Returning to Nature for the Design of Sorptive Phases in Solid-Phase Microextraction
Abstract
1. Introduction
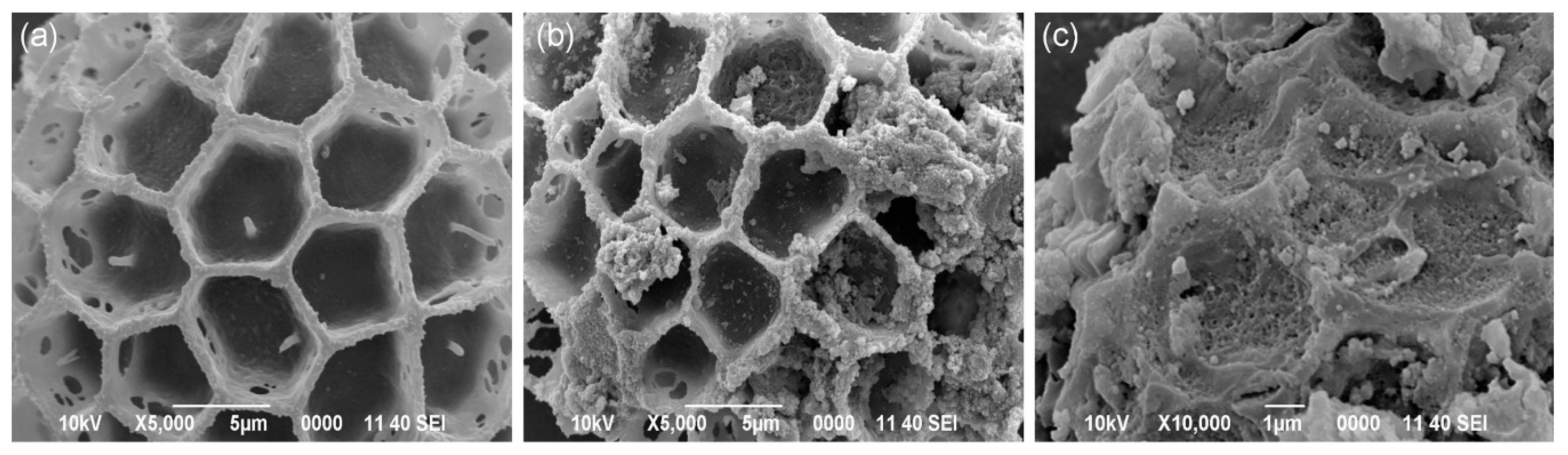
2. Cork
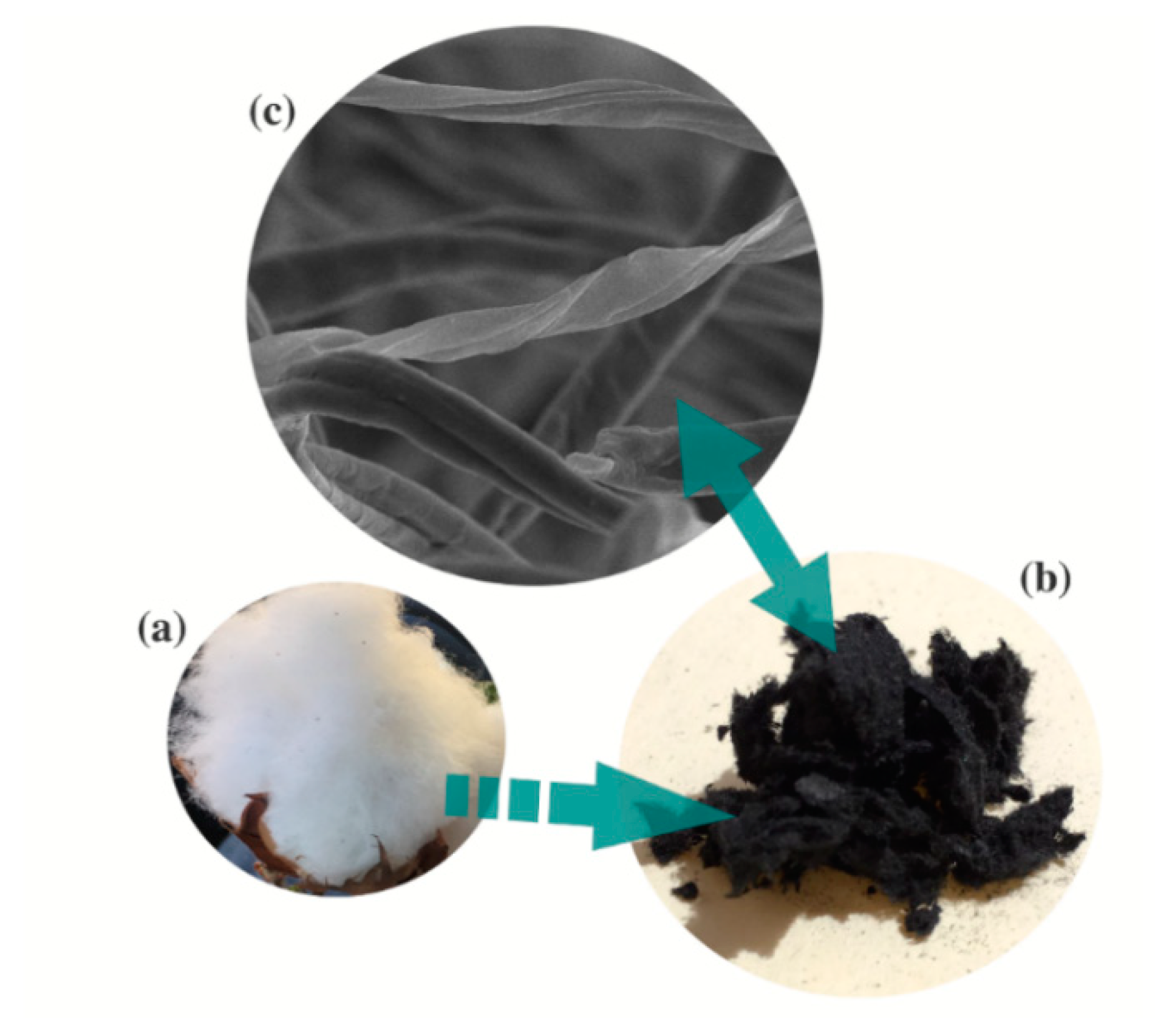
3. Cotton
4. Pollen
5. Agricultural By-Products
6. Trends, Natural Products for Simplifying the Analytical Workflow
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, R.B.; Karousos, N.G.; Cowham, E.; Davis, J.; Billington, S. Covert Approaches to Countering Adult Chemophobia. J. Chem. Educ. 2008, 85, 379. [Google Scholar] [CrossRef]
- Chalupa, R.; Nesměrák, K. Analytical chemistry as a tool for suppressing chemophobia: An introduction to the 5E-principle. Monatshefte für Chemie-Chemical Monthly 2018, 149, 1527–1534. [Google Scholar] [CrossRef]
- Valcárcel, M.; Christian, G.D.; Lucena, R. Teaching Social Responsibility in Analytical Chemistry. Anal. Chem. 2013, 85, 6152–6161. [Google Scholar] [CrossRef] [PubMed]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green analytical chemistry. TrAC Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Koel, M.; Kaljurand, M. Green Analytical Chemistry; RSC Publishing: Cambridge, UK, 2010; ISBN 978-1-84755-872-5. [Google Scholar]
- De la Guardia, M.; Garrigues, S. Handbook of Green Analytical Chemistry; John Wiley & Sons: Chichester, UK, 2012; ISBN 9780470972014. [Google Scholar]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Valcárcel, M.; Cárdenas, S.; Lucena, R. Microextraction techniques. Anal. Bioanal. Chem. 2014, 406, 1999–2000. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. TrAC Trends Anal. Chem. 2015, 71, 2–8. [Google Scholar] [CrossRef]
- Pacheco-Fernández, I.; Pino, V. Green solvents in analytical chemistry. Curr. Opin. Green Sustain. Chem. 2019, 18, 42–50. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Rutkowska, M.; Owczarek, K.; Tobiszewski, M.; Namieśnik, J. Extraction with environmentally friendly solvents. TrAC Trends Anal. Chem. 2017, 91, 12–25. [Google Scholar] [CrossRef]
- Marino, T.; Galiano, F.; Molino, A.; Figoli, A. New frontiers in sustainable membrane preparation: CyreneTM as green bioderived solvent. J. Membr. Sci. 2019, 580, 224–234. [Google Scholar] [CrossRef]
- de los Ángeles, M.; Boiteux, J.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Natural deep eutectic solvents-mediated extractions: The way forward for sustainable analytical developments. Anal. Chim. Acta 2018, 1038, 1–10. [Google Scholar] [CrossRef]
- Kirchherr, J.; Reike, D.; Hekkert, M. Conceptualizing the circular economy: An analysis of 114 definitions. Resour. Conserv. Recycl. 2017, 127, 221–232. [Google Scholar] [CrossRef]
- Silva, S.P.; Sabino, M.A.; Fernandes, E.M.; Correlo, V.M.; Boesel, L.F.; Reis, R.L. Cork: Properties, capabilities and applications. Int. Mater. Rev. 2005, 50, 345–365. [Google Scholar] [CrossRef]
- Lagorce-Tachon, A.; Karbowiak, T.; Loupiac, C.; Gaudry, A.; Ott, F.; Alba-Simionesco, C.; Gougeon, R.D.; Alcantara, V.; Mannes, D.; Kaestner, A.; et al. The cork viewed from the inside. J. Food Eng. 2015, 149, 214–221. [Google Scholar] [CrossRef]
