Assessment of Bioactive Compounds and Antioxidant Activity of Barley Sprouts
Abstract
1. Introduction
2. Materials and Methods
2.1. Barley Sprout Sample and Chemicals
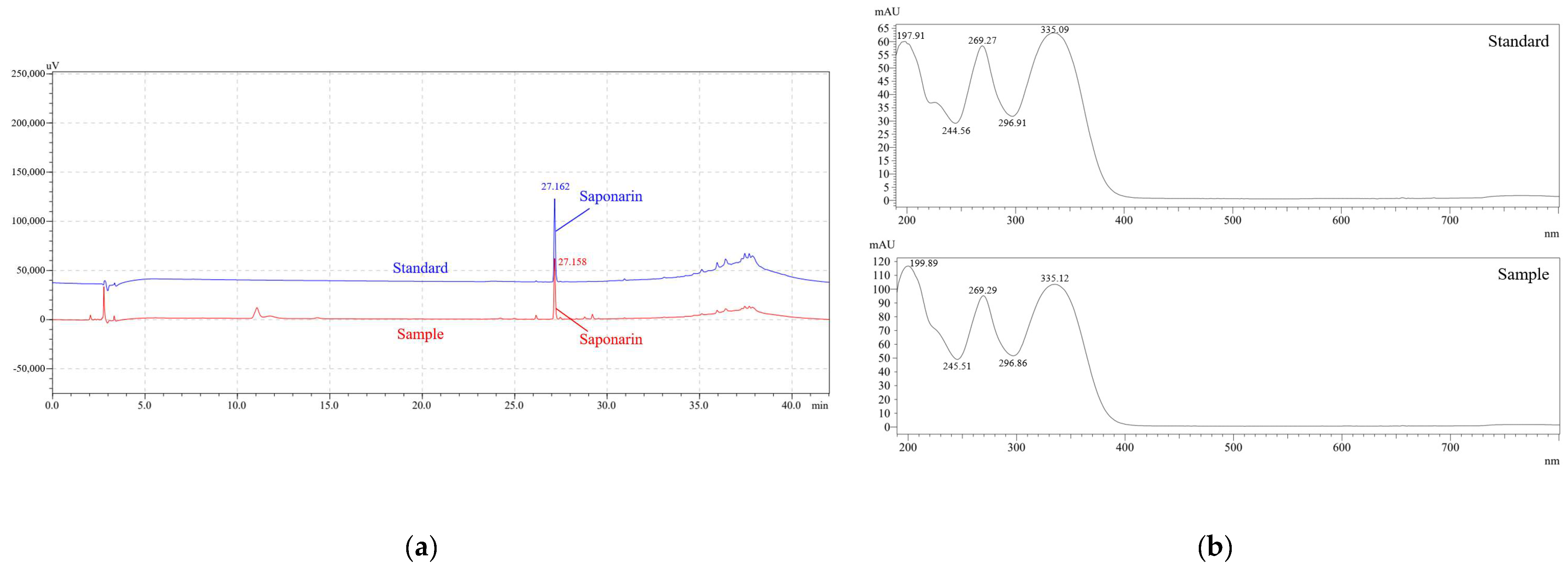
2.2. Analysis of Saponarin
2.3. Analysis of Chlorophylls
2.4. Analysis of Policosanols
2.5. Total Polyphenol and Flavonoid Content
2.6. DPPH Radical-Scavenging Activity
2.7. ABTS Radical-Scavenging Activity
2.8. FRAP Assay
2.9. Statistical Analyses
3. Results
3.1. Saponarin and Clorophyll Contents in Barley Sprout
3.2. Identification and Quantification of Policosanols
3.3. TP and TF Contents
3.4. Antioxidant Effects of Barley Sprout
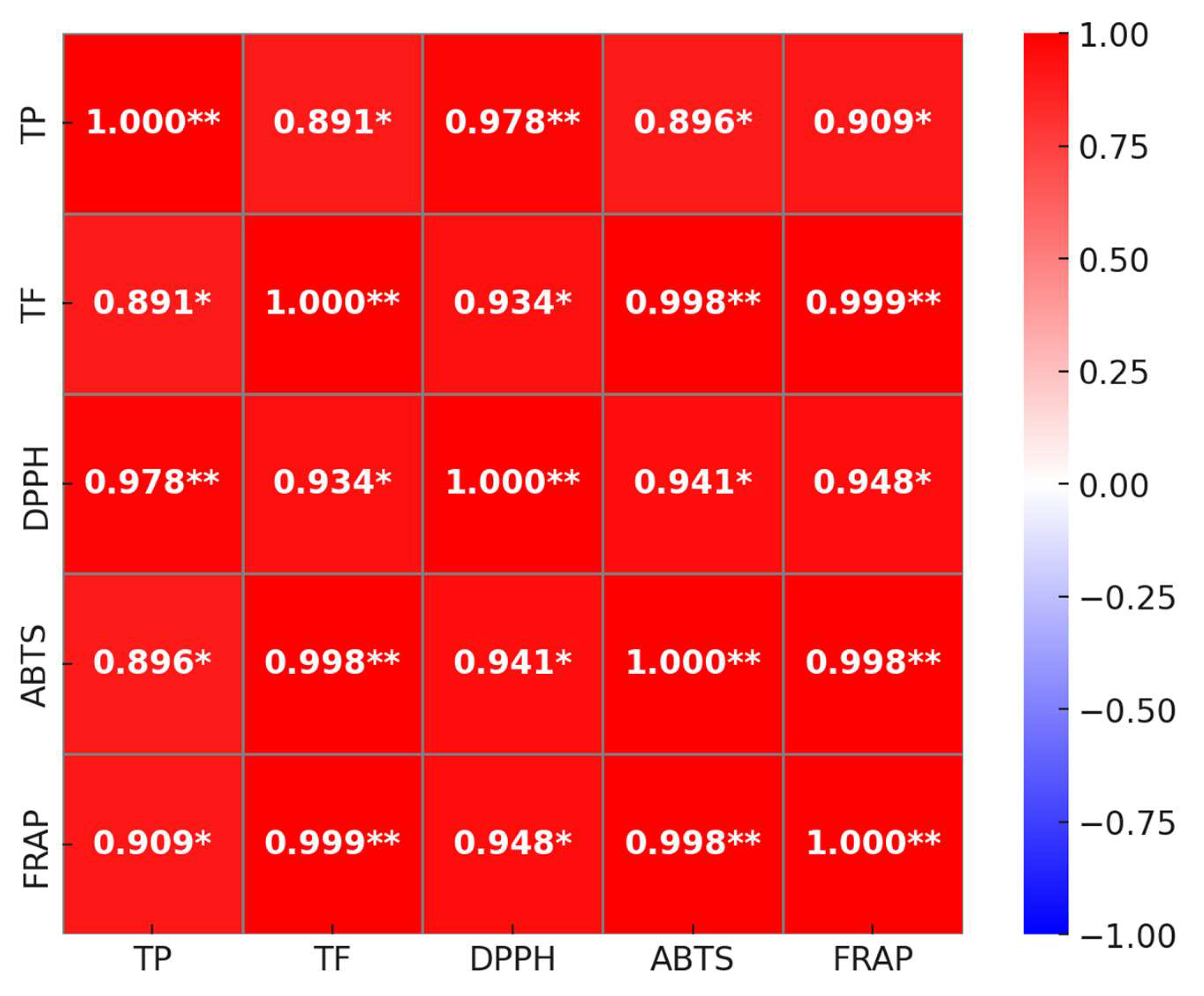
3.5. Relationship Between Antioxidant Activities and Phenolic Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peñaranda, J.D.; Bueno, M.; Álvarez, F.; Pérez, P.D.; Perezábad, L. Sprouted grains in product development. Case studies of sprouted wheat for baking flours and fermented beverages. Int. J. Gastr. Food Sci. 2021, 25, 100375. [Google Scholar] [CrossRef]
- Saithalavi, K.M.; Bhasin, A.; Yaqoob, M. Impact of sprouting on physicochemical and nutritional properties of sorghum: A review. J. Food Meas. Charact. 2021, 15, 4190–4204. [Google Scholar] [CrossRef]
- Drabińska, N.; Siger, A.; Jeleń, H.H. Metabolic Changes during Sprouting of Rapeseed and Their Consequences for the Volatilome of Cold-Pressed Oil. Eur. J. Lipid Sci. Technol. 2023, 125, 2200181. [Google Scholar] [CrossRef]
- Avezum, L.; Rondet, E.; Mestres, C.; Achir, N.; Madode, Y.; Gibert, O.; Lefevre, C.; Hemery, Y.; Verdeil, J.L.; Rajjou, L. Improving the nutritional quality of pulses via germination. Food Rev. Int. 2022, 39, 6011–6044. [Google Scholar] [CrossRef]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Miyahira, R.F.; Lopes, J.D.O.; Antunes, A.E.C. The use of sprouts to improve the nutritional value of food products: A brief review. Plant Foods Hum. Nutr. 2021, 76, 143–152. [Google Scholar] [CrossRef]
