the increase in the current, the enrichment effect decreases gradually. For the separation effect between organic solution and catholyte, as shown in Figure 2b, α(C/O) increases and approaches 1 as the current increases from 0 mA to 6 mA, which shows that the separation effect is gradually decreasing. When the current reaches 8 mA, a small increase in α(C/O) is observed, and it begins to exceed 1. However, the separation effect is still weak after the current is increased to 10 mA. As shown in Figure 2c, the Δ7Li(C/O) value is positive within 0–6 mA but decreases and approaches 0 as the current increases, indicating a weakening 7Li enrichment effect in the catholyte. When the current reaches 8 mA, Δ7Li becomes negative with a small absolute value, indicating that there is a very weak 6Li enrichment effect in the catholyte.
Thus, there is a transition in the enrichment effect in the catholyte at a current of 6–8 mA for which the α(C/O) and Δ7Li(C/O) values change from 0.998 and 1.9 (6 mA) to 1.001 and −1.3 (8 mA), respectively, indicating a change from 7Li enrichment (0–6 mA) to 6Li enrichment (≥8 mA). In other words, the separation effect between organic solution and catholyte is reduced with the increase in the current and tends to be very weak above 8 mA. Compared to the organic solution, the catholyte is enriched with 7Li at 0–6 mA and with 6Li above 8 mA. In our previous investigation, we proposed that in this method, when the voltage rises to a certain value, the probability of dissociation of chelated 6Li will exceed that of chelated 7Li around the C/O interface, resulting in 6Li enrichment in the catholyte. Here, as the current increases above the transition value, the weakening and instability of the separation effect are likely to be correlated to the corresponding voltage: it is observed in the experiment that although the current is maintained at 10 mA, the voltage always drops rapidly within the first 1 h after the start of the migration, and to below 10 V within 8 h, which is close to the ‘transition voltage’ [54].
Therefore, the separation of lithium isotopes in this method is more affected by voltage than by current. On the other hand, compared with constant voltage, the Li+ migration rate is larger under constant current, but the effect of lithium isotope separation is not easy to control. In view of this, we suggest a strategy of using constant current first and then turning to constant voltage mode at a certain point before the voltage drops to 10 V.
3.2. Influence of Migration Time
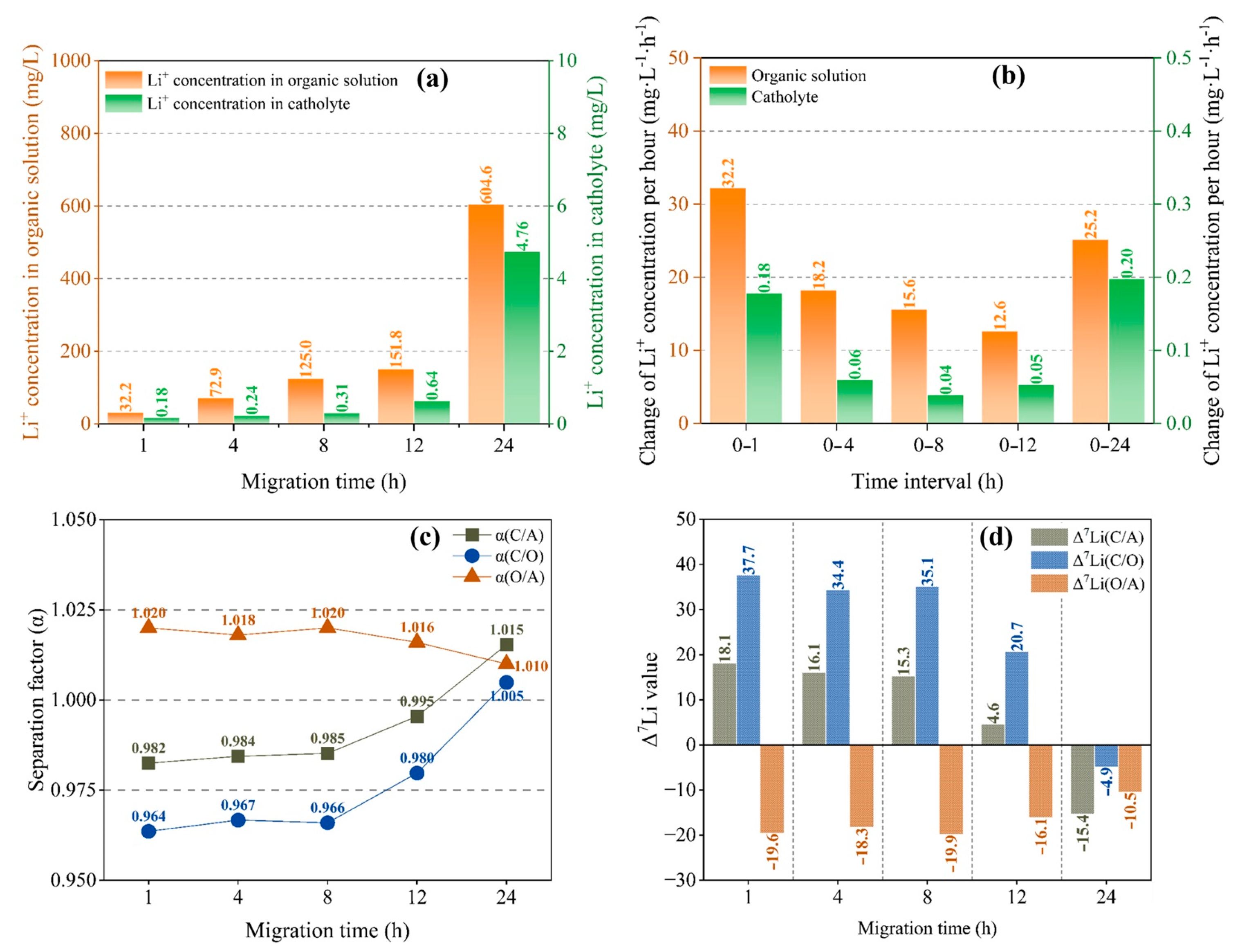
It can be seen from Figure 3a that the concentration of Li+ in an organic solution and catholyte changes with migration time in the same way, with slow growth at 0–12 h and rapid growth at 12–24 h. For the organic solution, although the concentration of Li+ increases with the duration of migration in the first 12 h (Figure 3b), the quantity of Li+ migrating into the organic solution per unit time decreases, which indicates a slowing down of the migration rate of lithium ions. However, as time goes by, the migration rate of lithium ions increases again. The change in Li+ content in the catholyte (Figure 3b) is similar to that in the organic solution.