- Pintor, A.M.A.; Ferreira, C.I.A.; Pereira, J.C.; Correia, P.; Silva, S.P.; Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Use of cork powder and granules for the adsorption of pollutants: A review. Water Res. 2012, 46, 3152–3166. [Google Scholar] [CrossRef]
- Monteiro, M.K.S.; Paiva, S.S.M.; da Silva, D.R.; Vilar, V.J.P.; Martínez-Huitle, C.A.; dos Santos, E.V. Novel cork-graphite electrochemical sensor for voltammetric determination of caffeine. J. Electroanal. Chem. 2019, 839, 283–289. [Google Scholar] [CrossRef]
- Novais, R.M.; Caetano, A.P.F.; Seabra, M.P.; Labrincha, J.A.; Pullar, R.C. Extremely fast and efficient methylene blue adsorption using eco-friendly cork and paper waste-based activated carbon adsorbents. J. Clean. Prod. 2018, 197, 1137–1147. [Google Scholar] [CrossRef]
- Domingues, V.; Alves, A.; Cabral, M.; Delerue-Matos, C. Sorption behaviour of bifenthrin on cork. J. Chromatogr. A 2005, 1069, 127–132. [Google Scholar] [CrossRef]
- Olivella, M.À.; Jové, P.; Oliveras, A. The use of cork waste as a biosorbent for persistent organic pollutants–Study of adsorption/desorption of polycyclic aromatic hydrocarbons. J. Environ. Sci. Health Part A 2011, 46, 824–832. [Google Scholar] [CrossRef]
- de Aguiar, T.R.; Guimarães Neto, J.O.A.; Şen, U.; Pereira, H. Study of two cork species as natural biosorbents for five selected pesticides in water. Heliyon 2019, 5, e01189. [Google Scholar] [CrossRef]
- Mallek, M.; Chtourou, M.; Portillo, M.; Monclús, H.; Walha, K.; ben Salah, A.; Salvadó, V. Granulated cork as biosorbent for the removal of phenol derivatives and emerging contaminants. J. Environ. Manag. 2018, 223, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Castellar, J.A.C.; Formosa, J.; Fernández, A.I.; Jové, P.; Bosch, M.G.; Morató, J.; Brix, H.; Arias, C.A. Cork as a sustainable carbon source for nature-based solutions treating hydroponic wastewaters—Preliminary batch studies. Sci. Total. Environ. 2019, 650, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Pirozzi, C.; Pontoni, L.; Fabbricino, M.; Bogush, A.; Campos, L.C. Effect of organic matter release from natural cork used on bisphenol a removal from aqueous solution. J. Clean. Prod. 2020, 244, 118675. [Google Scholar] [CrossRef]
- Silva, B.; Martins, M.; Rosca, M.; Rocha, V.; Lago, A.; Neves, I.C.; Tavares, T. Waste-based biosorbents as cost-effective alternatives to commercial adsorbents for the retention of fluoxetine from water. Sep. Purif. Technol. 2020, 235, 116139. [Google Scholar] [CrossRef]
- Neng, N.R.; Mestre, A.S.; Carvalho, A.P.; Nogueira, J.M.F. Cork-based activated carbons as supported adsorbent materials for trace level analysis of ibuprofen and clofibric acid in environmental and biological matrices. J. Chromatogr. A 2011, 1218, 6263–6270. [Google Scholar] [CrossRef]
- Neves Dias, A.; Simão, V.; Merib, J.; Carasek, E. Use of green coating (cork) in solid-phase microextraction for the determination of organochlorine pesticides in water by gas chromatography-electron capture detection. Talanta 2015, 134, 409–414. [Google Scholar] [CrossRef]
