Abstract
Understanding the effects of industrial lubricating oil and additives on desulfurization solution foaming is very important to both engineers and academic researchers. In this study, the influences of lubricating oil and its additive components on the foaming performance of desulfurization solution were examined, and the empirical model was established to predict the foam height of desulfurization solution containing contaminants. It is established that the additives of dodecenyl succinic acid and zinc dialkyl dithiophosphate have more significant influence on the foaming performance of desulfurization solution than the lubricant base oil. In addition, the effect can be enlarged at lower temperatures. The prediction model of the foaming performance of desulfurization solution was established, and the prediction deviation of the foam height was no more than 7%. The predicted foam height increases with increasing apparent air velocity, solution surface tension, and viscosity, and decreases with increasing solution density and average bubble radius. Additionally, the foam height and defoaming time of the contaminated industrial solution were largely reduced by employing an adsorption purification process using activated carbon as an adsorbent. The present study highlights the quantitative relationship between contaminant contents and foam height, as well as defoaming time.
1. Introduction
The absorption process based on alkanolamine solution is the most commonly used method in natural gas purification due to its advantages, including high processing capacity, good adaptability to sulfide concentration, and low equipment requirements and operating costs. With the increasing awareness of environmental protection, the requirement of sulfur content in natural gas is becoming more and more strict, and the development of efficient desulfurization solvent and its process technology is the key to cope with this issue. The extensive applications of alkanolamines in acid gas removal plants are due to their properties, including low viscosity, the application in a vast range of partial pressure of H2S and CO2, and approximately no absorption of hydrocarbons.
In the natural gas purification processes that remove CO2 and various sulfides by using aqueous solutions of different alkanolamines, solvent foaming is a very important negative factor affecting the operational stability of industrial purification plants [1]. Foaming compromises the stability of industrial operations by leading to a series of issues [2], such as significant solvent loss, premature flooding, reductions in production and overall efficiency, low-quality products, and oscillation in operating parameters; in severe cases, it can lead to an emergency stop in the plant [3,4]. Additionally, it escalates both capital and operational expenses due to the need for investments in foam mitigation and control strategies, including the installation of reclamation units or the application of antifoaming agents. Numerous gas treatment facilities utilizing alkanolamine-based absorption systems have faced challenges related to foaming. For example, the CASTOR plant in Esbjergværket (Denmark), which employs a gas absorption process using monoethanolamine (MEA) solution to capture CO2 from the flue gas of a coal-fired power plant experienced the foaming problem [5]. Foaming of solvent systems results from multiple aspects, including large fluctuations in the flow rate of raw gas, changes in operating pressure, as well as contamination of the solvent system [6,7,8]. AI-Qahtani and Garland [9] reported the foaming events of the onshore gas-treatment facilities at the Khursaniyah gas plant of the Karan nonassociated (NA) sour-gas field. Saudi Aramco sought to eliminate early foaming incidents during the commissioning stage and emphasized the need for rigorous process evaluation and the application of fundamental principles in plant troubleshooting. Sample results showed that heavy hydrocarbon formation/condensation was the primary reason for foaming. Rumaih [10] reported another example of operational problems in the Shaybah natural gas liquefaction (NGL) plant associated with foam in acid gas removal (AGR) unit, which has two identical trains processing 2.4 BSCFD of dry clean sour gas. The AGR unit experienced repetitive amine absorber foaming events due to the gas impurities in the form of heavy end liquid hydrocarbon (C6+) and suspended solids, including sulfur, iron sulfide, pyrite, and magnetite. The repetitive foaming led to frequent disturbances in plant operations, reducing the unit throughput and impacting the product quality. Edalatpour et al. [11] examined the impact of various operational factors, such as temperature, amine concentration, and flow rate, on the foaming characteristics of aqueous solutions containing N-methyldiethanolamine (MDEA) and isopropyl aminoethanol (IPAE). The combination of MDEA and IPAE demonstrated superior foam control compared to MDEA alone. Foaming experiments conducted with formaldehyde and formic acid revealed that IPAE functions as a foam suppressant even when contaminants are present. When subjected to contaminants present in the flue gas and the elevated temperatures within the reboiler, alkanolamine solvents are prone to both chemical and thermal breakdown. Additives change the physical properties of desulfurization solution, such as density, viscosity, and surface tension, which, in turn, leads to deterioration of the foaming performance of the solution. The primary byproducts of this degradation are heat stable salts (HSSs), which significantly affect the functionality and integrity of industrial equipment. These salts predominantly result from the ionic degradation of organic acids, including acetic and formic acids. Numerous investigations have been performed to examine the influence of operational parameters, such as process variables, degradation byproducts, contaminants, and additives, on the propensity for foaming and the stability of foam, and to explain the mechanism of foaming induced by various contaminants [12,13,14,15]. These contaminants could be degradation products of alkanolamine, dissolved hydrocarbons, organic acids, suspended solids, additives (for example, corrosion inhibitors and defoaming agents), water-soluble surfactants, or ionogenic substances in mining water. Studies on the effects of different kinds of contaminants on the foaming tendency of amine solutions have been widely conducted. The contaminant components examined involve solid particles, degradation products, corrosion inhibitors [13,16], and electrolytes [17]. The accumulation of degradation byproducts and contaminants can lead to significant operational difficulties and complications throughout the absorption process. In addition, lubricating oil and its additives, which are widely used in mechanical equipment at desulfurization units, such as solvent pumps, may leak into the solvent system due to equipment malfunction or other issues, thus causing solvent contamination and resulting in serious foaming of the solvent system. At present, the most commonly utilized purification methods for reclaiming solvent consist of ion exchange, electrodialysis, and thermal reclamation. Ion exchange and electrodialysis are specifically effective in eliminating ionic degradation byproducts, including HSSs and organic acids. Ju et al. [18] introduced an innovative purification method designed to isolate degradation byproducts and various contaminants from these solvents. This advanced reclamation technique effectively eliminates the majority of unwanted impurities and degradation residues, restoring the solvent nearly to its initial purity level. The process achieves a high recovery efficiency while maintaining low energy usage.
A foam model enables operators to strategically prioritize their efforts in addressing foaming issues and to assess the effects of foaming on overall plant efficiency. In several investigations by Thitakamol et al. [13,19,20,21], they established a relationship to estimate foam height based on process variables under steady-state pneumatic conditions. Furthermore, they designed a foam model specifically for a CO2 absorber utilizing alkanolamine solutions and equipped with sheet-metal structured packing. The simulation outcomes indicate that the model can predict foam quantities, identify potential locations and operational scenarios where foaming is likely to arise, and assess the impact of foaming on process efficiency. The inclusion of degradation byproducts and corrosion inhibitors was observed to significantly increase foam formation within the absorber. Despite these efforts, there remains a gap in research aimed at developing methods to forecast the volume of foam generated within industrial systems. Moreover, understanding the effect of industrial lubricating oil and its additives on solvent foaming is also very important to both engineers and academic researchers.
