Degradation of Acid Orange II by FeOCl/Biochar-Catalyzed Heterogeneous Fenton Oxidation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of Catalysts
2.2.1. Preparation of BC
2.2.2. Synthesis of FeOCl/BC Composites
2.3. Degradation Experiment Evaluation
2.4. Characterization
3. Results and Discussion
3.1. Degradation Efficiency of AO-II Under Different Reaction Systems
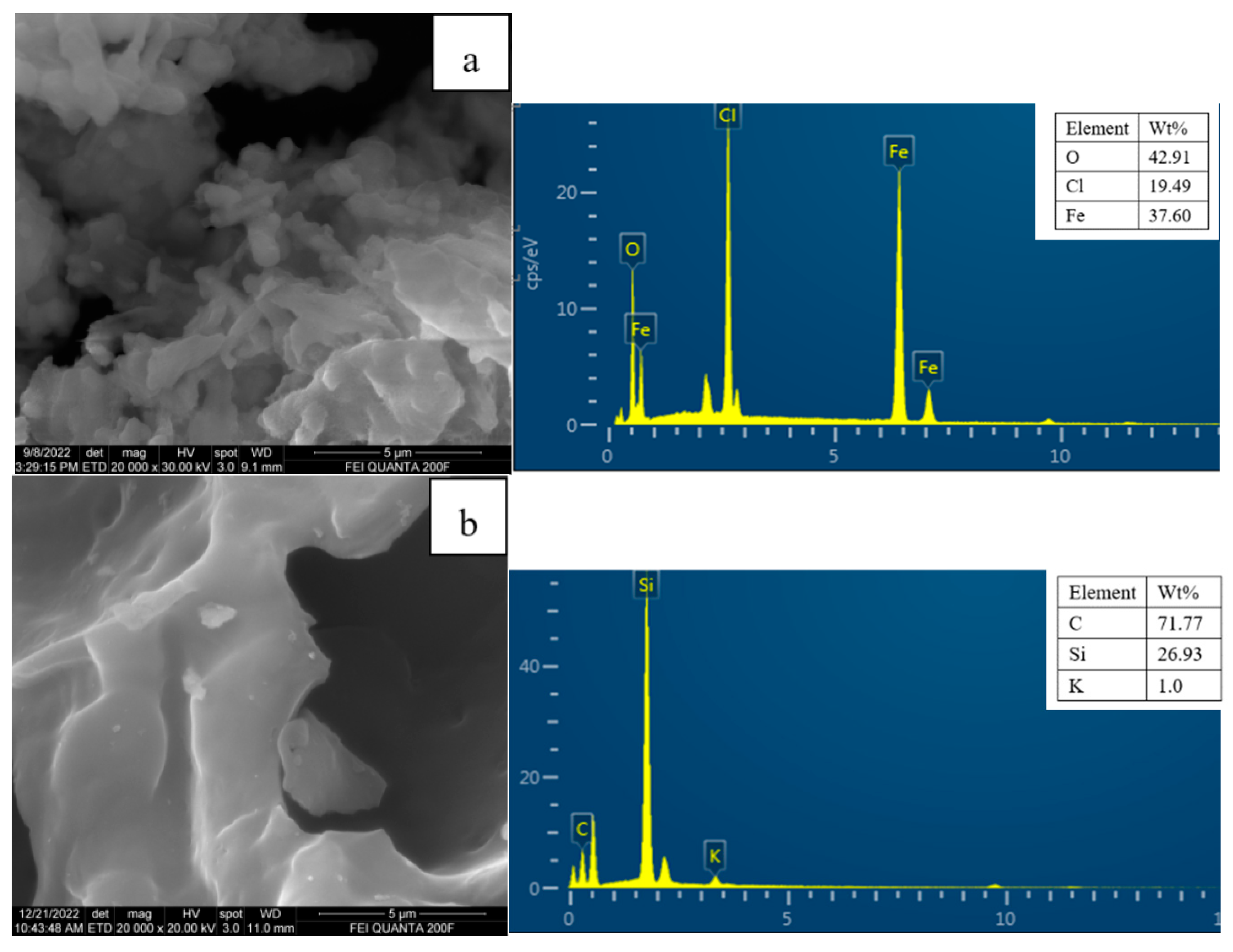
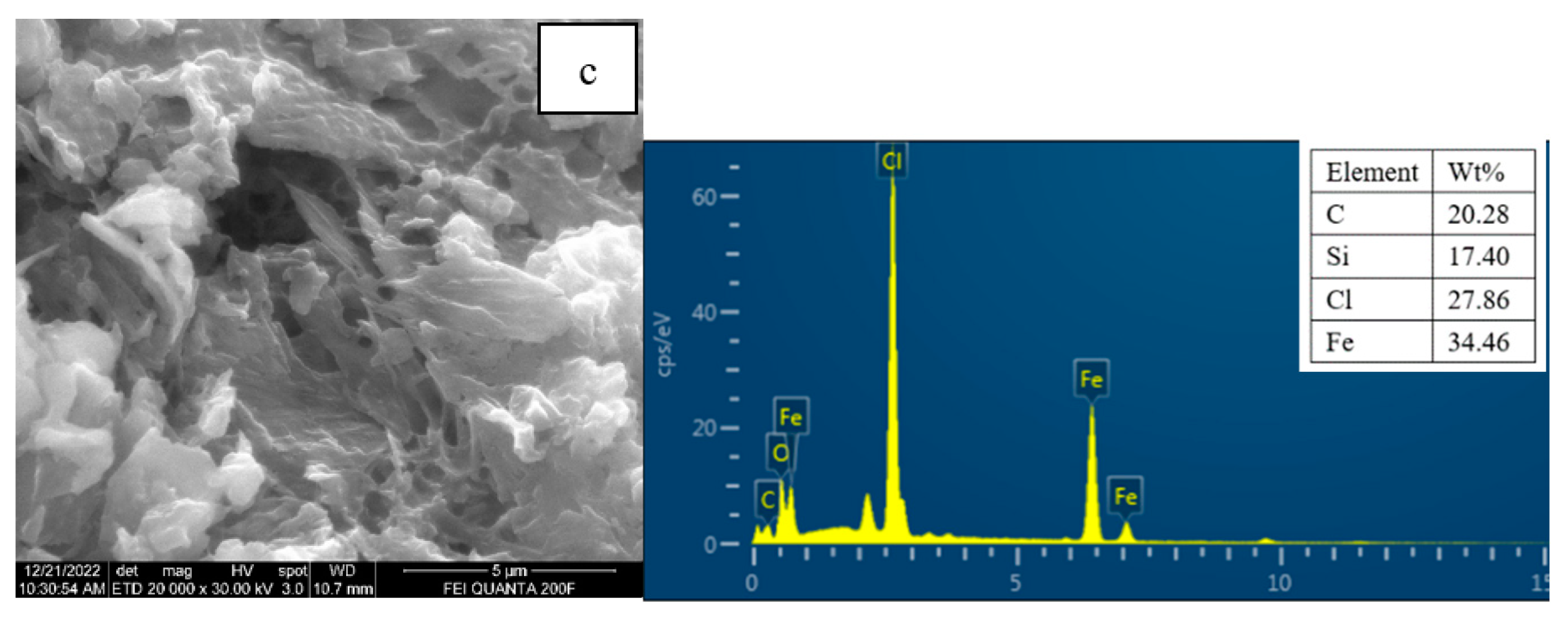
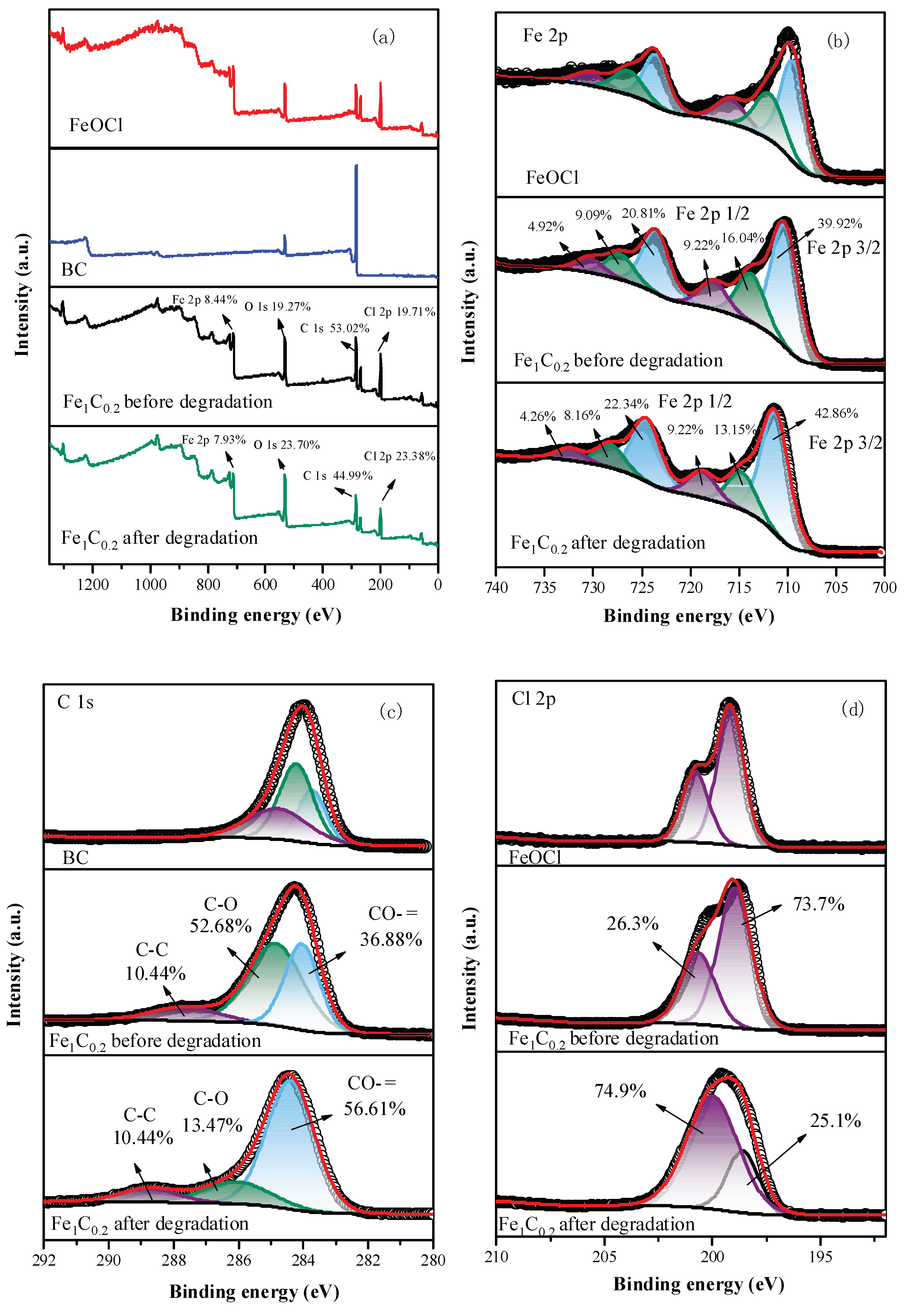
3.2. Sample Characterization
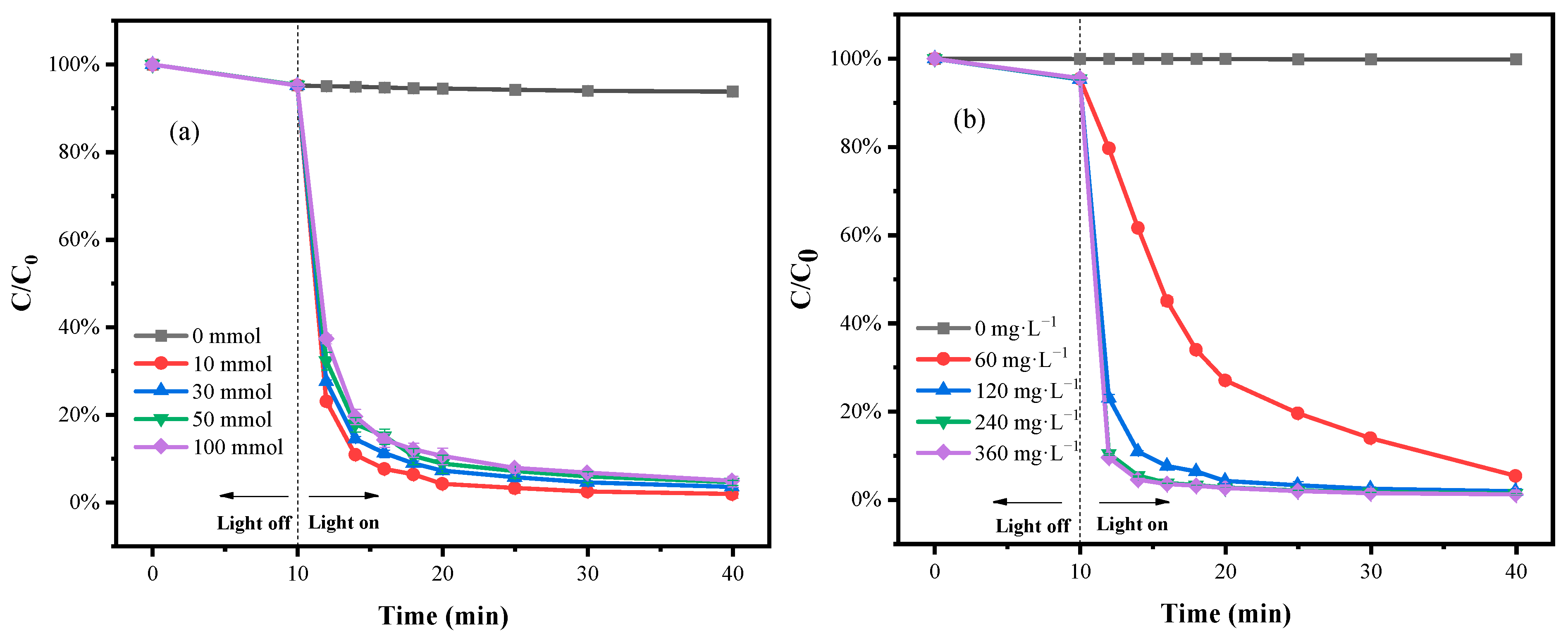
3.3. Effect of H2O2 Dosage and Fe1C0.2 Composite Concentrations
3.4. Effect of the Initial pH Value
3.5. Effect of Temperature
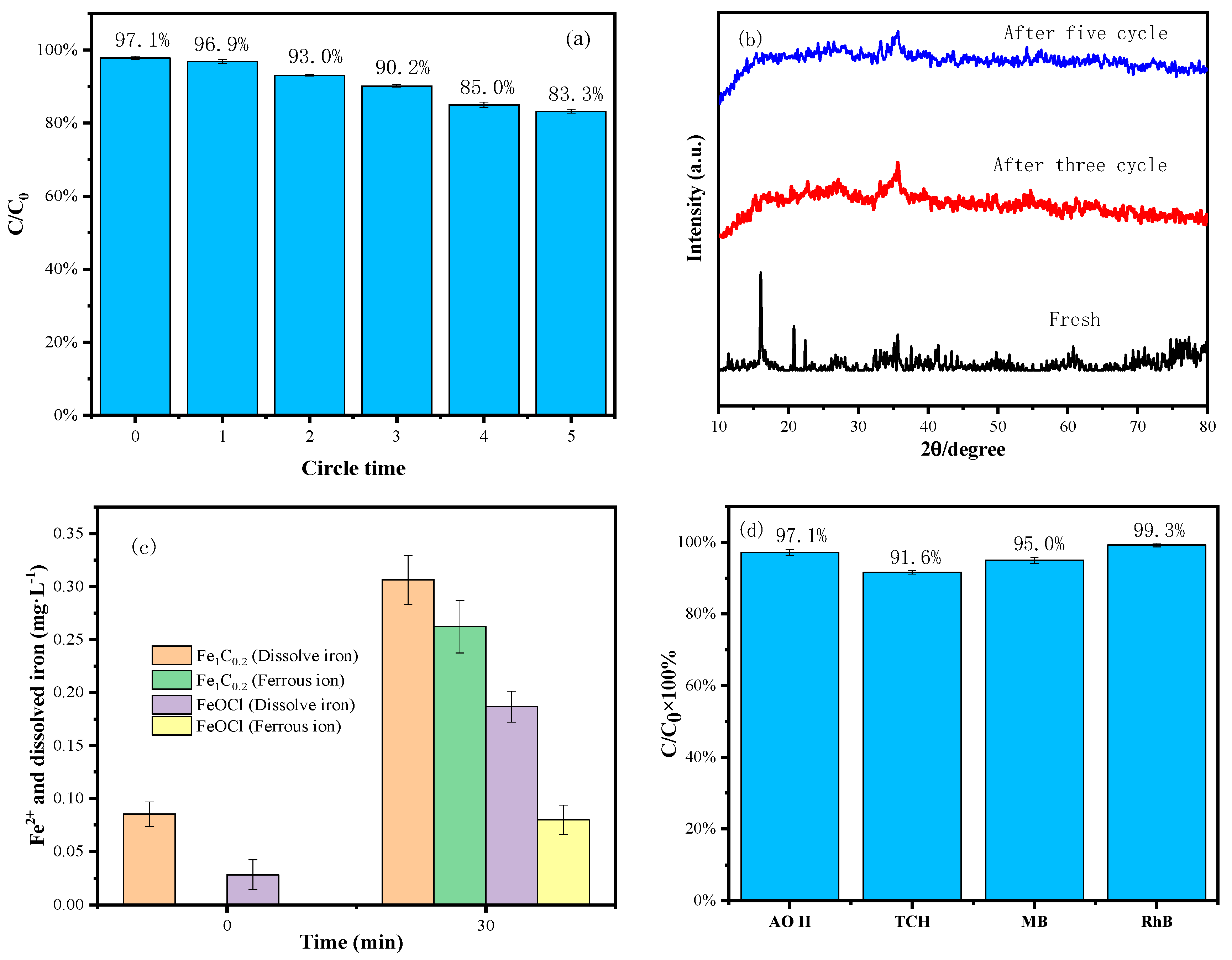
3.6. Reusability of the Fe1C0.2 Composite and Versatility
3.7. Effect of Coexisting Anions
3.8. Active Species Involved in the Fe1C0.2/H2O2 Fenton System
3.9. Possible Photocatalytic Principles of Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ranjbari, A.; Kim, J.; Yu, J.; Kim, J.; Park, M.; Kim, N.; Demeestere, K.; Heynderickx, P.M. Fundamental kinetic modeling of dye sensitization photocatalysis by oxygen vacancy enriched ZnO for the quantification of degradation by catalyst or dye sensitizer. Appl. Surf. Sci. 2024, 659, 159867. [Google Scholar] [CrossRef]
- Ranjbari, A.; Anbari, A.P.; Kashif, M.; Adhikary, K.K.; Kim, K.; Heynderickx, P.M. The adsorption/photocatalytic degradation kinetics of oxygen vacancy-enriched ZnO in relation to surface functional groups of cationic/anionic dyes. Chem. Eng. J. 2025, 505, 159526. [Google Scholar] [CrossRef]
- Ranjbari, A.; Kim, J.; Yu, J.; Kim, J.; Park, M.; Kim, N.; Demeestere, K.; Heynderickx, P.M. Effect of oxygen vacancy modification of ZnO on photocatalytic degradation of methyl orange: A kinetic study. Catal. Today 2024, 427, 114413. [Google Scholar] [CrossRef]