- Maqbool, Z.; Khalid, W.; Mahum; Khan, A.; Azmat, M.; Sehrish, A.; Zia, S.; Koraqi, H.; AL-Farga, A.; Aqlan, F.; et al. Cereal sprout-based food products: Industrial application, novel extraction, consumer acceptance, antioxidant potential, sensory evaluation, and health perspective. Food Sci. Nutr. 2024, 12, 707–721. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. microgreens as novel functional foods: Variation of nutritional and phytochemical profiles and their in vitro bioactive properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef]
- Kehinde, B.A.; Majid, I.; Hussain, S. Isolation of bioactive peptides and multiple nutraceuticals of antidiabetic and antioxidant functionalities through sprouting: Recent advances. J. Food Biochem. 2022, 46, e14317. [Google Scholar] [CrossRef]
- Islam, M.Z.; Shim, M.J.; Jeong, S.Y.; Lee, Y.T. Effects of soaking and sprouting on bioactive compounds of black and red pigmented rice cultivars. Int. J. Food Sci. Technol. 2022, 57, 201–209. [Google Scholar] [CrossRef]
- Vicentini, A.; Liberatore, L.; Mastrocola, D. Functional foods: Trends and development of the global market. Ital. J. Food Sci. 2016, 28, 338. [Google Scholar]
- Rico, D.; Peñas, E.; García, M.D.C.; Martínez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martín-Diana, A.B. Sprouted barley flour as a nutritious and functional ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, P.J.; Martínez-Villaluenga, C.; Amigo, L.; Frias, J. Maximising the phytochemical content and antioxidant activity of Ecuadorian brown rice sprouts through optimal germination conditions. Food Chem. 2014, 152, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Cáceres, P.J.; Peñas, E.; Martínez-Villaluenga, C.; Amigo, L.; Frias, J. Enhancement of biologically active compounds in germinated brown rice and the effect of sun-drying. J. Cereal Sci. 2017, 73, 1–9. [Google Scholar] [CrossRef]
- Ghahremaninejad, F.; Hoseini, E.; Jalali, S. The cultivation and domestication of wheat and barley in Iran, brief review of a long history. Bot. Rev. 2021, 87, 1–22. [Google Scholar] [CrossRef]
- Perera, W.N.U.; Abdollahi, M.R.; Zaefarian, F.; Wester, T.J.; Ravindran, V. Barley, an undervalued cereal for poultry diets: Limitations and opportunities. Animals 2022, 12, 2525. [Google Scholar] [CrossRef]