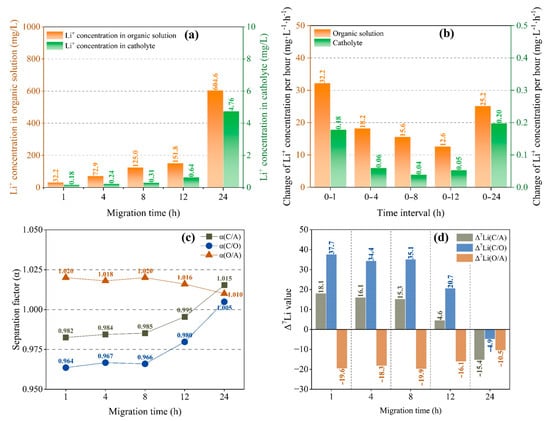
Figure 3.
Li+ concentration in organic solution and catholyte with different migration times (a); average Li+ concentration change in organic solution and catholyte per unit time (b); separation factor (c) and Δ7Li (d) with different migration times at a current of 4 mA and B12C4 and LiCl concentrations of 0.2 mol/L and 1 mol/L.
As shown in Figure 3, during the first 8 h, the α and Δ7Li did not change significantly. Furthermore, for the separation effect between anolyte and organic solution, α is always larger than 1 and decreases slightly with migration time. For the separation effect between organic solution and catholyte, α is initially less than 1, then rapidly approaches 1 as the migration time increases and exceeds 1 after 24 h (Figure 3c). Figure 3d shows that the Δ7Li remained almost invariant for the first 8 h. For organic solutions, Δ7Li(O/A) is always negative, indicating enrichment of 6Li in the organic solution, and its absolute value decreases slightly after 8 h, indicating that the enrichment effect of 6Li is slightly weakened. For the catholyte, Δ7Li(C/O) is initially greater than 0, indicating enrichment of 7Li in the catholyte. After 8 h, Δ7Li(C/O) rapidly decreases toward 0 and is negative at 24 h (20.7 at 12 h and −4.9 at 24 h), indicating enrichment of 6Li in the catholyte.
Hence, there is a change in the enrichment effect from 7Li enrichment to 6Li enrichment at a certain time point in 12–24 h in the catholyte. The change is potentially related to Li+ migration and dissociation of chelated Li+. As shown in Figure 3a, the migration of Li+ is greatly accelerated within 12 h, and Li+ concentration in catholyte also rapidly increases from 0.64 mg/L to 4.76 mg/L. When the migration time reaches 12–24 h, the dissociation of chelated Li+ starts becoming relatively easier; thus, in order to obtain both 6Li enrichment and high Li+ content in the catholyte, and considering the stability of the system, a migration time of at least 12 h is required.
3.3. Chelating Effect of B12C4
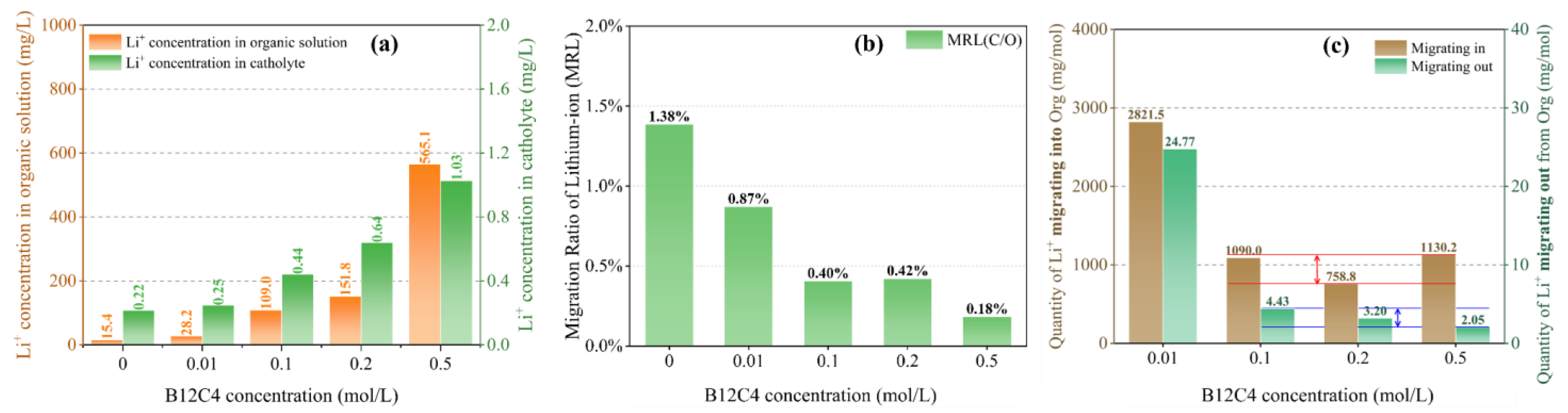
As shown in Figure 4a, the chelating effect of crown ether is conducive to the migration of Li+ into the organic solution. The concentration of lithium ions in the organic solution increases with the increase of B12C4 concentration. In order to further understand the rule of migration of Li+ from the organic solution, the ‘Migration Ratio of Lithium-ion (MRL)’ is defined to represent the ratio of total Li+ migrating from the organic solution (C/O) to total Li+ migrating into the organic solution (O/A). It is not difficult to see that although the concentration of lithium ions in the catholyte increases with the increase in the content of B12C4 (Figure 4a), the MRL of Li+ migrating from the organic solution to the catholyte actually decreases (Figure 4b). The chelating effect of crown ether promotes Li+ to migrate into the organic solution while hindering the out-migration of Li+, either under constant voltage or constant current conditions. Moreover, the increase in Li+ concentration in the catholyte can be accounted for by diffusion caused by the concentration difference between the organic solution and the catholyte, as well as the electric effect.