- Lassoued, A.; Liu, L.J.; Li, J.F. Removal of acid orange II azo dyes using Fe-based metallic glass catalysts by Fenton-like process. J. Mater. Sci. 2022, 57, 2039–2052. [Google Scholar] [CrossRef]
- Wang, T.; Li, Y.; Qu, G.; Liang, D.; Hu, S. Photocatalytic degradation of acid orange II using activated carbon fiber-supported cobalt phthalocyanine coupled with hydrogen peroxide. Water Air Soil Pollut. 2016, 227, 464. [Google Scholar] [CrossRef]
- Li, D.; Hua, T.; Yuan, J.; Xu, F. Methylene blue adsorption from an aqueous solution by a magnetic graphene oxide/humic acid composite. Colloids Surf. A 2021, 627, 127171. [Google Scholar] [CrossRef]
- Hou, Z.; Tian, Z.; Zhao, J.; Yang, X.; Liu, L. Seeded-secondary growth synthesis of ZIF-8@porous mullite beads for adsorption of dyes. J. Mater. Sci. 2023, 58, 7319–7332. [Google Scholar] [CrossRef]
- Hua, Z.; Pan, Y.; Hong, Q. Adsorption of Congo red dye in water by orange peel biochar modified with CTAB. RSC Adv. 2023, 13, 12502–12508. [Google Scholar] [CrossRef]
- Thoa, L.K.; Thao, T.T.P.; Nguyen-Thi, M.L.; Chung, N.D.; Ooi, C.W.; Park, S.M.; Lan, T.T.; Quang, H.T.; Khoo, K.S.; Show, P.L. Microbial biodegradation of recalcitrant synthetic dyes from textile-enriched wastewater by Fusarium oxysporum. Chemosphere 2023, 325, 138392. [Google Scholar] [CrossRef]
- Singh, A.L.; Kumar, S.S.; Kumar, A.; Singh, A.; Yadav, A. Biodegradation of reactive yellow-145 azo dye using bacterial consortium: A deterministic analysis based on degradable Metabolite, phytotoxicity and genotoxicity study. Chemosphere 2022, 300, 134504. [Google Scholar] [CrossRef]
- Maurya, K.L.; Swain, G.; Sonwani, R.K.; Verma, A. Biodegradation of Congo red dye using polyurethane foam-based biocarrier combined with activated carbon and sodium alginate: Batch and continuous study. Bioresour. Technol. 2022, 351, 126999. [Google Scholar]