A family of formulated solutions, named UDS, was developed in this lab and found wide applications in removing a variety of organosulfurs from natural gas, associated gas, and refinery gases [22,23,24]. In this work, the influences of lubricating oil and its additive components on the foaming performance of the UDS solvent were examined, and the empirical model was established to predict the foam height of the UDS solution containing contaminants. Moreover, activated carbon commonly used in a typical industrial amine purification unit was utilized to purify the contaminated UDS solution from an industrial plant. The present study highlights the quantitative relationship between contaminant contents and foam height, as well as defoaming time.
2. Materials and Methods
2.1. Materials and Reagents
The UDS solvent was obtained from Shandong Jinlu Environmental Protection Technology Co., Ltd. (Jining, China); L-FD7 lubricant was provided by Sinopec Great Wall Lubricant Company (Beijing, China); and 150N lubricant base oil was obtained from Dongguan Zhongxiang Fine Oil Co., Ltd. (Dongguan, China). Dodecyl succinic acid (>95%) was provided by Guangzhou Bifeng Trading Co., Ltd. (Guangzhou, China). Zinc dialkyl dithiophosphate (>95%) was purchased from Maya Reagent Co., Ltd. (Jiaxing, China). Coconut shell activated carbon was obtained from Shanghai Activated Carbon Factory (Shanghai, China).
2.2. Measurement of Kinematic Viscosity and Surface Tension
The kinematic viscosity of liquid samples was measured using an SYD-265C petroleum product kinematic viscosity tester (Shanghai Changji Geological Instrument Co., Ltd., Shanghai, China). The surface tension was measured using a BZY-1 automatic surface tension instrument (Shanghai Hengping Instrument Factory, Shanghai, China).
2.3. Foaming Performance Measurement
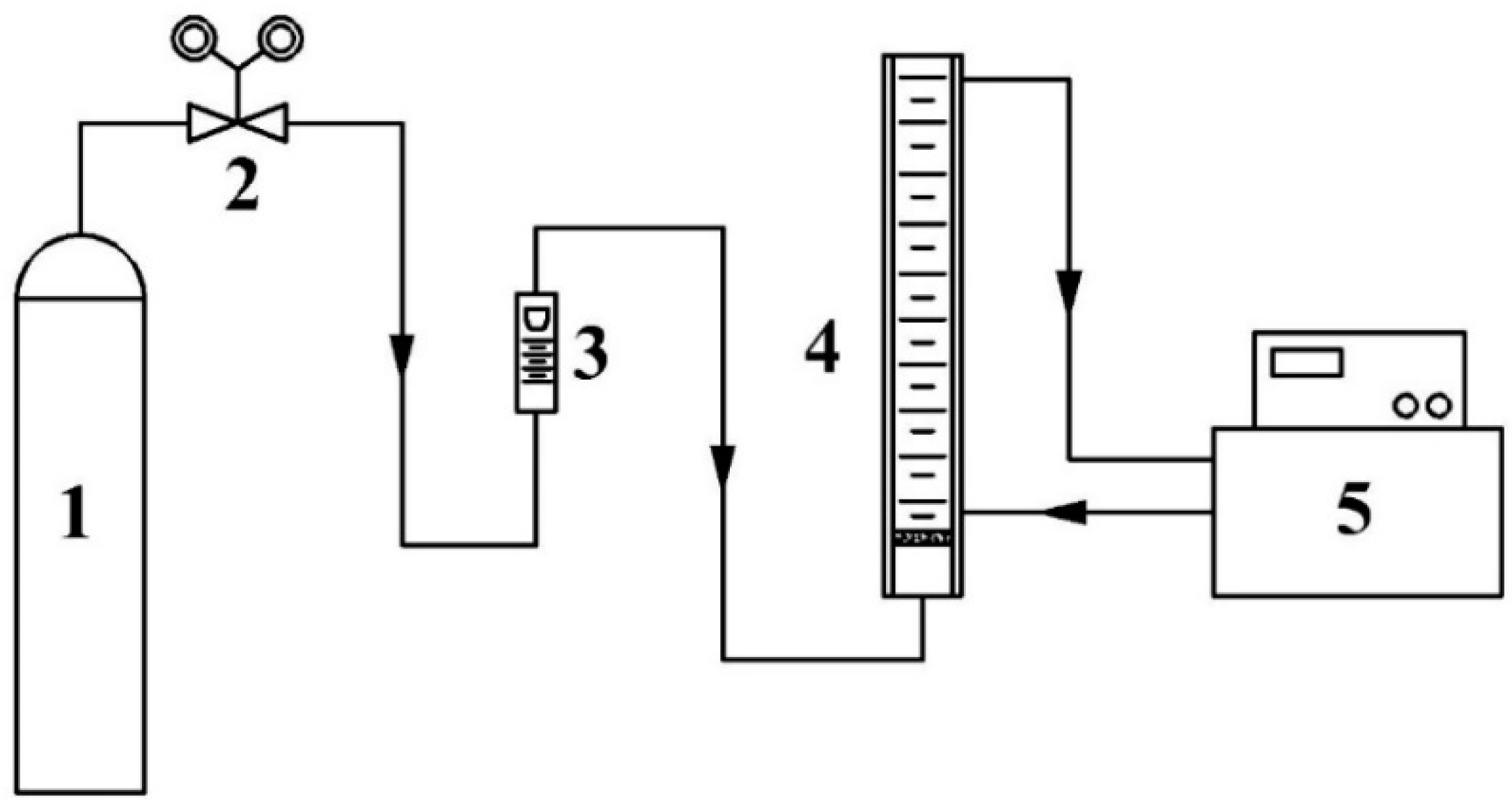
The foaming performance of the UDS solution was measured according to our previously reported method [25]. The experimental setup for the measurement of the foaming performance of the solution is shown in Figure 1. Approximately 100 mL of aqueous solution with a UDS mass fraction of 40% was added to the glass foaming tube. During the experiment, the temperature of solution was fixed at 40 °C by using a thermostatic water bath. An N2 gas flow of 250mL/min was introduced into the tube from the bottom. Once the foam height of the solution in the tube reached a constant value, the value was recorded as the foam height, H, in mm. Then the N2 gas flow was stopped, and timing started. The time for thoroughly defoaming was recorded as the defoaming time, t, in s.
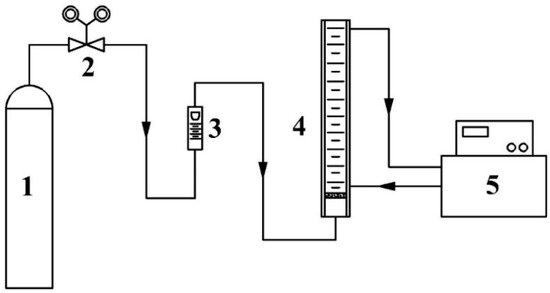
Figure 1.
Experimental flowchart for the foaming performance test of the solution (1—N2 cylinder, 2—pressure reducing valve, 3—gas flow meter, 4—foaming tube, and 5—thermostatic water bath).