- Martínez, M.; Motilva, M.J.; de Las Hazas, M.C.L.; Romero, M.P.; Vaculova, K.; Ludwig, I.A. Phytochemical composition and β-glucan content of barley genotypes from two different geographic origins for human health food production. Food Chem. 2018, 245, 61–70. [Google Scholar] [CrossRef]
- Raj, R.; Shams, R.; Pandey, V.K.; Dash, K.K.; Singh, P.; Bashir, O. Barley phytochemicals and health promoting benefits: A comprehensive review. J. Agric. Food Res. 2023, 14, 100677. [Google Scholar] [CrossRef]
- Castro, A.G.; Ortiz, F.A.G.; Hernández, G.H.; Román-Gutiérrez, A.D. Analysis of bioactive compounds in lyophilized aqueous extracts of barley sprouts. J. Food Meas. Charact. 2024, 18, 5327–5338. [Google Scholar] [CrossRef]
- Kim, J.S.; Jeong, E.; Jo, S.M.; Park, J.; Kim, J.Y. Comparative study of the effects of light-controlled germination conditions on saponarin content in barley sprouts and lipid accumulation suppression in HepG2 hepatocyte and 3T3-L1 adipocyte cells using barley sprout extracts. Molecules 2020, 25, 5349. [Google Scholar] [CrossRef]
- Seo, W.D.; Yuk, H.J.; Curtis-Long, M.J.; Jang, K.C.; Lee, J.H.; Han, S.I.; Kang, H.W.; Nam, M.H.; Lee, S.J.; Lee, J.H.; et al. Effect of the growth stage and cultivar on policosanol profiles of barley sprouts and their adenosine 5′-monophosphate-activated protein kinase activation. J. Agric. Food Chem. 2013, 61, 1117–1123. [Google Scholar] [CrossRef]
- Duval, B.; Shetty, K. The stimulation of phenolics and antioxidant activity in pea (Pisum sativum) elicited by genetically transformed anise root extract. J. Food Biochem. 2001, 25, 361–377. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis, and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Yoon, N.; Jeong, S.H.; Park, J.S.; Kim, W.J.; Lee, S. Comparative analysis of chemical composition and radical-scavenging activities in two wheat cultivars. Appl. Sci. 2024, 14, 10763. [Google Scholar] [CrossRef]
- Kohyama, N.; Chono, M.; Nakagawa, H.; Matsuo, Y.; Ono, H.; Matsunaka, H. Flavonoid compounds related to seed coat color of wheat. Biosci. Biotechnol. Biochem. 2017, 81, 2112–2118. [Google Scholar] [CrossRef]