- Dias, A.N.; Simão, V.; Merib, J.; Carasek, E. Cork as a new (green) coating for solid-phase microextraction: Determination of polycyclic aromatic hydrocarbons in water samples by gas chromatography–mass spectrometry. Anal. Chim. Acta 2013, 772, 33–39. [Google Scholar] [CrossRef]
- Dias, A.N.; da Silva, A.C.; Simão, V.; Merib, J.; Carasek, E. A novel approach to bar adsorptive microextraction: Cork as extractor phase for determination of benzophenone, triclocarban and parabens in aqueous samples. Anal. Chim. Acta 2015, 888, 59–66. [Google Scholar] [CrossRef]
- Oenning, A.L.; Morés, L.; Dias, A.N.; Carasek, E. A new configuration for bar adsorptive microextraction (BAμE) for the quantification of biomarkers (hexanal and heptanal) in human urine by HPLC providing an alternative for early lung cancer diagnosis. Anal. Chim. Acta 2017, 965, 54–62. [Google Scholar] [CrossRef]
- Mafra, G.; Spudeit, D.; Brognoli, R.; Merib, J.; Carasek, E. Expanding the applicability of cork as extraction phase for disposable pipette extraction in multiresidue analysis of pharmaceuticals in urine samples. J. Chromatogr. B 2018, 1102–1103, 159–166. [Google Scholar] [CrossRef]
- Morés, L.; Dias, A.N.; Carasek, E. Development of a high-throughput method based on thin-film microextraction using a 96-well plate system with a cork coating for the extraction of emerging contaminants in river water samples. J. Sep. Sci. 2018, 41, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.M.S.; Mazurkievicz, M.; Lopez Calvo, A.M.; Debatin, V.; Micke, G.A.; Richter, P.; Rosero-Moreano, M.; da Rocha, E.C. Exploiting green sorbents in rotating-disk sorptive extraction for the determination of parabens by high-performance liquid chromatography with tandem electrospray ionization triple quadrupole mass spectrometry. J. Sep. Sci. 2018, 41, 4047–4054. [Google Scholar] [CrossRef] [PubMed]
- Cadorim, H.R.; Schneider, M.; Hinz, J.; Luvizon, F.; Dias, A.N.; Carasek, E.; Welz, B. Effective and High-Throughput Analytical Methodology for the Determination of Lead and Cadmium in Water Samples by Disposable Pipette Extraction Coupled with High-Resolution Continuum Source Graphite Furnace Atomic Absorption Spectrometry (HR-CS GF AAS). Anal. Lett. 2019, 52, 2133–2149. [Google Scholar] [CrossRef]
- Morés, L.; da Silva, A.C.; Merib, J.; Dias, A.N.; Carasek, E. A natural and renewable biosorbent phase as a low-cost approach in disposable pipette extraction technique for the determination of emerging contaminants in lake water samples. J. Sep. Sci. 2019, 42, 1404–1411. [Google Scholar] [CrossRef]
- Manzo, V.; Goya-Pacheco, J.; Arismendi, D.; Becerra-Herrera, M.; Castillo-Aguirre, A.; Castillo-Felices, R.; Rosero-Moreano, M.; Carasek, E.; Richter, P. Cork sheet as a sorptive phase to extract hormones from water by rotating-disk sorptive extraction (RDSE). Anal. Chim. Acta 2019, 1087, 1–10. [Google Scholar] [CrossRef]