3. Results and Discussion
3.1. Effect of Lubricant Content on Foaming Performance of UDS Solution
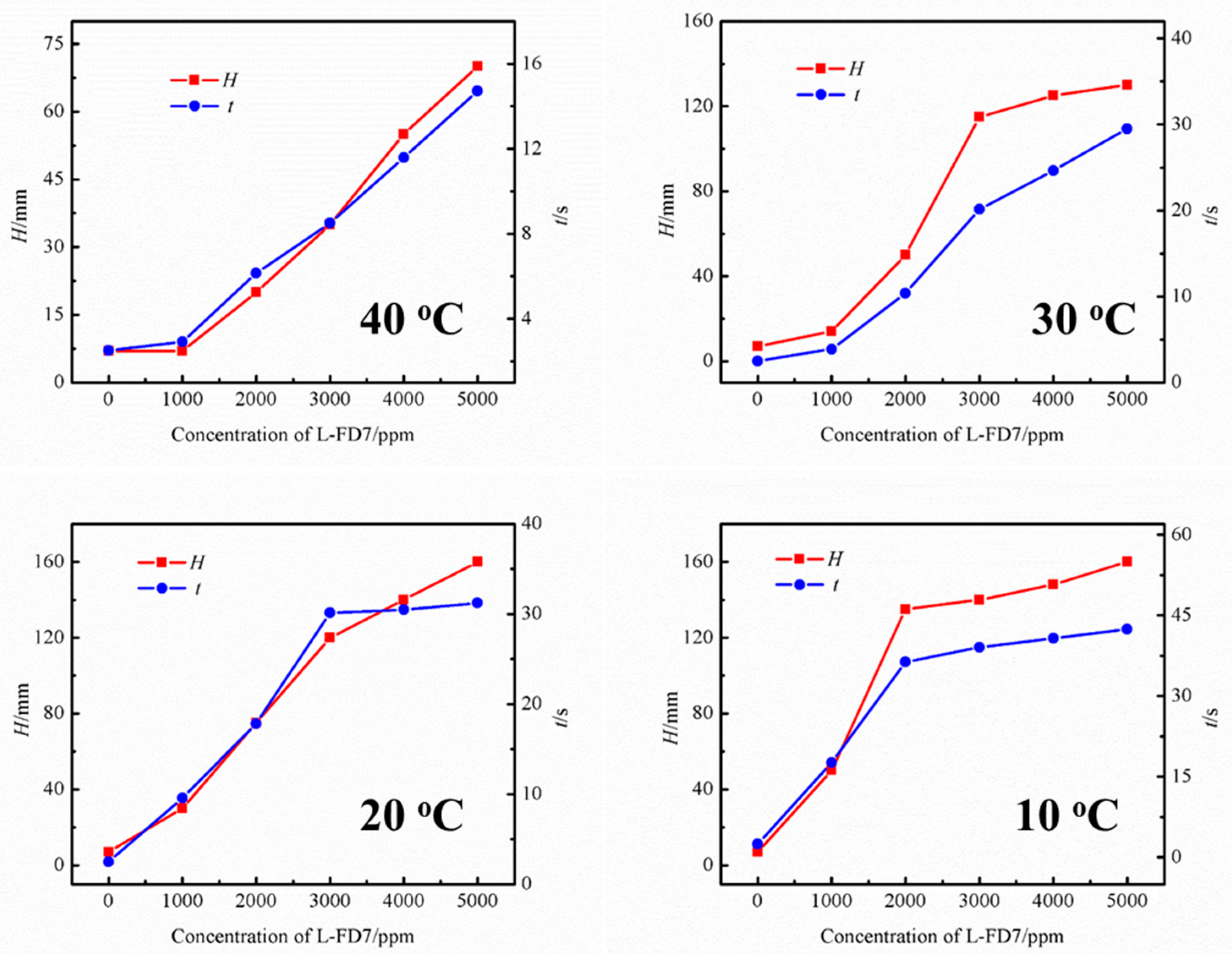
The effect of lubricants on the foaming performance of the UDS solution was investigated using the commercial L-FD7 lubricant at different temperatures. Figure 2 shows the effect of lubricant content on the foam height and defoaming time of the UDS solvent at different temperatures. In each temperature case, it can be observed that the foam height of the solution increases continuously, with the lubricant concentration in the UDS solvent rising to 5000 ppm. Also, the defoaming time increases monotonically, indicating an increased tendency to foaming. At 40 °C, compared to the fresh UDS solution with the same concentration, the solution containing lubricant shows significantly increasing tendency to foaming as the concentration of lubricant increases to 3000 ppm, where the foam height is 35 mm and the defoaming time is 8.50 s. When the concentration of lubricant is reduced to 1000 ppm, the foam height drops to 7 mm, which is very close to that of the UDS solution without lubricant. Accordingly, the solution indicates a short defoaming time of 2.91 s, suggesting the negligible effect of low content of L-FD7 lubricant on the foaming performance of the UDS solution.
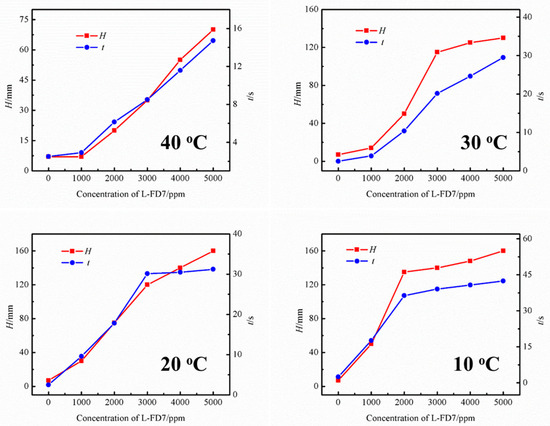
Figure 2.
Effect of lubricant content on the foaming performance of the UDS solvent at different temperatures.
At 30 °C, the foam height was 13 mm with the lubricant content in the solution of 1000 ppm. As the lubricant concentration in the solution increased to 2000 ppm, the solution showed serious foaming phenomenon, at which time, the foam height reached 50 mm and the corresponding defoaming time was 10.37 s. When the concentration increases from 1000 to 3000 ppm, the foaming tendency of the solution shows rapid growth. However, once the concentration exceeds 3000 ppm, the foaming tendency plateaus, indicating that the solubility of the lubricant in the solution has reached saturation. As for 20 °C, at the concentration of lubricant in the solution of 1000 ppm, the foam height of the solution was 30 mm and the corresponding defoaming time was 9.59 s. And the changing trend was very similar to that at 30 °C. In the range of 1000–3000 ppm, the foam height and defoaming time show greater variation. In the case of 10 °C, the foam height of the solution grows rapidly as the concentration rises to 2000 ppm; the growth rate of the foam height of the solution starts to decrease as the concentration rises higher than 2000 ppm. Furthermore, the cut-off point of the growth rate decreases from 3000 ppm at 20 and 30 °C to 2000 ppm at 10 °C. The main reason is that as the temperature decreases, the solubility of the lubricant in the UDS solution also decreases.
In conclusion, lubricants significantly impact the foaming performance of the UDS solution. The foaming performance of the solution gradually increased with the increase of lubricant content in the solution in all four temperature conditions. It is clearly indicated that the temperature has a significant effect on the foaming performance of the solution, and the foaming performance of the solution is greatly enlarged when the temperature is 20 °C or lower. Therefore, further experiments were conducted at 20 °C.