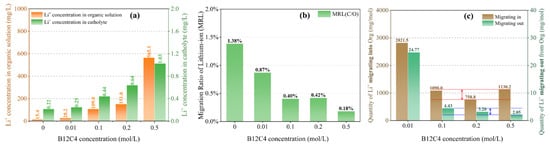
Figure 4.
Li+ concentration in organic solution and catholyte (a); the MRL (b) and change in Li+ quantity per mole of crown ether (c) with different B12C4 concentrations at a current of 4 mA, a migration time of 12 h and a LiCl concentration of 1 mol/L (The red and blue lines indicate the fluctuation ranges).
Another interesting phenomenon here is the relationship between the quantity of Li+ migrating in the organic solution and the concentration of B12C4. When the concentration of crown ether goes beyond 0.1 mol/L, the quantity of Li+ migrating into and out of the organic solution per mole of B12C4 is generally invariant, i.e., it is independent of the content of B12C4 (Figure 4c). However, the concentration of B12C4 is much higher than that of Li+ in organic solution (0.1 mol/L B12C4 vs. 0.016 mol/L Li+, 0.2 mol/L B12C4 vs. 0.022 mol/L Li+, and 0.5 mol/L B12C4 vs. 0.081 mol/L Li+), indicating that most crown ethers are not complexed with Li+. This is the reason for the above phenomenon: although the content of B12C4 is increased, the amount that can chelate with Li+ is limited.
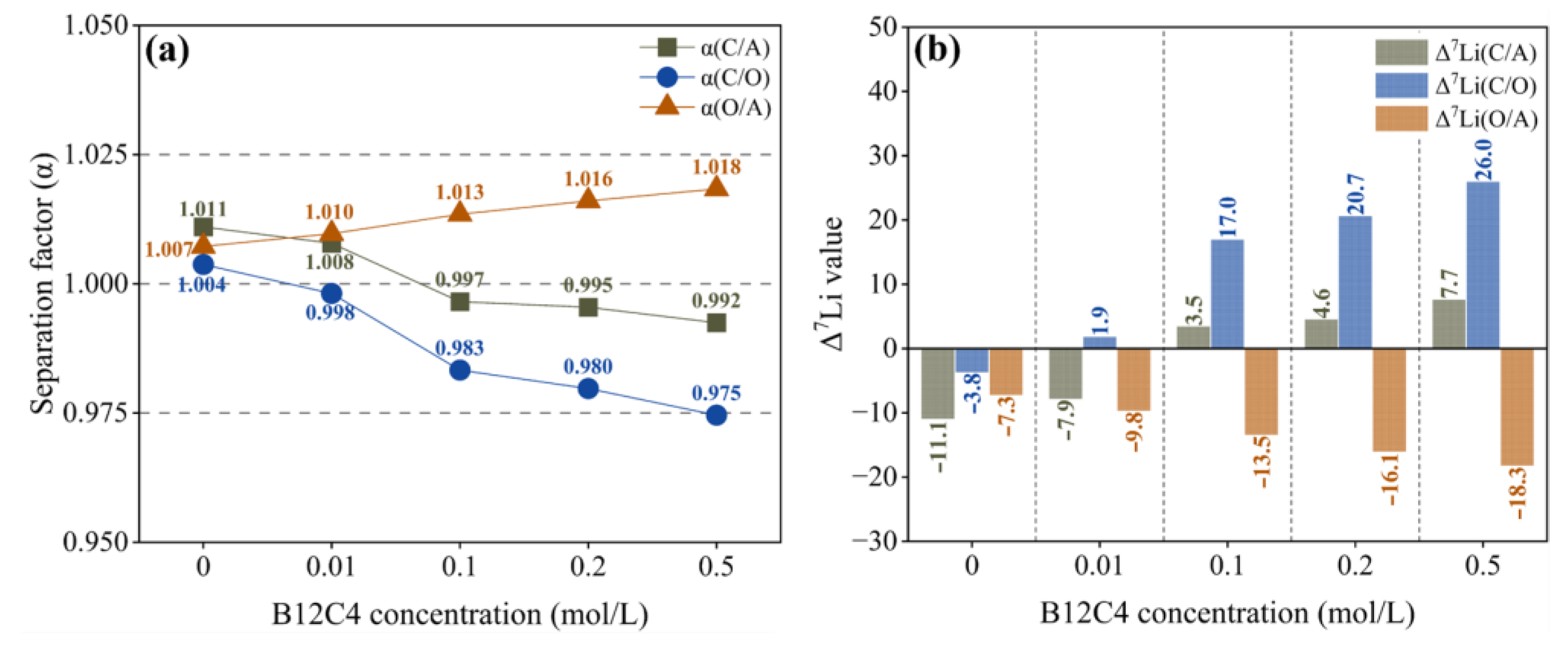
As shown in Figure 5a, α(O/A) changes little with the increase in B12C4 concentration, indicating that the separation effect between anolyte and organic solution is hardly affected by the concentration of B12C4. When the concentration of crown ether exceeds 0.01 mol/L, α(C/O) decreases with the increase in crown ether content, indicating that the separation effect becomes stronger and stronger. However, the Δ7Li(C/O) is greater than 0, indicating enrichment of 7Li in the catholyte (Figure 5b). In other words, with the increase in B12C4 concentration in the organic solution, the catholyte becomes more and more enriched with 7Li, which is caused by the enhanced ability of the organic solution to retain 6Li. Considering lithium ion migration and lithium isotope separation together, we recommend a B12C4 concentration of 0.2 mol/L.