- Vieira, C.M.S.; Mafra, G.; Brognoli, R.; Richter, P.; Rosero-Moreano, M.; Carasek, E. A high throughput approach to rotating-disk sorptive extraction (RDSE) using laminar cork for the simultaneous determination of multiclass organic micro-pollutants in aqueous sample by GC-MS. Talanta 2019, 120459. [Google Scholar] [CrossRef]
- Menachem, L. Handbook of Fiber Chemistry; CRC Press: Boca Raton, FL, USA, 2006; ISBN 9780824725655. [Google Scholar]
- Heidari, N.; Ghiasvand, A.; Abdolhosseini, S. Amino-silica/graphene oxide nanocomposite coated cotton as an efficient sorbent for needle trap device. Anal. Chim. Acta 2017, 975, 11–19. [Google Scholar] [CrossRef]
- Hong, K.H. Preparation and properties of phenolic compound/BTCA treated cotton fabrics for functional textile applications. Cellulose 2015, 22, 2129–2136. [Google Scholar] [CrossRef]
- Ragab, S.; El Nemr, A. Zirconyl chloride as a novel and efficient green Lewis acid catalyst for direct acetylation of cotton cellulose in the presence and absence of solvent. J. Polym. Res. 2019, 26, 156. [Google Scholar] [CrossRef]
- Harms, H.; Bernt, I.; Roggenstein, W. Regenerated Cellulose fiber 2013. International Application No. PCT/EP2012/072387, 6 June 2013. [Google Scholar]
- Edwards, J.; Fontenot, K.; Prevost, N.; Pircher, N.; Liebner, F.; Condon, B. Preparation, Characterization and Activity of a Peptide-Cellulosic Aerogel Protease Sensor from Cotton. Sensors 2016, 16, 1789. [Google Scholar] [CrossRef]
- Edwards, J.V.; Prevost, N.T.; Condon, B.; French, A.; Wu, Q. Immobilization of lysozyme-cellulose amide-linked conjugates on cellulose I and II cotton nanocrystalline preparations. Cellulose 2012, 19, 495–506. [Google Scholar] [CrossRef]
- Gao, L.; Wei, Y. Fabrication of boronate-decorated polyhedral oligomeric silsesquioxanes grafted cotton fiber for the selective enrichment of nucleosides in urine. J. Sep. Sci. 2016, 39, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Monier, M.; Ibrahim, A.A.; Metwally, M.M.; Badawy, D.S. Surface ion-imprinted amino-functionalized cellulosic cotton fibers for selective extraction of Cu(II) ions. Int. J. Biol. Macromol. 2015, 81, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, I.; Sfakianaki, A.; Gallego, M.; Stalikas, C.D. Graphene-coated cotton fibers as a sorbent for the extraction of multiclass pesticide residues from water and their determination by gas chromatography with mass spectrometry. J. Sep. Sci. 2015, 38, 836–843. [Google Scholar] [CrossRef]
- Karimi, M.; Dadfarnia, S.; Shabani, A.M.H. Application of Deep Eutectic Solvent Modified Cotton as a Sorbent for Online Solid-Phase Extraction and Determination of Trace Amounts of Copper and Nickel in Water and Biological Samples. Biol. Trace Elem. Res. 2017, 176, 207–215. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, X.; Ge, B.; Men, X.; Li, P.; Zhang, Z. Versatile fabrication of magnetic carbon fiber aerogel applied for bidirectional oil–water separation. Appl. Phys. A 2015, 120, 949–957. [Google Scholar] [CrossRef]
- Yue, X.; Jiang, F.; Zhang, D.; Lin, H.; Chen, Y. Preparation of adsorbent based on cotton fiber for removal of dyes. Fibers Polym. 2017, 18, 2102–2110. [Google Scholar] [CrossRef]