3.2. Effect of Different Components on the Foaming Performance of Amine Liquid
The lubricant is composed of three main components: base oil, dodecyl succinic acid (a rust inhibitor), and zinc dialkyl dithiophosphate (an anti-wear agent). In the consideration of the compositional complexity of the lubricant, experiments were conducted to distinguish the effect of each component of the lubricant on the properties of the UDS solution in order to reveal the different roles of each lubricant component in determining the foaming performance of the UDS solution.
3.2.1. Influence of Lubricant Base Oil
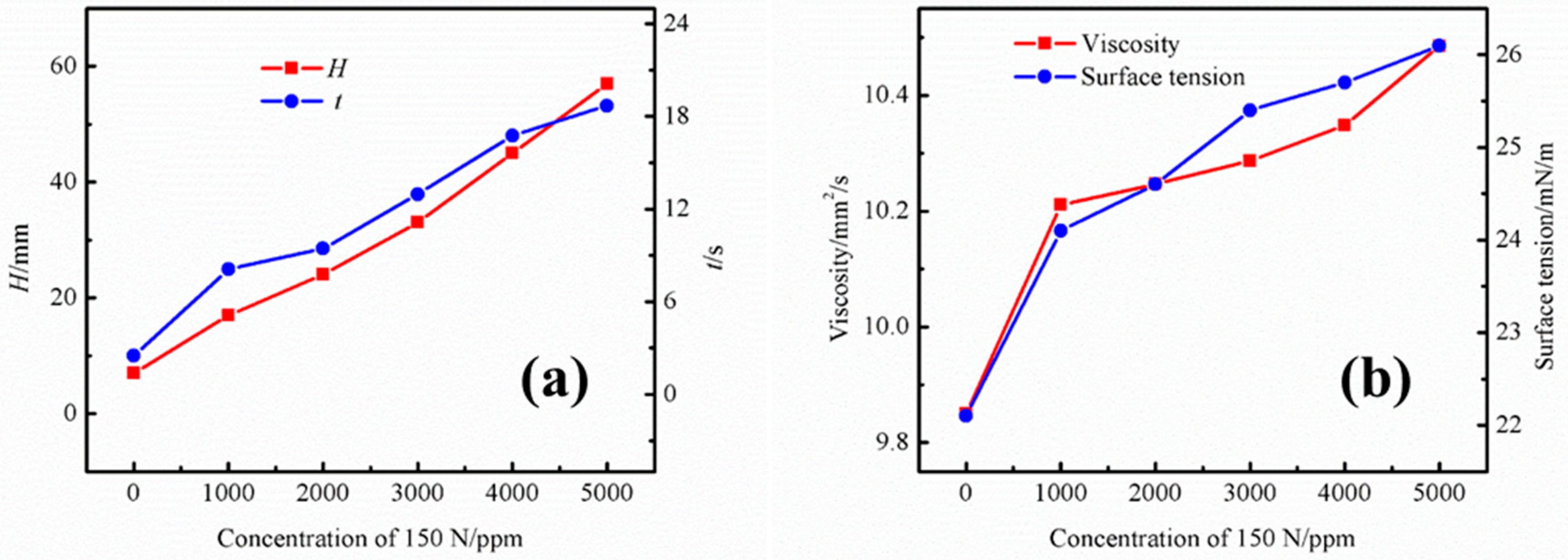
To examine the effect of base oil on the foaming performance of the UDS solution, the base oil of the commercial 150 N lubricant was used in the experiment. Figure 3a shows the effect of different concentrations of base oil on the foaming performance of the UDS solution. After the addition of base oil components into the UDS solution, it can be observed that the foaming tendency of the solution increases significantly with the increasing concentration of the base oil. The foaming height is 17 mm at the concentration of 1000 ppm, and the corresponding defoaming time is 8.1 s. As the concentration increases to 5000 ppm, the foam height increases significantly to 57 mm, and the corresponding defoaming time is 18.69 s. Figure 3b shows the effect of different concentrations of base oil on the physical properties of the UDS solution. It is apparent that the viscosity and surface tension of the solution also increases with an increase of base oil content in the solution. As the concentration is increased to 5000 ppm, the viscosity and surface tension rise to 10.49 mm2/s and 26.1 mN/m, respectively. The increase in viscosity causes a significant increase in the film strength of the foam, which, in turn, leads to a significant decrease in the film decay rate, resulting in remarkably increased foaming tendency.
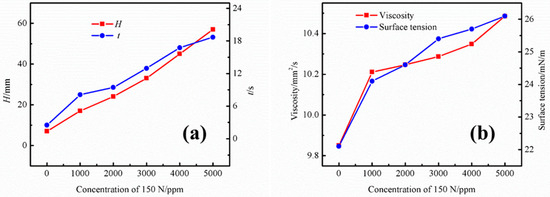
Figure 3.
Effects of different lubricant base oil concentrations on the (a) foaming performance and (b) physical properties of the UDS solution.
3.2.2. Effect of Dodecyl Succinic Acid
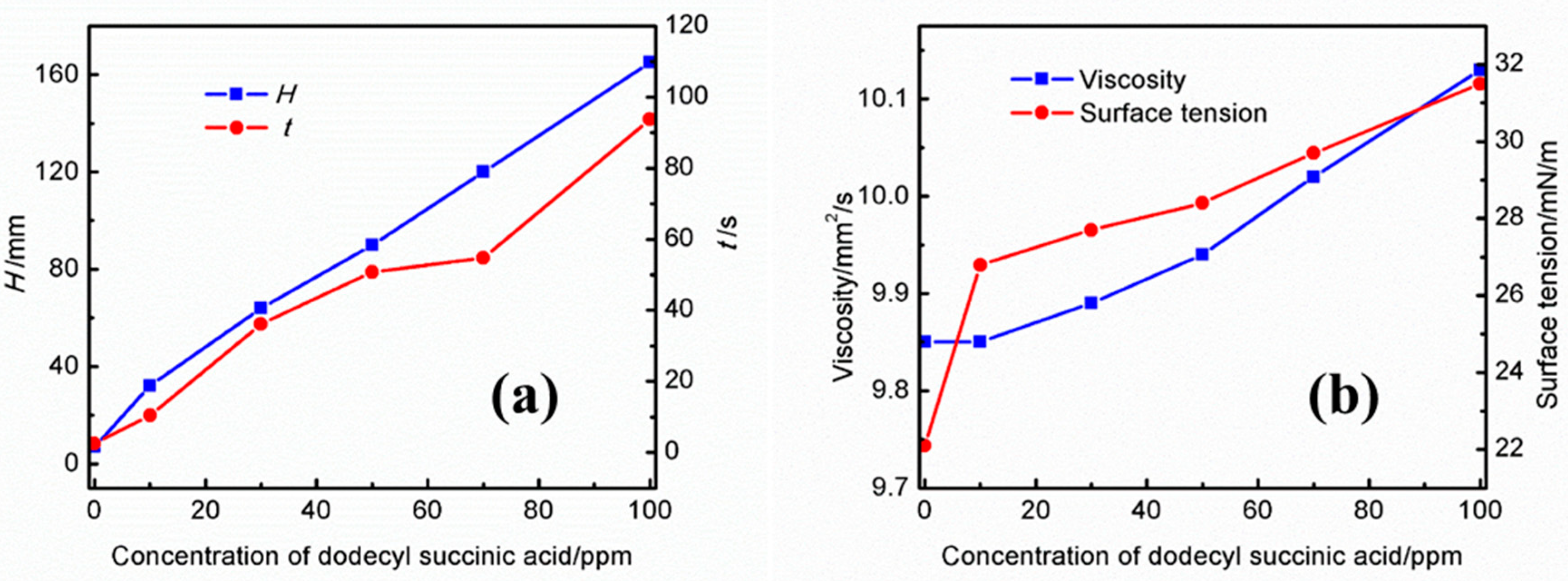
Effects of different concentrations of dodecyl succinic acid on the foaming performance of the UDS solution are presented in Figure 4a. As shown in this figure, the foaming height and the defoaming time increase up to 100 ppm with the concentration of dodecyl succinic acid in the UDS solution. At a concentration of 10 ppm, the foam height is 32 mm and the defoaming time is 10.47 s. The foam height and defoaming time increase to 165 mm and 93.71 s as the concentration of dodecyl succinic acid in the solution increases to 100 ppm. Figure 4b shows the effect of different concentrations of dodecyl succinic acid on the physical properties of the solution. With an increase in the concentration of dodecyl succinic acid, although the increase of surface tension makes the foaming tendency of the amine solution decrease, the increase of viscosity causes an increase in foam stability and a decayed defoaming rate in the solution, which leads to increasing the foaming tendency of the UDS solution. Moreover, the long water-repellent chain of dodecyl succinic acid reduces the gas diffusion rate, which also contributes to the high foam stability.