- Ielciu, I.; Mouithys-Mickalad, A.; Franck, T.; Angenot, L.; Ledoux, A.; Păltinean, R.; Cieckiewicz, E.; Etienne, D.; Tits, M.; Crişan, G.; et al. Flavonoid composition, cellular antioxidant activity and (myelo) peroxidase inhibition of a Bryonia alba L. (Cucurbitaceae) leaves extract. J. Pharm. Pharmacol. 2019, 71, 230–239. [Google Scholar] [CrossRef]
- Yoo, K.O.; Lim, H.T.; Kim, J.H. Studies on the flavonoids of the Hibiscus syriacus L. complex. Korean J. Plant Resour. 1996, 9, 224–229. [Google Scholar]
- Dang, K.; Mu, J.; Tian, H.; Gao, D.; Zhou, H.; Guo, L.; Shao, X.; Geng, Y.; Zhang, Q. Zinc regulation of chlorophyll fluorescence and carbohydrate metabolism in saline-sodic stressed rice seedlings. BMC Plant Biol. 2024, 24, 464. [Google Scholar] [CrossRef]
- Datt, B. Remote sensing of chlorophyll a, chlorophyll b, chlorophyll a + b, and total carotenoid content in Eucalyptus leaves. Remote Sens. Environ. 1998, 66, 111–121. [Google Scholar] [CrossRef]
- Burns, J.; Fraser, P.D.; Bramley, P.M. Identification and quantification of carotenoids, tocopherols and chlorophylls in commonly consumed fruits and vegetables. Phytochemistry 2003, 62, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.S.; Chen, B.H. Formation of pyrochlorophylls and their derivatives in spinach leaves during heating. Food Chem. 1999, 65, 367–373. [Google Scholar] [CrossRef]
- Turkmen, N.; Poyrazoglu, E.S.; Sari, F.; Velioglu, Y.S. Effects of cooking methods on chlorophylls, pheophytins and colour of selected green vegetables. Int. J. Food Sci. Technol. 2006, 41, 281–288. [Google Scholar] [CrossRef]
- Kim, K.C.; Kim, J.S. Comparative analysis of hydroponically cultivated barley sprouts yield, polyphenol and mineral content by nutrient solution treatment. J. Plant Biotechnol. 2021, 48, 193–200. [Google Scholar] [CrossRef]
- Ra, J.E.; Woo, S.Y.; Lee, K.S.; Lee, M.J.; Kim, H.Y.; Ham, H.M.; Chung, I.M.; Kim, D.H.; Lee, J.H.; Seo, W.D. Policosanol profiles and adenosine 5′-monophosphate-activated protein kinase (AMPK) activation potential of Korean wheat seedling extracts according to cultivar and growth time. Food Chem. 2020, 317, 126388. [Google Scholar] [CrossRef]