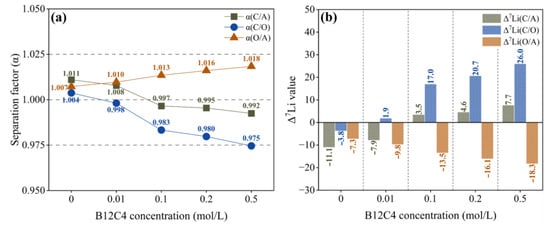
Figure 5.
Separation factor (a) and Δ7Li (b) with different B12C4 concentrations.
3.4. Diffusion of Li+ with Different LiCl Concentrations
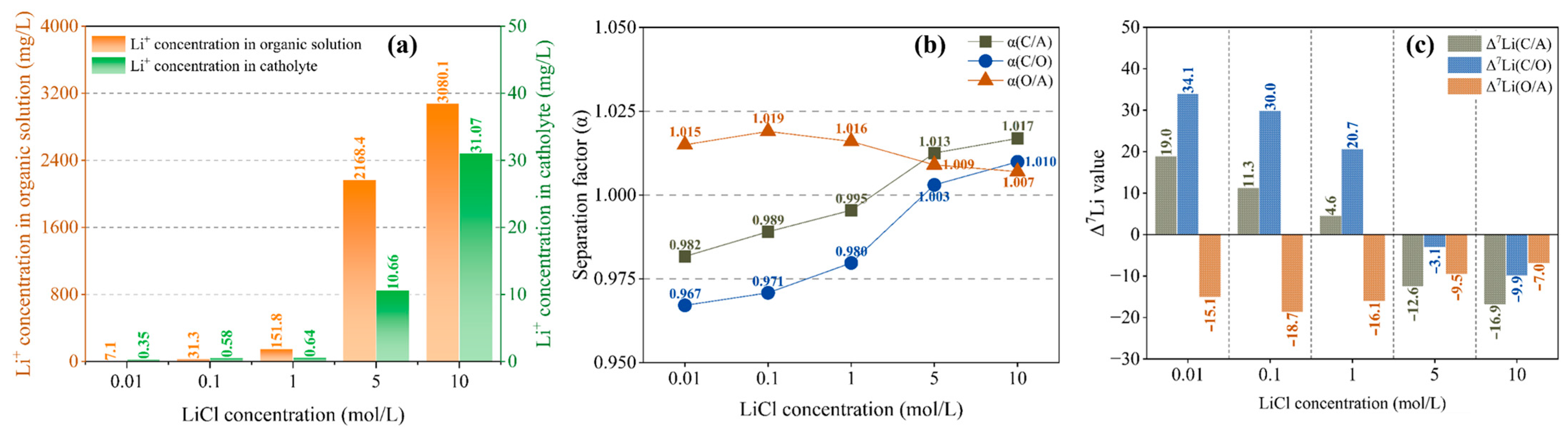
In addition to chelating effect and electric field force, the migration of Li+ is also affected by diffusion effect, which is closely correlated to the Li+ concentration difference on both sides of the interface. Therefore, increasing the initial concentration of lithium salt in the anolyte is conducive to Li+ migration. It is not difficult to see from Figure 6a that with the increase in LiCl concentration, Li+ molecules moving into the organic solution continue to increase. When the LiCl concentration is lower than 1 mol/L, the change is not obvious, but when it reaches 5 mol/L, there is a significant increase (from 151.8 mol/L to 2168.4 mol/L). As shown in Figure 6a, Li+ concentration in the catholyte is closely correlated to that in the organic solution, which also increases significantly with an increase in Li+ concentration in the organic solution. This suggests that the diffusion effect becomes dominant after the initial LiCl concentration reaches about 5 mol/L.
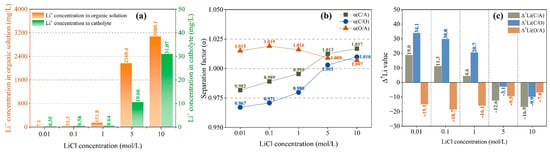
Figure 6.
Li+ concentration in organic solution and catholyte (a); separation factor (b) and Δ7Li (c) with different LiCl concentrations at a current of 4 mA, a migration time of 12 h and a B12C4 concentration of 0.2 mol/L.
As shown in Figure 6b, α(O/A) is always greater than 1, and with the increase in LiCl concentration, α(O/A) does not change much but only slightly decreases, indicating that the separation effect between anolyte and organic solution is slightly weakened. For the separation effect between organic solution and catholyte, α(C/O) is less than 1 when c(LiCl) ≤ 1 mol/L, and with the increase in LiCl concentration, α(C/O) gradually approaches 1 and exceeds 1 as the LiCl concentration reaches 5 mol/L, indicating a transition of separation effect. As shown in Figure 6c, when LiCl concentration is less than 1, Δ7Li(O/A) is negative, indicating that the organic solution is enriched with 6Li compared with the anolyte, and Δ7Li(C/O) is greater than 0, indicating that the catholyte is enriched with 7Li compared with the organic solution. When the LiCl concentration reaches 5 mol/L, the Δ7Li(C/O) value changes from positive to negative, indicating conversion of 7Li enrichment to 6Li enrichment in the catholyte.