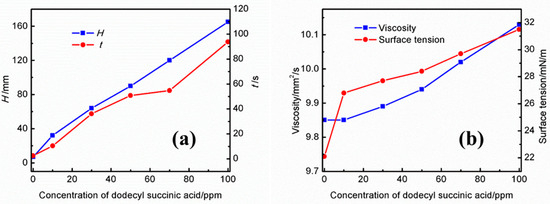
Figure 4.
Effects of different concentrations of dodecyl succinic acid on the (a) foaming performance and (b) physical properties of the UDS solution.
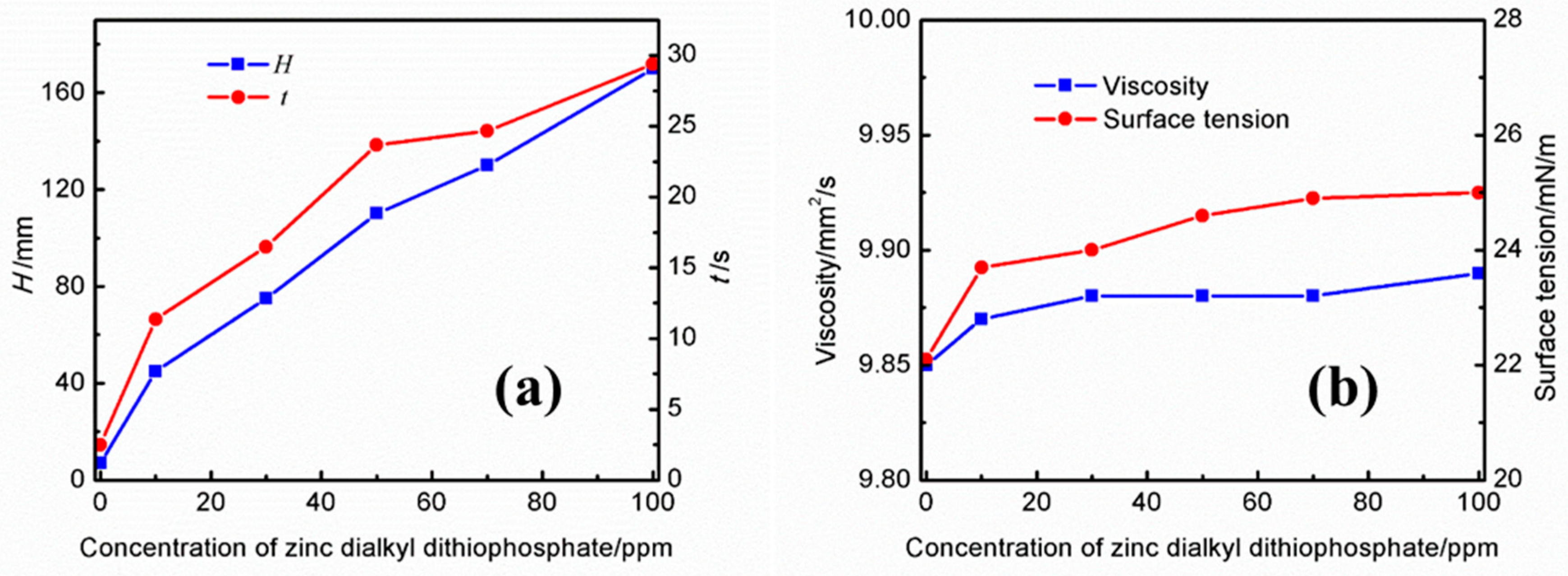
3.2.3. Effect of Zinc Dialkyl Dithiophosphate
The effects of different concentrations of zinc dialkyl dithiophosphate on the foaming performance and physical properties of the UDS solution are shown in Figure 5a. It can be observed that both the foaming height and the defoaming time increase with an increase in the concentration of zinc dialkyl dithiophosphate in the UDS solution. At a concentration of 10 ppm, the foam height was 45 mm and the defoaming time reached 11.37 s. When the concentration increased to 100 ppm, the foam height increased to 170 mm and the defoaming time reached 29.37 s. From Figure 5b, it can be noted that the viscosity of the solution increases slightly with the increase in the concentration of zinc dialkyl dithiophosphate. In addition, although the addition of zinc dialkyl dithiophosphate significantly increases the foaming height of the solution, the defoaming time is less than that of the solution containing the same concentration of dodecyl succinic acid.
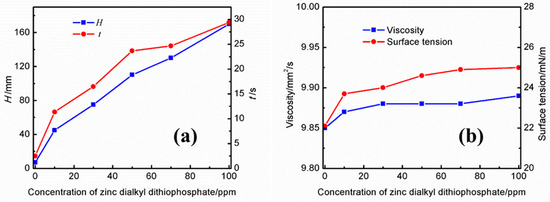
Figure 5.
Effects of different concentrations of zinc dialkyl dithiophosphate on the (a) foaming performance and (b) physical properties of the UDS solution.