- Shao, L.; Liu, H.; Zeng, W.; Zhou, C.; Li, D.; Wang, L.; Lan, Y.; Xu, F.; Liu, G. Immobilized and photocatalytic performances of PDMS-SiO2-chitosan@TiO2 composites on pumice under simulated sunlight irradiation. Appl. Surf. Sci. 2019, 478, 1017–1026. [Google Scholar] [CrossRef]
- Tian, G.D.; Duan, C.; Zhou, B.X.; Tian, C.C.; Wang, Q.; Chen, J.C. Lignin-based electrospun nanofiber membrane decorated with photo-Fenton Ag@MIF-100(Fe) heterojunctions for complex wastewater remediation. Front. Chem. Sci. Eng. 2023, 17, 930–941. [Google Scholar] [CrossRef]
- Wang, H.H.; Yu, S.W.; Meng, X.G.; Wang, Z.Y.; Gao, T.; Xiao, S.J. Facile synthesis of fumarate-type iron-cobalt bimetallic MOFs and its application in photo-Fenton degradation of organic dyes. J. Solid State Chem. 2023, 314, 123431. [Google Scholar] [CrossRef]
- Qu, X.; Tomatis, M.; Payne, B.; Daly, H.; Chansai, S.; Fan, X.; Agostino, C.D.; Azapagic, A.; Hardacre, C. Fracking wastewater treatment: Catalytic performance and life cycle environmental impacts of cerium-based mixed oxide catalysts for catalytic wet oxidation of organic compounds. Sci. Total Environ. 2023, 860, 160480. [Google Scholar]
- Sethi, N.K.; Kobayashi, H.; Wu, B.L.; Li, R.H.; Fan, J. Novel route to erucamide: Highly selective synthesis from acetonitrile at room temperature via a photo-Fenton process. ACS Sustain. Chem. Eng. 2018, 6, 11380–11385. [Google Scholar] [CrossRef]
- Xu, F.; Tan, W.; Liu, H.; Li, D.; Li, Y.; Wang, M. Immobilization of PDMS-SiO2-TiO2 composite for the photocatalytic degradation of dye AO-7. Water Sci. Technol. 2016, 74, 1680–1688. [Google Scholar] [CrossRef]
- Li, D.; Liu, H.; Niu, C.; Yuan, J.; Xu, F. Mpg-C3N4-ZIF-8 composites for the degradation of tetracycline hydrochloride using visible light. Water Sci. Technol. 2020, 80, 2206–2217. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.; Lv, S.; Li, X.; Shao, L.; Zhou, C.; Xu, F. Insights into the degradation mechanisms of TCH by magnetic Fe3S4/Cu2O composite. Inorg. Chem. 2023, 62, 10713–10726. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, M.; Li, D.; Hu, H.; Xia, D. Quantitative investigation into the enhancing utilization efficiency of H2O2 catalyzed by FeOCl under visible light. J. Photochem. Photobiol. A Chem. 2020, 386, 112072. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Xu, X.; Xu, J.; Wang, H.; Semiat, R.; Han, Y. Modeling and kinetics study of Bisphenol A (BPA) degradation over an FeOCl/SiO2 Fenton-like catalyst. Catal. Today 2016, 276, 85–96. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Xu, J.; Han, Y. Iron oxychloride (FeOCl): An efficient Fenton-like catalyst for producing hydroxyl radicals in degradation of organic contaminants. J. Am. Chem. Soc. 2013, 135, 16058–16061. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Tang, C.; Li, D.; Yuan, J.; Xu, F. FeOCl/WS2 composite as a heterogeneous Fenton catalyst to efficiently degrade acid orange II. Inorg. Chem. Comm. 2023, 156, 111085. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, P.; Zhang, P.; Meng, S.; Zhou, C.; Liu, Y.; Zhang, H.; Xiong, Z.; Duan, X.; Lai, B. New insight into carbon materials enhanced Fenton oxidation: A strategy for green iron(III)/iron(II) cycles. Chem. Eng. J. 2022, 450, 138423. [Google Scholar] [CrossRef]
- Bhuyan, P.M.; Borah, S.; Bhuyan, B.K.; Hazarika, S.; Gogoi, N.; Gogoi, A.; Gogoi, P. Fe3S4/biochar catalysed heterogeneous Fenton oxidation of organic contaminants: Hydrogen peroxide activation and biochar enhanced reduction of Fe (III) to Fe (II). Sep. Purif. Techno. 2023, 312, 123387. [Google Scholar] [CrossRef]
- Huang, D.; Luo, H.; Zhang, C.; Zeng, G.; Lai, C.; Cheng, M.; Wang, R.; Deng, R.; Xue, W.; Gong, X.; et al. Nonnegligible role of biomass types and its compositions on the formation of persistent free radicals in biochar: Insight into the influences on Fenton-like process. Chem. Eng. J. 2019, 361, 353–363. [Google Scholar] [CrossRef]