- Zuo, L.; Zhang, Y.; Zhang, L.; Miao, Y.-E.; Fan, W.; Liu, T. Polymer/Carbon-Based Hybrid Aerogels: Preparation, Properties and Applications. Materials (Basel) 2015, 8, 6806–6848. [Google Scholar] [CrossRef]
- Liu, R.-L.; Mao, S.; Wang, Y.; Wang, L.; Ge, Y.-H.; Xu, X.-Y.; Fu, Q. A mussel-inspired hybrid copolymer adhered to chitosan-coated micro-sized carbon fiber aerogels for highly efficient nanoparticle scavenging. Environ. Sci. Nano 2017, 4, 2164–2174. [Google Scholar] [CrossRef]
- Chiu, K.-L.; Ng, D.H.L. Synthesis and characterization of cotton-made activated carbon fiber and its adsorption of methylene blue in water treatment. Biomass Bioenergy 2012, 46, 102–110. [Google Scholar] [CrossRef]
- Duan, X.; Srinivasakannan, C.; Wang, X.; Wang, F.; Liu, X. Synthesis of activated carbon fibers from cotton by microwave induced H3PO4 activation. J. Taiwan Inst. Chem. Eng. 2017, 70, 374–381. [Google Scholar] [CrossRef]
- Sun, Y.; Yue, Q.; Gao, B.; Li, Q.; Huang, L.; Yao, F.; Xu, X. Preparation of activated carbon derived from cotton linter fibers by fused NaOH activation and its application for oxytetracycline (OTC) adsorption. J. Colloid Interface Sci. 2012, 368, 521–527. [Google Scholar] [CrossRef]
- Hina, K.; Zou, H.; Qian, W.; Zuo, D.; Yi, C. Preparation and performance comparison of cellulose-based activated carbon fibres. Cellulose 2018, 25, 607–617. [Google Scholar] [CrossRef]
- Li, Z.; Jia, Z.; Ni, T.; Li, S. Adsorption of methylene blue on natural cotton based flexible carbon fiber aerogels activated by novel air-limited carbonization method. J. Mol. Liq. 2017, 242, 747–756. [Google Scholar] [CrossRef]
- Wanassi, B.; Ben Hariz, I.; Ghimbeu, C.M.; Vaulot, C.; Jeguirim, M. Green Carbon Composite-Derived Polymer Resin and Waste Cotton Fibers for the Removal of Alizarin Red S Dye. Energies 2017, 10, 1321. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Nayl, A.A.; El. Shanshory, A.A.; Soliman, H.M.A.; Aly, H.F. Decontamination of organic pollutants from aqueous media using cotton fiber–graphene oxide composite, utilizing batch and filter adsorption techniques: A comparative study. RSC Adv. 2019, 9, 5770–5785. [Google Scholar] [CrossRef]
- Wu, Y.; Qi, H.; Li, B.; Zhanhua, H.; Li, W.; Liu, S. Novel hydrophobic cotton fibers adsorbent for the removal of nitrobenzene in aqueous solution. Carbohydr. Polym. 2017, 155, 294–302. [Google Scholar] [CrossRef]
- García-Valverde, M.T.; Lucena, R.; Cárdenas, S.; Valcárcel, M. In-syringe dispersive micro-solid phase extraction using carbon fibres for the determination of chlorophenols in human urine by gas chromatography/mass spectrometry. J. Chromatogr. A 2016, 1464, 42–49. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, L.; Liu, T. A magnetic cellulose-based carbon fiber hybrid as a dispersive solid-phase extraction material for the simultaneous detection of six bisphenol analogs from environmental samples. Analyst 2018, 143, 3100–3106. [Google Scholar] [CrossRef]