3.3. Foaming Performance of Contaminated Amine Solution at Different Temperatures
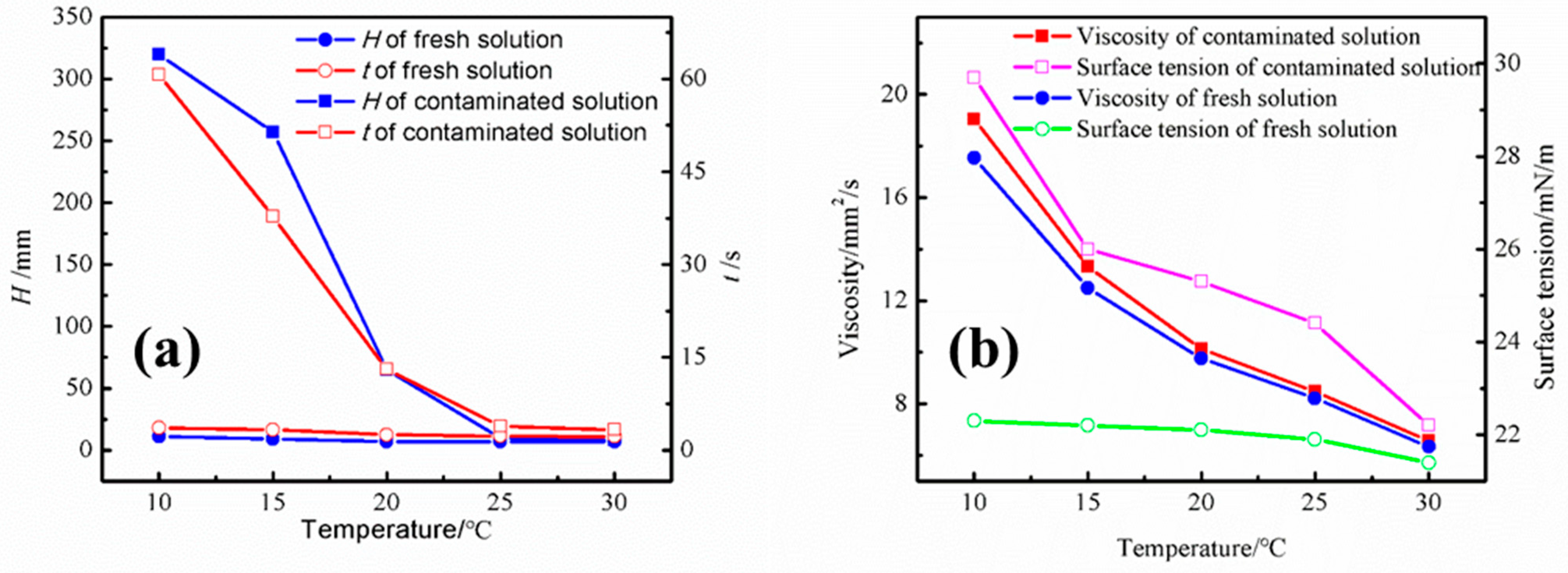
Figure 6 shows the foaming performance and physical properties of the contaminated amine solution at different temperatures. To better represent foaming behavior, we set the temperature within the low-temperature range (10–30 °C), where amine solutions are more prone to foaming. The foaming height of the contaminated amine solution at 25 °C and 30 °C is not very high, and the defoaming time is within the normal range. Therefore, to amplify the foaming phenomenon of the contaminated amine solution under laboratory conditions, the temperature of the foaming experiment was reduced to below 25 °C. At 20 °C, with a gas velocity of 250 mL/min, the foaming height of the contaminated amine solution reached 65 mm, and the defoaming time significantly increased from 7 s in the fresh solution to 13.1 s in the contaminated solution. The foaming phenomenon became even more pronounced at 10 °C. The figure shows that the foaming of the amine solution is closely related to temperature, with the foaming tendency decreasing as the temperature rises. The viscosity and surface tension of the contaminated amine solution at different temperatures are also presented in Figure 6. As compared to the fresh solution, the contaminated solution shows higher viscosity and surface tension. At a temperature of 10 °C, the surface tension increases from 22.3 mN/m in the fresh solution to 29.7 mN/m in the contaminated solution.
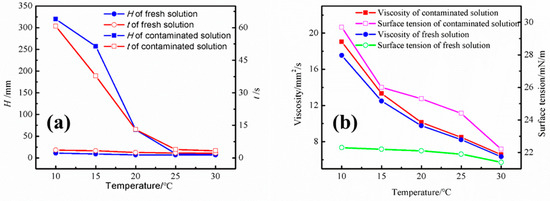
Figure 6.
The (a) foaming performance and (b) physical properties of the contaminated amine solution at different temperatures.
3.4. The Prediction Model of Foam Height of UDS Solution
When contaminants are introduced into the desulfurization solution, they change the physical properties of the solution, such as surface tension, viscosity, and density, which are closely related to the formation, merging, discharging, and rupture of bubbles, thus affecting the foaming performance of the desulfurization solution. Pilon et al. [26] obtained two dimensionless parameters, ∏1 and ∏2, via dimensional analysis using Equations (1) and (2). Re, Fr, and Ca are the Reynolds number, Froude number, and capillary number, respectively, with a dimension of 1, and the expressions are described in Equation (3). The semi-empirical Equation (4) for predicting the foam thickness in steady-state and isothermal conditions is obtained by correlating ∏1 and ∏2 by the power law relationship equation. The model correlates the foam thickness with the physical properties of the solution, such as density, viscosity, and surface tension, the average bubble radius, and the gas velocity.
where H is the foam height, m; ρ is the liquid density, g/mL; μ is the liquid viscosity, mPa/s; σ is the liquid surface tension, mN/m; j is the gas flow rate, m/s; r is Average bubble radius, mm; g is the gravitational acceleration, m/s2; Re is the Reynolds number; Fr is the Froude number; Ca is the Capillary number; K is the constant parameter determined from experimental data; n is the constant parameter determined from experimental data; ∏1 is the ratio of the gravitational force to the viscous force; and ∏2 is the ratio of the viscous force to the surface tension force times the ratio of the steady-state foam characteristic height to the bubble characteristic dimension.
Equation (4) can transfer the influence of macroscopic factors (such as different types of contaminants) on foaming performance into the influence of the physical properties of the solution. It can both mechanistically explain the causes of the changes in the foaming properties of the solution and predict the foaming properties of the desulfurization solution to guide engineers to take reasonable measures to regulate the foaming properties of the solution and ensure the safe and stable operation of desulfurization units.
Alhseinat et al. [14] combined the foaming experimental data with the model proposed by Pilon et al. The values of model constants K and n were obtained using mathematical iterations and multiple stochastic nonlinear regression techniques. A model for predicting the foaming performance of the amine solution in the steady state of the gas phase was developed as in Equation (5).
Table 1 summarizes the physical properties and foaming results of the UDS solution with different contaminant contents at two apparent gas velocities, and the specific values of parameters K and n can be fitted to Equations (3) and (4), which are 25.6 and −1.3, respectively. Therefore, the prediction model of the foaming performance of the UDS solution can be given as Equation (6).

Table 1.
The physical properties and foaming data of the UDS solution containing different amounts of contaminants at different gas velocities (under 1 atm and 25 °C).