- Xu, X.; Huang, H.; Zhang, Y.; Zhang, Y.; Xu, Z.; Cao, X. Biochar as both electron donor and electron shuttle for the reduction transformation of Cr(VI) during its sorption. Environ. Pollut. 2019, 244, 423–430. [Google Scholar] [CrossRef]
- Matos, I.; Bernardo, M.; Fonseca, I. Porous carbon: A versatile material for catalysis. Catal. Today 2017, 285, 194–203. [Google Scholar] [CrossRef]
- Jia, Y.; Qu, Y.; Khanal, S.K.; Sun, L.; Shu, W.; Lu, H. Biochar-based strategies for antibiotics removal: Mechanisms, factors, and application. ACS EST Eng. 2024, 4, 1256–1274. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Jin, L.; Lu, Z.; Xiang, M.; Wang, C.; Wang, W.; Zhang, J.; Li, C.; Xie, H. Activation of peroxymonosulfate by magnetic Fe3S4/biochar composites for the efficient degradation of 2,4,6-trichlorophenol: Synergistic effect and mechanism. J. Environ. Chem. Eng. 2022, 10, 107085. [Google Scholar] [CrossRef]
- Sun, M.; Chu, C.; Geng, F.; Lu, X.; Qu, J.; Crittenden, J.; Elimelech, M.; Kim, J. Reinventing Fenton chemistry: Iron oxychloride nanosheet for pH-Insensitive H2O2 activation. Environ. Sci. Tech. Lett. 2018, 5, 186–191. [Google Scholar] [CrossRef]
- Peimyoo, N.; Shang, J.; Cong, C.; Shen, X.; Wu, X.; Yeow, E.K.L.; Yu, T. Nonblinking, intense two-dimensional light emitter: Monolayer WS2 triangles. ACS Nano 2013, 7, 10985–10994. [Google Scholar] [CrossRef]
- Nawaz, A.; Goudarzi, S.; Saravanan, P.; Zarrin, H. Z-scheme induced g-C3N4/WS2 heterojunction photocatalyst with improved electron mobility for enhanced solar photocatalysis. Sol. Energy 2021, 228, 53–67. [Google Scholar] [CrossRef]
- Kang, J.; Tongay, S.; Zhou, J.; Li, J.; Wu, J. Band offsets and heterostructures of two-dimensional semiconductors. Appl. Phys. Lett 2013, 102, 12111. [Google Scholar] [CrossRef]