The change in enrichment effect is consistent with the change in Li+ concentration (Figure 6a), and both are correlated to the migration rate of lithium ions. The migration of Li+ caused by concentration diffusion often occurs when the Li+ concentration suddenly increases in the organic solution, accompanied by 6Li enrichment in the catholyte, which is due to the fact that chelated 6Li is more easily dissociated than chelated 7Li. It can also be inferred that a rather high concentration difference between the organic solution and the catholyte is required if sustained 6Li enrichment is to be obtained in the catholyte; this was demonstrated in a 5-stage electromigration experiment [55]. For this reason, we recommend that the initial LiCl concentration be at least 1 mol/L.
3.5. Li+ Migration Along with Isotope Separation
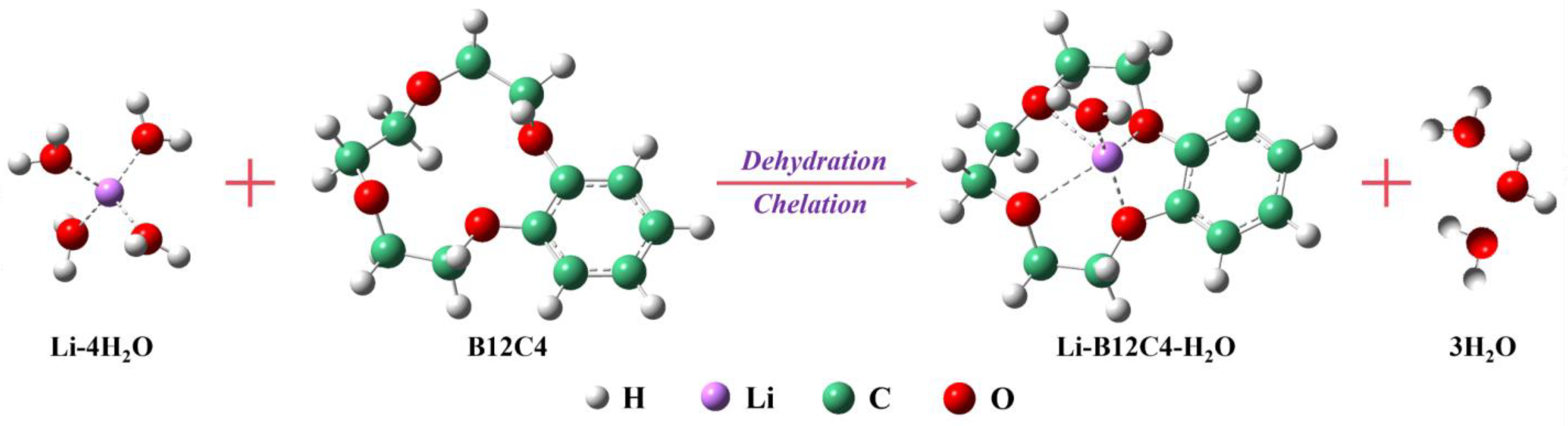
According to our previous research [42], in this method, the separation of lithium isotopes usually occurs during lithium-ion migration. During the process of migration, Li+ is subjected to chelating, the electric field and diffusion. The direction of the electric field and diffusion is the same as that of Li+ migration, which promotes the transfer of Li+. However, chelating always points towards the organic solution, such that chelating promotes Li+ migration into the organic solution but impedes Li+ migration out of the organic solution. The process of complex formation is shown in Figure 7.
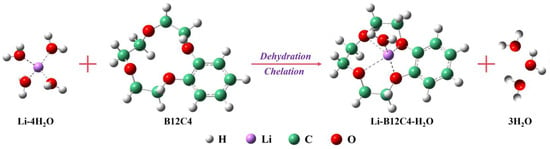
Figure 7.
Formation of Li-B12C4-H2O complex.
According to the theory of isotope separation [57,58], the difference in the strength of the Li-O bond leads to the enrichment of the heavy isotope (7Li) in the phase with a strong Li-O bond (aqueous phase) and the enrichment of the light isotope (6Li) in the phase with a weak Li-O bond (organic phase). When the electric field is weak, the catholyte is enriched with 7Li (Figure 2c), and the migration of Li+ is mainly dominated by diffusion and chelating. The electric field begins to play a dominant role in the migration of Li+ when the current exceeds a certain value. At this moment, the chelated lithium ions begin to dissociate significantly, and the dissociation of chelated 6Li under the electric field is easier than chelated 7Li; thus, the catholyte begins to enrich 6Li, as previously analyzed [54]. Similarly, the change in enrichment effect with migration time can also be attributed to the electric field; that is, with the extension of ‘electrifying time’, the catholyte changes from 7Li enrichment to 6Li enrichment.
To sum up, the concentration diffusion and chelating by crown ethers seem to play a decisive role in the migration of Li+, while the electric field plays a decisive role in the separation of lithium isotopes. In order to obtain better 6Li enrichment and a higher Li+ concentration at the same time, it is necessary to pay attention to the concentration of lithium salt and the intensity of the electric field. We recommend constant voltage conditions rather than constant current. In addition, compared with one of our previous works [17], in single-phase systems, strong separation effects can usually be obtained at the beginning of migration; however, the separation factor decreases rapidly with the migration of lithium ions. In the biphasic system we presented, the separation factor of the whole system is relatively stable (α(C/A)), which is an advantage of this work.