Process contaminants, such as lubricant and additives, affect the foaming properties of the UDS solution via changing the surface tension, viscosity, density, and other physical properties of the solution, which will seriously affect the operation of the desulfurization unit. In order to avoid this unfavorable situation, various measures should be taken to reduce the introduction of various contaminants into the desulfurization solution.
3.5. Regeneration of Contaminated Amine Solution via Activated Carbon Adsorption
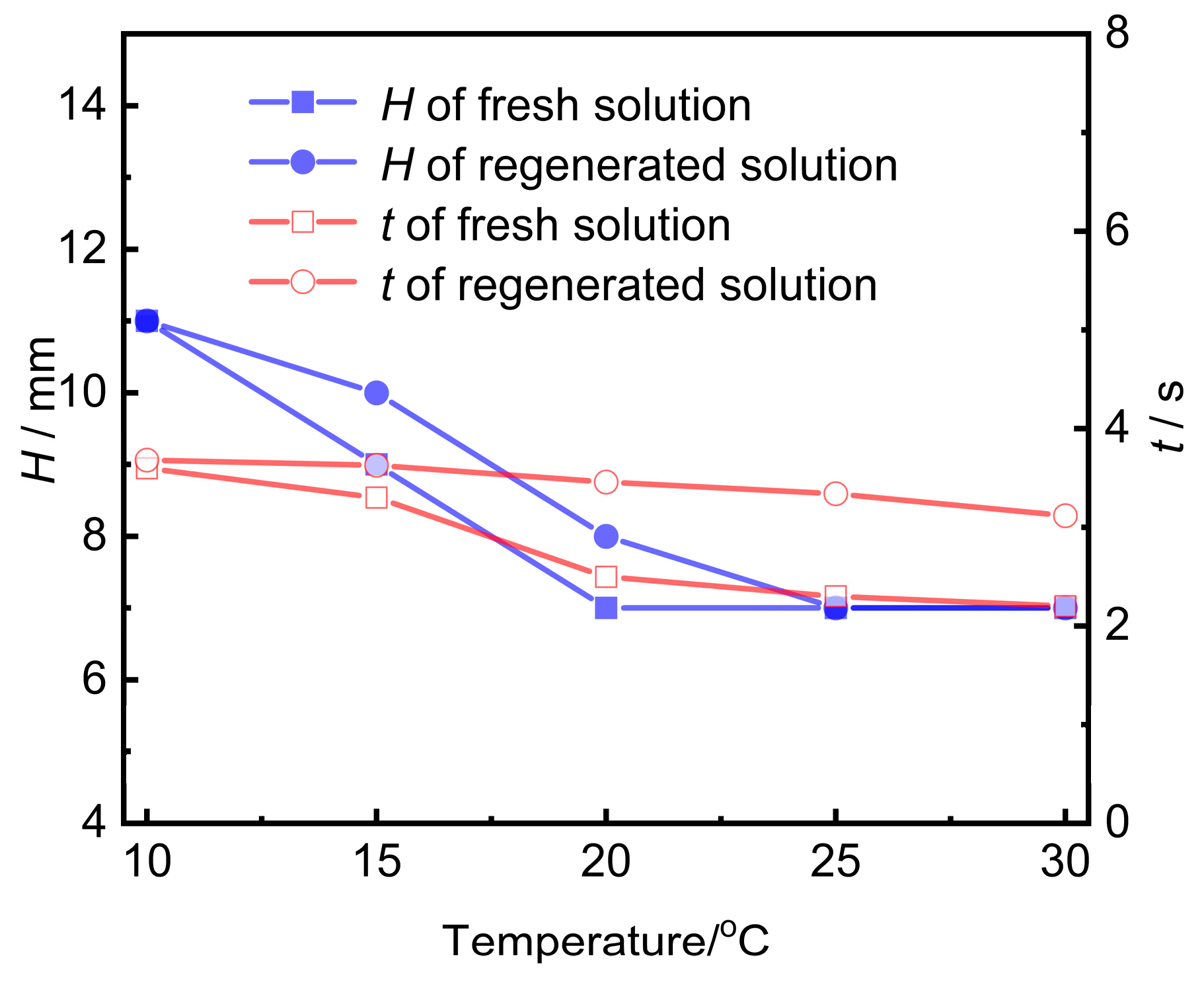
Activated carbon was utilized to purify the contaminated UDS solution. The contaminated amine solution was subjected to adsorption regeneration using 20–40 mesh coconut shell activated carbon. The foaming performance of the amine solution undergoing post-adsorption treatment was evaluated and compared with that of a fresh amine solution, as illustrated in Figure 7. It can be observed from Figure 7 that the foam height of the amine solution after activated carbon treatment is quite similar to that of the fresh amine solution, and the defoaming time was reduced from 60.7 s before treatment (see Figure 6a) to less than 3.6 s.
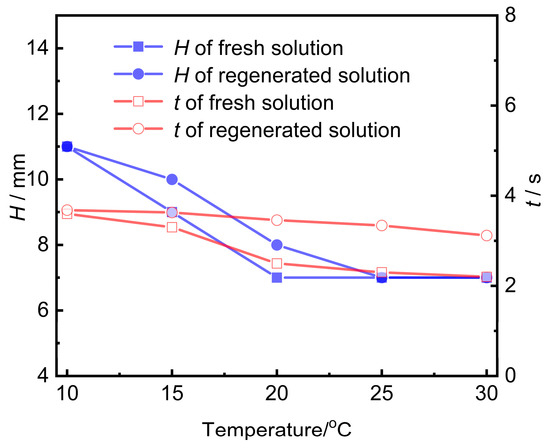
Figure 7.
The foaming performance of the regenerated amine solution at different temperatures.
4. Conclusions
This study systematically investigated the effects of lubricant contaminants and their components (base oil, dodecyl succinic acid, and zinc dialkyl dithiophosphate) on the foaming performance of the UDS solvent. The experimental results revealed that increased lubricant content significantly enhances foam height and defoaming time, with temperature playing a critical role—lower temperatures (≤20 °C) markedly amplify foaming tendencies due to reduced solubility and elevated viscosity. The additives of dodecenyl succinic acid and zinc dialkyl dithiophosphate have a more significant influence on the foaming performance of desulfurization solution than the lubricant base oil. Additives change the physical properties of the desulfurization solution, such as density, viscosity, and surface tension, which, in turn, lead to the deterioration of the foaming performance of the solution. A predictive model was established, correlating foam height with solution properties (surface tension, viscosity), and the model parameters K and n were observed to be 25.6 and −1.3, respectively. The deviation of the predicted foam height from the experimental foam height is no more than 7%. Additionally, activated carbon adsorption effectively regenerated the contaminated UDS solvent, reducing defoaming time from 60.7 s to <3.6 s, restoring performance close to that of the fresh solvent. Amine solution foaming can lead to reduced plant processing capacity, increased amine loss, higher equipment operating costs, elevated energy consumption, and shortened equipment lifespans, all of which significantly impact the economic performance of purification units. This study provides actionable insights for optimizing solvent stability and operational efficiency in natural gas purification processes.