- Yang, J.; Voiry, D.; Ahn, S.J.; Kang, D.; Kim, A.Y.; Chhowalla, M.; Shin, H.S. Two-dimensional hybrid nanosheets of tungsten disulfide and reduced graphene oxide as catalysts for enhanced hydrogen evolution. Angew Chem Int Edit 2013, 52, 13751–13754. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Zhang, L.; Xie, P.; Wang, Z.; Zhou, A.; Fang, Z.; Ma, J. Efficient degradation of imipramine by iron oxychloride-activated peroxymonosulfate process. J. Hazard. Mater. 2018, 353, 18–25. [Google Scholar] [CrossRef]
- Ye, S.; Zeng, G.; Tan, X.; Wu, H.; Liang, J.; Song, B.; Tang, N.; Zhang, P.; Yang, Y.; Chen, Q.; et al. Nitrogen-doped biochar fiber with graphitization from Boehmeria nivea for promoted peroxymonosulfate activation and non-radical degradation pathways with enhancing electron transfer. Appl. Catal. B Environ. 2020, 269, 118850. [Google Scholar] [CrossRef]
- Wei, J.; Feng, X.; Hu, X.; Yang, J.; Yang, C.; Liu, B. Cu(II) doped FeOCl as an efficient photo-Fenton catalyst for phenol degradation at mild pH. Colloids Surf. A 2021, 631, 127754. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.S.; Wu, J. Characterization and application of chars produced from pinewood pyrolysis and hydrothermal treatment. Fuel 2010, 89, 510–514. [Google Scholar] [CrossRef]
- Wang, C.; Sun, R.; Huang, R. Highly dispersed iron-doped biochar derived from sawdust for Fenton-like degradation of toxic dyes. J. Clean. Prod. 2021, 297, 126681. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Wang, Q.; Cheng, H. High-efficiency photo-Fenton Fe/g-C3N4/kaolinite catalyst for tetracycline hydrochloride degradation. Appl. Clay Sci. 2021, 212, 106213. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.; Fang, Y.; Cao, Z.; Chen, D.; Li, N.; Xu, Q.; Lu, J. TiO2 nanoparticles anchored onto the metal-organic frame work NH2-MIL-88B(Fe) as an adsorptive photocatalyst with enhanced Fenton-like degradation of organic pollutants under visible light irradiation. ACS Sustain. Chem. Eng. 2018, 6, 16186–16197. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Yang, Y.; Han, Y.; Wang, T.; Chen, J.; Tsang, D.C.W. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 2020, 383, 121240. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, W.; Huang, J.; Zhou, Y.; Zhu, Y.; Li, C. Rapid degradation of methylene blue in a novel heterogeneous Fe3O4@rGO@TiO2-catalyzed photo-Fenton system. Sci. Rep. 2015, 5, 10632. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.E.M.; Gad-Allah, T.A.; Badawy, M.I. Heterogeneous Fenton process using steel industry wastes for methyl orange degradation. Appl. Water Sci. 2013, 3, 263–270. [Google Scholar] [CrossRef]
- Araujo, F.V.F.; Yokoyama, L.; Teixeira, L.A.C.; Campos, J.C. Heterogeneous Fenton process using the mineral hematite for the discolouration of a reactive dye solution. Braz. J. Chem. Eng. 2011, 28, 605–616. [Google Scholar] [CrossRef]
- Wang, S.; Long, J.; Jiang, T.; Shao, L.; Li, D.; Xie, X.; Xu, F. Magnetic Fe3O4/CeO2/g-C3N4 composites with a visible-light response as a high efficiency Fenton photocatalyst to synergistically degrade tetracycline. Sep. Purif. Technol. 2022, 278, 119609. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, S.; Jiang, J.; Shao, L.; Li, D.; Yuan, J.; Xu, F. Magnetic Fe3S4/MoS2 with visible-light response as an efficient photo-Fenton-like catalyst: Validation in degrading tetracycline hydrochloride under mild pH conditions. J. Alloys Compd. 2022, 921, 166023. [Google Scholar] [CrossRef]
- Prabavathi, S.L.; Saravanakumar, K.; Mamba, G.; Muthuraj, V. 1D/2D MnWO4 nanorods anchored on g-C3N4 nanosheets for enhanced photocatalytic degradation ofloxacin under visible light irradiation. Colloids Surf. A 2019, 581, 123845. [Google Scholar]
- Chen, H.; Wang, X.; Bi, W.; Wu, Y.; Dong, W. Photodegradation of carbamazepine with BiOCl/Fe3O4 catalyst under simulated solar light irradiation. J. Colloid Interf. Sci. 2017, 502, 89–99. [Google Scholar] [CrossRef]