4. Conclusions
In this article, the rules of lithium isotope separation and Li+ migration were investigated in a three-stage electromigration system consisting of ‘LiCl aqueous solution (anolyte)|B12C4-[EMIm][NTf2] organic solution|NH4Cl aqueous solution (catholyte)’. The main conclusions are as follows: (1) The current, migration time and LiCl concentration mainly affect the separation effect of the C/O interface and the enrichment effect of the catholyte, while the O/A interface and organic solution are less affected. (2) There is a transition from 7Li enrichment to 6Li enrichment in the catholyte as the current, migration time and LiCl concentration increase. (3) Taking both lithium-ion transport efficiency and lithium isotope separation effect into consideration, a current larger than 6 mA, a duration of migration larger than 12 h, a LiCl concentration larger than 1 mol/L and a B12C4 concentration of about 0.2 mol/L are preferable in the electromigration process. The work provides an important reference for the electromigration separation of lithium isotopes with a biphasic system and promotes the industrial application of this method.
Author Contributions
Conceptualization, J.S. and Z.Z.; methodology, J.S. and Z.Z.; software, P.Z.; validation, Z.Z., L.M. and T.Z.; formal analysis, X.L.; investigation, C.Y.; resources, H.L.; data curation, W.S.; writing—original draft preparation, Z.Z.; writing—review and editing, Z.Z., J.S. and W.S.; visualization, Z.Z. and W.S.; supervision, J.S.; project administration, J.S.; funding acquisition, W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 22378409), CAS Project for Young Scientists in Basic Research (YSBR-039), and The Open Project of Salt Lake Chemical Engineering Research Complex, Qinghai University (2024-DXSSKF-02).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors thank Hao Xuan and Shiwu Xuan from Beijing Extraction Application Technology Research Institute for technical support on the electromigration device.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Michiels, E.; De Bièvre, P. Absolute isotopic composition and the atomic weight of a natural sample of lithium. Int. J. Mass Spectrom. Ion Phys. 1983, 49, 265–274. [Google Scholar] [CrossRef]
- Symons, E.A. Lithium Isotope Separation: A Review of Possible Techniques. Sep. Sci. Technol. 1985, 20, 633–651. [Google Scholar] [CrossRef]
- Zinkle, S.J.; Busby, J.T. Structural materials for fission & fusion energy. Mater. Today 2009, 12, 12–19. [Google Scholar]
- Le Calvar, M.; De Curières, I. 15—Corrosion issues in pressurized water reactor (PWR) systems. In Nuclear Corrosion Science and Engineering; Féron, D., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 473–547. [Google Scholar]
- Lewis, G.N.; Macdonald, R.T. The Separation of Lithium Isotopes. J. Am. Chem. Soc. 1936, 58, 2519–2524. [Google Scholar] [CrossRef]
- Palko, A.A.; Drury, J.S.; Begun, G.M. Lithium isotope separation factors of some two-phase equilibrium systems. J. Chem. Phys. 1976, 64, 1828–1837. [Google Scholar] [CrossRef]
- Chen, D.; Chang, Z.; Nomura, M.; Fujii, Y. Isotope effects of magnesium in amalgam/organic solution systems. J. Chem. Soc. Faraday Trans. 1997, 93, 2395–2398. [Google Scholar] [CrossRef]
- Black, J.R.; Umeda, G.; Dunn, B.; McDonough, W.F.; Kavner, A. Electrochemical Isotope Effect and Lithium Isotope Separation. J. Am. Chem. Soc. 2009, 131, 9904–9905. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.I.; Urey, H.C. Fractionation of the Lithium and Potassium Isotopes by Chemical Exchange with Zeolites. J. Chem. Phys. 1938, 6, 429–438. [Google Scholar] [CrossRef]
- Okuyama, K.; Okada, I.; Saito, N. The isotope effects in the isotope exchange equilibria of lithium in the amalgam-solution system. J. Inorg. Nucl. Chem. 1973, 35, 2883–2895. [Google Scholar] [CrossRef]
- Okada, I.; Okuyama, K.; Miyamoto, T.; Tomita, I.; Saito, N. Enrichment of 7Li by countercurrent electromigration of molten lithium nitrate. J. Inorg. Nucl. Chem. 1973, 35, 2957–2969. [Google Scholar] [CrossRef]
- Hoshino, T.; Terai, T. Basic technology for 6Li enrichment using an ionic-liquid impregnated organic membrane. J. Nucl. Mater. 2011, 417, 696–699. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, Q.; Zou, W.; Fang, J.; Yang, Z.; Xu, T. Dibenzo-15-crown-5-based Tröger’s Base membrane for 6Li+/7Li+ separation. Sep. Purif. Technol. 2023, 309, 122990. [Google Scholar] [CrossRef]
- Honda, S.; Shin-Mura, K.; Sasaki, K. Lithium isotope enrichment by electrochemical pumping using solid lithium electrolytes. J. Ceram. Soc. Jpn. 2018, 126, 331–335. [Google Scholar] [CrossRef]
- Takami, Y.; Yanase, S.; Oi, T. Observation of Lithium Isotope Effects Accompanying Electrochemical Release from Lithium Cobalt Oxide. Z. Fur Naturforschung A 2013, 68, 73–78. [Google Scholar] [CrossRef]