- García-Valverde, M.; Ledesma-Escobar, C.; Lucena, R.; Cárdenas, S. Tunable Polarity Carbon Fibers, a Holistic Approach to Environmental Protection. Molecules 2018, 23, 1026. [Google Scholar] [CrossRef]
- Mehmandost, N.; Soriano, M.L.; Lucena, R.; Goudarzi, N.; Chamjangali, M.A.; Cardenas, S. Recycled polystyrene-cotton composites, giving a second life to plastic residues for environmental remediation. J. Environ. Chem. Eng. 2019, 7, 103424. [Google Scholar] [CrossRef]
- Cheng, H.; Song, Y.; Bian, Y.; Ji, R.; Wang, F.; Gu, C.; Yang, X.; Jiang, X. Sustainable synthesis of nanoporous carbons from agricultural waste and their application for solid-phase microextraction of chlorinated organic pollutants. RSC Adv. 2018, 8, 15915–15922. [Google Scholar] [CrossRef]
- Silva, T.L.; Cazetta, A.L.; Souza, P.S.C.; Zhang, T.; Asefa, T.; Almeida, V.C. Mesoporous activated carbon fibers synthesized from denim fabric waste: Efficient adsorbents for removal of textile dye from aqueous solutions. J. Clean. Prod. 2018, 171, 482–490. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, Z.; Zhou, J.; Zhang, Y. Reuse of waste cotton cloth for the extraction of cellulose nanocrystals. Carbohydr. Polym. 2017, 157, 945–952. [Google Scholar] [CrossRef]
- Boudrahem, N.; Delpeux-Ouldriane, S.; Khenniche, L.; Boudrahem, F.; Aissani-Benissad, F.; Gineys, M. Single and mixture adsorption of clofibric acid, tetracycline and paracetamol onto Activated carbon developed from cotton cloth residue. Process. Saf. Environ. Prot. 2017, 111, 544–559. [Google Scholar] [CrossRef]
- Wang, S.; Wei, M.; Xu, Q.; Jia, H. Functional porous carbons from waste cotton fabrics for dyeing wastewater purification. Fibers Polym. 2016, 17, 212–219. [Google Scholar] [CrossRef]
- Blanco, A.; Monte, M.C.; Campano, C.; Balea, A.; Merayo, N.; Negro, C. Nanocellulose for Industrial Use. In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 74–126. [Google Scholar]
- Harper, B.J.; Clendaniel, A.; Sinche, F.; Way, D.; Hughes, M.; Schardt, J.; Simonsen, J.; Stefaniak, A.B.; Harper, S.L. Impacts of chemical modification on the toxicity of diverse nanocellulose materials to developing zebrafish. Cellulose 2016, 23, 1763–1775. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Wang, Y.-G.; Ouyang, X.-K.; Yang, L.-Y. Surface-Imprinted Magnetic Carboxylated Cellulose Nanocrystals for the Highly Selective Extraction of Six Fluoroquinolones from Egg Samples. ACS Appl. Mater. Interfaces 2017, 9, 1759–1769. [Google Scholar] [CrossRef]
- Gopakumar, D.A.; Pasquini, D.; Henrique, M.A.; de Morais, L.C.; Grohens, Y.; Thomas, S. Meldrum’s Acid Modified Cellulose Nanofiber-Based Polyvinylidene Fluoride Microfiltration Membrane for Dye Water Treatment and Nanoparticle Removal. ACS Sustain. Chem. Eng. 2017, 5, 2026–2033. [Google Scholar] [CrossRef]
- Bradley, R.S. Pollen. In Paleoclimatology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 405–451. [Google Scholar]
- Domínguez, E.; Mercado, J.A.; Quesada, M.A.; Heredia, A. Pollen sporopollenin: Degradation and structural elucidation. Sex. Plant Reprod. 1999, 12, 171–178. [Google Scholar] [CrossRef]