- Dugandžić, A.M.; Tomašević, A.V.; Radišić, M.M.; Šekuljica, N.Ž.; Mijin, D.Ž.; Petrović, S.D. Effect of inorganic ions, photosensitisers and scavengers on the photocatalytic degradation of nicosulfuron. J. Photochem. Photobiol. A 2017, 336, 146–155. [Google Scholar] [CrossRef]
- Hou, Z.; Chen, F.; Wang, J.; Francois-Xavier, C.P.; Wintgens, T. Novel Pd/GdCrO3 composite for photo-catalytic reduction of nitrate to N2 with high selectivity and activity. Appl. Catal. B Environ. 2018, 232, 124–134. [Google Scholar] [CrossRef]
- Liang, L.; Gao, S.; Zhu, J.; Wang, L.; Xiong, Y.; Xia, X.; Yang, L. The enhanced photocatalytic performance toward carbamazepine by nitrogen-doped carbon dots decorated on BiOBr/CeO2: Mechanism insight and degradation pathways. Chem. Eng. J. 2020, 391, 123599. [Google Scholar] [CrossRef]
- Qu, S.; Li, C.; Sun, X.; Wang, J.; Luo, H.; Wang, S.; Ta, J.; Li, D. Enhancement of peroxymonosulfate activation and utilization efficiency via iron oxychloride nanosheets in visible light. Sep. Purif. Technol. 2019, 224, 132–141. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, J.; Xie, D.; Li, D.; Xu, F. Degradation of Acid Orange II by FeOCl/Biochar-Catalyzed Heterogeneous Fenton Oxidation. Separations 2025, 12, 101. https://doi.org/10.3390/separations12040101
Yuan J, Xie D, Li D, Xu F. Degradation of Acid Orange II by FeOCl/Biochar-Catalyzed Heterogeneous Fenton Oxidation. Separations. 2025; 12(4):101. https://doi.org/10.3390/separations12040101
Chicago/Turabian StyleYuan, Jiren, Dongao Xie, Dan Li, and Feigao Xu. 2025. "Degradation of Acid Orange II by FeOCl/Biochar-Catalyzed Heterogeneous Fenton Oxidation" Separations 12, no. 4: 101. https://doi.org/10.3390/separations12040101
APA StyleYuan, J., Xie, D., Li, D., & Xu, F. (2025). Degradation of Acid Orange II by FeOCl/Biochar-Catalyzed Heterogeneous Fenton Oxidation. Separations, 12(4), 101. https://doi.org/10.3390/separations12040101