- Lee, H.G.; Woo, S.Y.; Ahn, H.J.; Yang, J.Y.; Lee, M.J.; Kim, H.Y.; Seo, W.D. Comparative analysis of policosanols related to growth times from the seedlings of various Korean oat (Avena sativa L.) cultivars and screening for adenosine 5′-monophosphate-activated protein kinase (AMPK) activation. Plants 2022, 11, 1844. [Google Scholar] [CrossRef]
- El Jemli, M.; Kamal, R.; Marmouzi, I.; Zerrouki, A.; Cherrah, Y.; Alaoui, K. Radical-scavenging activity and ferric reducing ability of Juniperus thurifera (L.), J. oxycedrus (L.), J. phoenicea (L.) and Tetraclinis articulata (L.). Adv. Pharmacol. Pharm. Sci. 2016, 2016, 6392656. [Google Scholar] [CrossRef]
- Schlesier, K.; Harwat, M.; Böhm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Conditions | ||
|---|---|---|---|
| HPLC system | Shimadzu LC system LC-40B XR | ||
| Detection system | Shimadzu SPD-M40 PDA | ||
| Column | CAPCELL PAK C18 UG120 (4.6 mm × 250 mm, 5 µm) | ||
| Column temperature | 40 °C | ||
| Flow rate | 1.0 mL/min | ||
| Wavelength | 254 nm | ||
| Injection volume | 5 µL | ||
| Mobile phase | A: DDW containing 1% formic acid | ||
| B: Acetonitrile containing 1% formic acid | |||
| Gradient | Time (min) | A (%) | B (%) |
| 0 | 100 | 0 | |
| 20 | 90 | 10 | |
| 32 | 60 | 40 | |
| 36 | 40 | 60 | |
| 42 | 90 | 10 | |
| Parameters | Conditions | |
|---|---|---|
| GC system | 7890A series GC system coupled with 5975C single quadrupole MS | |
| Column | HP-5MS capillary column (30 m × 0.25 µm × 0.25 µm film thickness) | |
| Carrier gas | Helium, 1.8 mL/min | |
| Temperature program | 150 °C to 325 °C with a 4 °C/min heating rate, and then maintained at 320 °C for 5 min | |
| Inlet | Temperature | 280 °C |
| Injection volume | 1 µL | |
| Split ratio | 1:5 | |
| Mass spectra | Ionization mode | Electron impact |
| Ionization energy | 70 Ev | |
| Ion source and transfer line temperatures | 200 and 280 °C | |
| Mass range | 40–500 amu | |
| Analytical mode | SCAN and SIM | |
| Barley Sprout (mg/g) | |
|---|---|
| Saponarin | 8.14 ± 0.02 (RSD 1) 0.25%) |
| Chlorophyll a | 13.10 ± 0.29 (RSD 2.21%) |
| Chlorophyll b | 2.25 ± 0.09 (RSD 4.00%) |
| Total chlorophyll | 15.36 ± 0.18 (RSD 1.17%) |
| Compound | Peak Number | Retention Time | Policosanol—TMS Derivative Mass (m/z) | Contents (mg/100g) | Ratio (%) |