- Thio, B.J.R.; Clark, K.K.; Keller, A.A. Magnetic pollen grains as sorbents for facile removal of organic pollutants in aqueous media. J. Hazard. Mater. 2011, 194, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Wu, J.-H.; Yu, Q.-W.; Feng, Y.-Q. Using pollen grains as novel hydrophilic solid-phase extraction sorbents for the simultaneous determination of 16 plant growth regulators. J. Chromatogr. A 2014, 1367, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhao, Q.; Yu, Q.-W.; Feng, Y.-Q. Use of Pollen Solid-Phase Extraction for the Determination of trans -Resveratrol in Peanut Oils. J. Agric. Food Chem. 2015, 63, 4771–4776. [Google Scholar] [CrossRef] [PubMed]
- Syed Yaacob, S.F.F.; Kamboh, M.A.; Wan Ibrahim, W.A.; Mohamad, S. New sporopollenin-based β-cyclodextrin functionalized magnetic hybrid adsorbent for magnetic solid-phase extraction of nonsteroidal anti-inflammatory drugs from water samples. R. Soc. Open Sci. 2018, 5, 171311. [Google Scholar] [CrossRef]
- Abd Wahib, S.M.; Wan Ibrahim, W.A.; Sanagi, M.M.; Kamboh, M.A.; Abdul Keyon, A.S. Magnetic sporopollenin-cyanopropyltriethoxysilane-dispersive micro-solid phase extraction coupled with high performance liquid chromatography for the determination of selected non-steroidal anti-inflammatory drugs in water samples. J. Chromatogr. A 2018, 1532, 50–57. [Google Scholar] [CrossRef]
- Kurniawan, A.; Kosasih, A.N.; Febrianto, J.; Ju, Y.-H.; Sunarso, J.; Indraswati, N.; Ismadji, S. Evaluation of cassava peel waste as lowcost biosorbent for Ni-sorption: Equilibrium, kinetics, thermodynamics and mechanism. Chem. Eng. J. 2011, 172, 158–166. [Google Scholar] [CrossRef]
- Mallampati, R.; Valiyaveettil, S. Apple Peels—A Versatile Biomass for Water Purification? ACS Appl. Mater. Interfaces 2013, 5, 4443–4449. [Google Scholar] [CrossRef]
- Gonzalez, M.H.; Araújo, G.C.L.; Pelizaro, C.B.; Menezes, E.A.; Lemos, S.G.; de Sousa, G.B.; Nogueira, A.R.A. Coconut coir as biosorbent for Cr(VI) removal from laboratory wastewater. J. Hazard. Mater. 2008, 159, 252–256. [Google Scholar] [CrossRef]
- Das, S.K.; Guha, A.K. Biosorption of chromium by Termitomyces clypeatus. Colloids Surf. B Biointerfaces 2007, 60, 46–54. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Tao, Y.; Yu, Y.; Jiang, H.; Lian, H. Comparative study of adsorption of Pb(II) on native garlic peel and mercerized garlic peel. Environ. Sci. Pollut. Res. 2014, 21, 2054–2063. [Google Scholar] [CrossRef]
- Memon, J.R.; Memon, S.Q.; Bhanger, M.I.; Memon, G.Z.; El-Turki, A.; Allen, G.C. Characterization of banana peel by scanning electron microscopy and FT-IR spectroscopy and its use for cadmium removal. Colloids Surf. B Biointerfaces 2008, 66, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Castro, R.S.D.; Caetano, L.; Ferreira, G.; Padilha, P.M.; Saeki, M.J.; Zara, L.F.; Martines, M.A.U.; Castro, G.R. Banana Peel Applied to the Solid Phase Extraction of Copper and Lead from River Water: Preconcentration of Metal Ions with a Fruit Waste. Ind. Eng. Chem. Res. 2011, 50, 3446–3451. [Google Scholar] [CrossRef]