- Okano, K.; Takami, Y.; Yanase, S.; Oi, T. Lithium Isotope Effects upon Electrochemical Release from Lithium Manganese Oxide. Energy Procedia 2015, 71, 140–148. [Google Scholar] [CrossRef]
- Zhang, Z.; Murali, A.; Sarswat, P.K.; Free, M.L. High-efficiency lithium isotope separation by electrochemical deposition and intercalation with electrochemical isotope effect in propylene carbonate and [BMIM][DCA] ionic liquid. Electrochim. Acta 2020, 361, 137060. [Google Scholar] [CrossRef]
- Taylor, T.I.; Urey, H.C. On the Electrolytic and Chemical Exchange Methods for the Separation of the Lithium Isotopes. J. Chem. Phys. 1937, 5, 597–598. [Google Scholar] [CrossRef]
- Martin, F.S.; Holt, R.J.W. Liquid-liquid extraction in inorganic chemistry. Q. Rev. Chem. Soc. 1959, 13, 327–352. [Google Scholar] [CrossRef]
- Nishizawa, K.; Ishino, S.-I.; Watanabe, H.; Shinagawa, M. Lithium Isotope Separation by Liquid-Liquid Extraction Using Benzo-15-Crown-5. J. Nucl. Sci. Technol. 1984, 21, 694–701. [Google Scholar] [CrossRef]
- Zhang, Q.; Jia, Y.; Sun, J.; Zhang, P.; Huang, C.; Wang, M.; Xue, Z.; Wang, C.; Shao, F.; Tong, F.; et al. Lithium isotope separation effect of N-phenylaza-15-crown-5. J. Mol. Liq. 2021, 330, 115467. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Kang, J.; Yin, C.; Guo, Y.; He, H.; Cheng, F. A novel ion-pair strategy for efficient separation of lithium isotopes using crown ethers. Sep. Purif. Technol. 2021, 274, 118989. [Google Scholar] [CrossRef]
- Yan, F.; Pei, H.; Pei, Y.; Li, T.; Li, J.; He, B.; Cheng, Y.; Cui, Z.; Guo, D.; Cui, J. Preparation and Characterization of Polysulfone-graft-4′-aminobenzo-15-crown-5-ether for Lithium Isotope Separation. Ind. Eng. Chem. Res. 2015, 54, 3473–3479. [Google Scholar] [CrossRef]
- Inoue, Y.; Kanzaki, Y.; Abe, M. Isotopic separation of Lithium using Inorganic Ion Exchangers. J. Nucl. Sci. Technol. 1996, 33, 671–672. [Google Scholar] [CrossRef]
- Lee, D.A.; Begun, G.M. The Enrichment of Lithium Isotopes by Ion-exchange Chromatography. I. The Influence of the Degree of Crosslinking on the Separation Factor. J. Am. Chem. Soc. 1959, 81, 2332–2335. [Google Scholar] [CrossRef]
- Kakihana, H.; Nomura, T.; Mori, Y. The separation factor of lithium isotopes with ion exchangers. J. Inorg. Nucl. Chem. 1962, 24, 1145–1151. [Google Scholar] [CrossRef]
- Fujine, S.; Saito, K.; Shiba, K. Lithium Isotope Separation by Displacement Chromatography Using Cryptand Resin. J. Nucl. Sci. Technol. 1983, 20, 439–440. [Google Scholar] [CrossRef]
- Nishizawa, K.; Watanabe, H.; Ishino, S.-i.; Shinagawa, M. Lithium Isotope Separation by Cryptand (2B, 2, 1) Polymer. J. Nucl. Sci. Technol. 1984, 21, 133–138. [Google Scholar] [CrossRef]
- Suzuki, T.; Zhang, M.H.; Nomura, M.; Tsukahara, T.; Tanaka, M. Engineering study on lithium isotope separation by ion exchange chromatography. Fusion Eng. Des. 2021, 168, 112478. [Google Scholar] [CrossRef]
- Shimazu, M.; Takubo, Y.; Maeda, Y. Selective Two-Step Photoionization of Lithium Atoms. Jpn. J. Appl. Phys. 1977, 16, 1275. [Google Scholar] [CrossRef]
- Arisawa, T.; Maruyama, Y.; Suzuki, Y.; Shiba, K. Lithium isotope separation by laser. Appl. Phys. B 1982, 28, 73–76. [Google Scholar] [CrossRef]
- Saleem, M.; Hussain, S.; Rafiq, M.; Baig, M.A. Laser isotope separation of lithium by two-step photoionization. J. Appl. Phys. 2006, 100, 053111. [Google Scholar] [CrossRef]
- Saleem, M.; Hussain, S.; Zia, M.A.; Baig, M.A. An efficient pathway for Li6 isotope enrichment. Appl. Phys. B 2007, 87, 723–726. [Google Scholar] [CrossRef]
- Arisawa, T.; Suzuki, Y.; Maruyama, Y.; Shiba, K. Isotope separation by laser-enhanced chemical reaction. Chem. Phys. 1983, 81, 473–479. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, P.P.; Zhou, P.P.; Chen, S.L.; Zhou, Z.Q.; Huang, Y.; Qi, X.Q.; Yan, Z.C.; Shi, T.Y.; Drake, G.W.F.; et al. Measurement of Hyperfine Structure and the Zemach Radius in 6Li+ Using Optical Ramsey Technique. Phys. Rev. Lett. 2023, 131, 103002. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Fan, Y.; Kang, J.; Yin, C.; Ding, W.; He, H.; Cheng, F. Novel class of crown ether functionalized ionic liquids with multiple binding sites for efficient separation of lithium isotopes. J. Mol. Liq. 2023, 376, 121412. [Google Scholar] [CrossRef]
- Cui, L.; Gao, R.; Zhang, Q.; Jiang, K.; He, H.; Ding, W.-L.; Cheng, F. Benzo-15-Crown-5 Functionalized Ionic Liquids with Enhanced Stability for Effective Separation of Lithium Isotopes: The Effect of Alkyl Chain Length. ACS Sustain. Chem. Eng. 2024, 12, 1221–1232. [Google Scholar] [CrossRef]
- Yan, F.; Liu, Y.; Wang, M.; Yang, B.; Pei, H.; Li, J.; Cui, Z.; He, B. Preparation of polysulfone-graft-monoazabenzo-15-crown-5 ether porous membrane for lithium isotope separation. J. Radioanal. Nucl. Chem. 2018, 317, 111–119. [Google Scholar] [CrossRef]