|---|---|---|---|---|---|
| Eicosanol | 1 | 19.475 | 355.30 [M-15]+ | 1.32 | 0.33 |
| Heneicosanol | 2 | 21.395 | 369.30 [M-15]+ | 0.45 | 0.11 |
| Docosanol | 3 | 23.287 | 383.40 [M-15]+ | 15.28 | 3.84 |
| Tricosanol | 4 | 24.840 | 397.40 [M-15]+ | 3.46 | 0.87 |
| Tetracosanol | 5 | 26.901 | 411.40 [M-15]+ | 46.65 | 11.75 |
| Hexacosanol | 6 | 30.289 | 439.40 [M-15]+ | 299.39 | 75.41 |
| Heptacosanol | 7 | 31.701 | 453.50 [M-15]+ | 8.35 | 2.10 |
| Octacosanol | 8 | 33.310 | 467.50 [M-15]+ | 19.82 | 4.99 |
| Triacontanol | 9 | 36.218 | 495.50 [M-15]+ | 2.27 | 0.57 |
| Total | 396.99 |
| Sample | Total Polyphenol Content (mg GAE 1/g) | Total Flavonoid Content (mg RE 2/g) |
|---|---|---|
| Barley sprouts | 12.64 ± 0.04 | 5.99 ± 0.09 |
| Compound | DPPH (IC50, mg/mL) | ABTS (IC50, mg/mL) | FRAP (IC50, mg/mL) |
|---|---|---|---|
| Barley sprout | 21.04 ± 0.00 | 88.53 ± 2.75 | 2.50 ± 0.00 |
| Ascorbic acid | 0.03 ± 0.00 | 0.44 ± 0.01 | 0.01 ± 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, G.; La, I.-J.; Lee, D.-S.; Chae, J.-W.; Im, J.-H.; Park, S.W.; Fu, X.; Lim, J.-S.; Kim, M.-H.; Seong, Y.-S.; et al. Assessment of Bioactive Compounds and Antioxidant Activity of Barley Sprouts. Separations 2025, 12, 68. https://doi.org/10.3390/separations12030068
Oh G, La I-J, Lee D-S, Chae J-W, Im J-H, Park SW, Fu X, Lim J-S, Kim M-H, Seong Y-S, et al. Assessment of Bioactive Compounds and Antioxidant Activity of Barley Sprouts. Separations. 2025; 12(3):68. https://doi.org/10.3390/separations12030068
Chicago/Turabian StyleOh, Geon, Im-Joung La, Do-Sang Lee, Jong-Woo Chae, Ji-Hyun Im, Seon Woo Park, Xiaolu Fu, June-Seok Lim, Min-Hye Kim, Yeon-Seok Seong, and et al. 2025. "Assessment of Bioactive Compounds and Antioxidant Activity of Barley Sprouts" Separations 12, no. 3: 68. https://doi.org/10.3390/separations12030068
APA StyleOh, G., La, I.-J., Lee, D.-S., Chae, J.-W., Im, J.-H., Park, S. W., Fu, X., Lim, J.-S., Kim, M.-H., Seong, Y.-S., Park, D., & Lee, O.-H. (2025). Assessment of Bioactive Compounds and Antioxidant Activity of Barley Sprouts. Separations, 12(3), 68. https://doi.org/10.3390/separations12030068