Author Contributions
Conceptualization, J.Z. and H.S.; methodology, J.Z. and C.L.; validation, J.Z., C.L. and X.X; formal analysis, J.Z. and X.X.; investigation, J.Z. and Y.Y.; resources, H.S.; data curation, J.Z. and X.X.; writing—original draft preparation, J.Z. and C.L.; writing—review and editing, H.S.; visualization, X.X.; supervision, H.S.; project administration, H.S.; funding acquisition, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sinopec, grant number: No. 34450521-24-ZC0699-0002.
Data Availability Statement
Requests for additional information related to the study can be directed to the corresponding author.
Conflicts of Interest
Authors Jiandong Zhang, Changchun Li and Yang Yang were employed by the company Sinopec Guangyuan Natural Gas Purification Co., Ltd., Guangyuan 618400, China. Author Xiaolong Xu was employed by the company Sinopec Shanghai Gaoqiao Petrochemical Co., Ltd., Shanghai 200129, China. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Liu, Y.; Wu, D.; Chen, M.; Zhang, B.; Chen, J.; Liu, Y. Identification of methyldiethanolamine degradation products and their influence on foaming properties during the desulfurization process for high-sulfurous natural gas. Ind. Eng. Chem. Res. 2015, 54, 5836–5841. [Google Scholar] [CrossRef]
- Chang, H.G. Gas desulfurization device amine solution foaming causes and understanding. Chem. Eng. Oil Gas 1995, 24, 60–63. [Google Scholar]
- Pauley, C.R. Face the facts about amine foaming. Chem. Eng. Prog. 1991, 87, 33–38. [Google Scholar]
- Stewart, E.J.; Lanning, R.A. Reduce amine plant solvent losses, Part 1. Hydrocarb. Process. 1994, 73, 67–81. [Google Scholar]
- Knudsen, J.N.; Jensen, J.N.; Vilhelmsen, P.J.; Biede, O. Experience with CO2 capture from coal flue gas in pilot-scale: Testing of different amine solvents. Energy Procedia 2009, 1, 783–790. [Google Scholar] [CrossRef]
- Liebermann, N.P. Amine appearance signals condition of system. Oil Gas J. 1980, 78, 115–120. [Google Scholar]
- Engel, D.B.; Williams, S.; Heinen, A. Activated carbon impact on MDEA amine solutions. Filter Separat. 2015, 52, 38–42. [Google Scholar]
- Abdi, M.A.; Golkar, M.M.; Meisen, A. Improve Contaminant Control in Amine Systems. Hydrocarb. Process. 2001, 80, 102C–102I. [Google Scholar]
- AI-Qahtani, M.H.; Garland, S.A.P. Khursaniyah gas plant experience with foaming during startup of Karan non-associated gas. In Proceedings of the International Petroleum Technology Conference, Beijing, China, 26–28 March 2013. [Google Scholar]
- Rumaih, M.A. Acid Gas Removal Unit Successful Switch from Silicon to Polyglycol Antifoam to Eliminate Foaming. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 11–14 November 2019. [Google Scholar]
- Edalatpour, A.; Abbasi, M.; Riahi, S.; Sabet, N.S.M.; Tavakoli, O. Investigation of foaming tendency of aqueous mixture of MDEA+IPAE for carbon dioxide absorption. J. CO2 Util. 2022, 62, 102079. [Google Scholar] [CrossRef]
- Al-Dhafeeri, M.A. Identifying sources key to detailed troubleshooting of amine foaming. Oil Gas J. 2007, 105, 56–67. [Google Scholar]
- Thitakamol, B.; Veawab, A. Foaming behavior in CO2 absorption process using aqueous solutions of single and blended alkanolamines. Ind. Eng. Chem. Res. 2008, 47, 216–225. [Google Scholar] [CrossRef]
- Alhseinat, E.; Pal, P.; Keewan, M. Foaming study combined with physical characterization of aqueous MDEA gas sweetening solutions. J. Nat. Gas Sci. Eng. 2014, 17, 49–57. [Google Scholar] [CrossRef]
- Pal, P.; Banat, F.; AlShoaibi, A. Adsorptive removal of heat stable salt anions from industrial lean amine solvent using anion exchange resins from gas sweetening unit. J. Nat. Gas Sci. Eng. 2013, 15, 14–21. [Google Scholar] [CrossRef]
- Alhseinat, E.; Pal, P.; Ganesan, A.; Banat, F. Effect of MDEA degradation products on foaming behavior and physical properties of aqueous MDEA solutions. Int. J. Greenh. Gas Con. 2015, 37, 280–286. [Google Scholar] [CrossRef]
- Chen, X.; Freeman, S.A.; Rochelle, G.T. Foaming of aqueous piperazine and monoethanolamine for CO2 capture. Int. J. Greenh. Gas Con. 2011, 5, 381–386. [Google Scholar] [CrossRef]
- Ju, H.T.; ElMoudir, W.; Aboudheir, A.; Mahinpey, N. Development of a facile reclaiming process for degraded alkanolamine and glycol solvents used for CO2 capture systems. Int. J. Greenh. Gas Con. 2018, 74, 174–181. [Google Scholar] [CrossRef]
- Thitakamol, B.; Veawab, A.; Aroonwilas, A. Development of a mechanistic foam model for alkanolamine-based CO2 absorbers fitted with sheet-metal structured packing. Int. J. Greenh. Gas Con. 2015, 33, 77–88. [Google Scholar] [CrossRef]
- Thitakamol, B.; Veawab, A. Foaming model for CO2 absorption process using aqueous monoethanolamine solutions. Colloids Surf. A Physicochem. Eng. Asp. 2009, 349, 125–136. [Google Scholar] [CrossRef]
- Thitakamol, B.; Veawab, A.; Aroonwilas, A. Foaming in amine-based CO2 capture process: Experiment, modeling and simulation. Energy Procedia 2009, 1, 1381–1386. [Google Scholar] [CrossRef]
- Zhang, J.H.; Shen, B.X.; Sun, H.; Liu, J.C. A study on the desulfurization performance of solvent UDS for purifying high sour natural gas. Petrol. Sci. Technol. 2011, 29, 48–58. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, B.X.; Sun, H.; Liu, J.C. Removal of organosulfurs from liquefied petroleum gas in a fiber film contactor using a new formulated solvent. Fuel Process. Technol. 2015, 140, 76–81. [Google Scholar] [CrossRef]
- Zhang, F.; Shen, B.X.; Sun, H.; Liu, J.C.; Liu, L. Rational Formulation Design and Commercial Application of a New Hybrid Solvent for Selectively Removing H2S and Organosulfurs from Sour Natural Gas. Energy Fuel 2016, 30, 12–19. [Google Scholar] [CrossRef]
- Ke, Y.; Shen, B.X.; Sun, H.; Liu, J.C.; Xu, X.L. Study on foaming of formulated solvent UDS and improving foaming control in acid natural gas sweetening process. J. Nat. Gas Sci. Eng. 2016, 28, 271–279. [Google Scholar] [CrossRef]
- Pilon, L.; Fedorov, A.G.; Viskanta, R. Steady-state thickness of liquid-gas foams. J. Colloid Interface Sci. 2001, 242, 425–436. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).