- Zhang, Z.; Sarswat, P.K.; Murali, A.; Free, M.L. Investigation on Lithium Isotope Fractionation with Diffusion, Electrochemical Migration, and Electrochemical Isotope Effect in PEO-PC Based Gel Electrolyte. J. Electrochem. Soc. 2019, 166, E145. [Google Scholar] [CrossRef]
- Zhang, Z.; Murali, A.; Sarswat, P.K.; Free, M.L. High-efficiency lithium isotope separation in an electrochemical system with 1-butyl-3-methylimidazolium dicyanamide, 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, and diethyl carbonate as the solvents. Sep. Purif. Technol. 2020, 253, 117539. [Google Scholar] [CrossRef]
- Wang, M.; Sun, J.; Zhang, P.; Huang, C.; Zhang, Q.; Shao, F.; Jing, Y.; Jia, Y. Lithium isotope separation by electromigration. Chem. Phys. Lett. 2020, 746, 137290. [Google Scholar] [CrossRef]
- Huang, C.; Sun, J.; Wang, C.; Zhang, Q.; Wang, M.; Zhang, P.; Xue, Z.; Jing, Y.; Jia, Y.; Shao, F. Lithium Isotope Electromigration Separation in an Ionic Liquid–Crown Ether System: Understanding the Role of Driving Forces. Ind. Eng. Chem. Res. 2022, 61, 4910–4919. [Google Scholar] [CrossRef]
- Wang, C.; Ju, H.; Zhou, X.; Zhang, P.; Xue, Z.; Mao, L.; Shao, F.; Jing, Y.; Jia, Y.; Sun, J. Separation of lithium isotopes: Electromigration coupling with crystallization. J. Mol. Liq. 2022, 355, 118911. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, P.; Huang, C.; Zhang, Q.; Ju, H.; Xue, Z.; Shao, F.; Li, B.; Mao, L.; Jing, Y.; et al. Electromigration Separation of Lithium Isotopes: The Effect of Electrode Solutions. J. Electrochem. Soc. 2022, 169, 016516. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, P.; Ju, H.; Xue, Z.; Zhou, X.; Mao, L.; Shao, F.; Zou, X.; Jing, Y.; Jia, Y.; et al. Electromigration separation of lithium isotopes: The multiple roles of crown ethers. Chem. Phys. Lett. 2022, 787, 139265. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, P.; Meng, Q.; Xue, Z.; Zhou, X.; Ju, H.; Mao, L.; Shao, F.; Jing, Y.; Jia, Y.; et al. Electromigration separation of lithium isotopes: The effect of electrolytes. J. Environ. Chem. Eng. 2023, 11, 109933. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, C.; Xue, Z.; Mao, L.; Sun, J.; Shao, F.; Qi, M.; Jing, Y.; Jia, Y. Extraction separation of lithium isotopes with Bromobenzene-15-crown-5/ionic liquids system: Experimental and theoretical study. J. Mol. Liq. 2022, 364, 120020. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, M.; Sun, J.; Shao, F.; Jia, Y.; Jing, Y. Lithium Isotope Green Separation Using Water Scrubbing. Chem. Lett. 2019, 48, 1541–1543. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, Z.; Wang, C.; Sun, J.; Shao, F.; Zou, X.; Li, B.; Qi, M.; Jing, Y.; Jia, Y. Mechanisms of ionic liquids on the enhancement of interfacial transport of lithium ions in crown ether system. J. Clean. Prod. 2022, 366, 132782. [Google Scholar] [CrossRef]
- Ju, H.; Wang, C.; Meng, Q.; Mao, L.; Zhou, X.; Zhang, P.; Xue, Z.; Shao, F.; Jing, Y.; Jia, Y.; et al. Electromigration separation of lithium isotopes: The effect of ionic liquid ratios. J. Mol. Liq. 2024, 393, 123526. [Google Scholar] [CrossRef]
- Mao, L.; Zhang, P.; Ju, H.; Zhou, X.; Xue, Z.; Wang, C.; Sun, J.; Jia, Y.; Shao, F.; Zou, X.; et al. Solvent extraction for lithium isotope separation by 4-NO2-B15C5/[BMIm][NTf2] system. J. Mol. Liq. 2022, 367, 120357. [Google Scholar] [CrossRef]
- Mao, L.; Zhou, X.; Zheng, T.; Li, X.; Wang, X.; Zhao, Z.; Sun, W.; Zhang, P.; Sun, J. A novel strategy for water content analysis in (B12C4/B15C5/B18C6-[EMIm][NTf2])-LiNTf2 extraction system: Quantitative calculation and theoretical study. J. Mol. Liq. 2024, 414, 126157. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, P.; Meng, Q.; Mao, L.; Ju, H.; Shao, F.; Jing, Y.; Jia, Y.; Wang, S.; Zou, X.; et al. The extraction method for the separation of lithium isotopes using B12C4/B15C5/B18C6-ionic liquid systems. N. J. Chem. 2023, 47, 1916–1924. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, X.; Meng, Q.; Zhang, P.; Shao, F.; Li, X.; Li, H.; Mao, L.; Zheng, T.; Jing, Y.; et al. Electromigration separation of lithium isotopes with B12C4, B15C5 and B18C6 systems. N. J. Chem. 2024, 48, 6676–6687. [Google Scholar] [CrossRef]
- Ju, H. Separation System and Separation Law of Lithium Isotope Byelectromigration Method. Master’s Thesis, University of Chinese Academy of Sciences, Xining, China, 2023. [Google Scholar]
- Flesch, G.D.; Anderson, A.R.; Svec, H.J. A secondary isotopic standard for 6Li/7Li determinations. Int. J. Mass Spectrom. Ion Phys. 1973, 12, 265–272. [Google Scholar] [CrossRef]
- Urey, H.C. The thermodynamic properties of isotopic substances. J. Chem. Soc. 1947, 562–581. [Google Scholar] [CrossRef]
- Betts, R.H.; Bron, J. A Discussion of Partial Isotope Separation by Means of Solvent Extraction. Sep. Sci. 1977, 12, 635–639. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).