- Khodarahmi, M.; Eftekhari, M.; Gheibi, M.; Chamsaz, M. Preconcentration of trace levels of cadmium (II) ion using Descurainia Sophia seeds as a green adsorbent for solid phase extraction followed by its determination by flame atomic absorption spectrometry. J. Food Meas. Charact. 2018, 12, 1485–1492. [Google Scholar] [CrossRef]
- Hameed, B.H.; Ahmad, A.A. Batch adsorption of methylene blue from aqueous solution by garlic peel, an agricultural waste biomass. J. Hazard. Mater. 2009, 164, 870–875. [Google Scholar] [CrossRef]
- Asfaram, A.; Fathi, M.R.; Khodadoust, S.; Naraki, M. Removal of Direct Red 12B by garlic peel as a cheap adsorbent: Kinetics, thermodynamic and equilibrium isotherms study of removal. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 127, 415–421. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, W.; Liu, J.; Huang, K.; Wu, C.; Shao, H.; Chen, H.; Liu, X. Modification of garlic peel by nitric acid and its application as a novel adsorbent for solid-phase extraction of quinolone antibiotics. Chem. Eng. J. 2017, 326, 745–755. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Alrozi, R. Removal of malachite green dye from aqueous solution using rambutan peel-based activated carbon: Equilibrium, kinetic and thermodynamic studies. Chem. Eng. J. 2011, 171, 510–516. [Google Scholar] [CrossRef]
- Li, M.; Jiao, C.; Yang, X.; Wang, C.; Wu, Q.; Wang, Z. Solid phase extraction of carbamate pesticides with banana peel derived hierarchical porous carbon prior to high performance liquid chromatography. Anal. Methods 2017, 9, 593–599. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Li, Z.; Zhang, S.; Wang, C.; Wang, Z. A nanoporous carbon material derived from pomelo peels as a fiber coating for solid-phase microextraction. RSC Adv. 2016, 6, 113951–113958. [Google Scholar] [CrossRef]
- Chisvert, A.; Cárdenas, S.; Lucena, R. Dispersive micro-solid phase extraction. TrAC Trends Anal. Chem. 2019, 112, 226–233. [Google Scholar] [CrossRef]
- Huang, Y.; Peng, J.; Huang, X. One-pot preparation of magnetic carbon adsorbent derived from pomelo peel for magnetic solid-phase extraction of pollutants in environmental waters. J. Chromatogr. A 2018, 1546, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Zhang, W.; Cao, S.; Wang, G.; Zhou, Z. Carbon-Based Fe3O4 Nanocomposites Derived from Waste Pomelo Peels for Magnetic Solid-Phase Extraction of 11 Triazole Fungicides in Fruit Samples. Nanomaterials 2018, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ríos, G.A.; Mirabelli, M.F. Solid Phase Microextraction-mass spectrometry: Metanoia. TrAC Trends Anal. Chem. 2019, 112, 201–211. [Google Scholar] [CrossRef]
- Gómez-Ríos, G.A.; Pawliszyn, J. Development of Coated Blade Spray Ionization Mass Spectrometry for the Quantitation of Target Analytes Present in Complex Matrices. Angew. Chem. Int. Ed. 2014, 53, 14503–14507. [Google Scholar] [CrossRef]
- Gómez-Ríos, G.A.; Tascon, M.; Reyes-Garcés, N.; Boyacı, E.; Poole, J.; Pawliszyn, J. Quantitative analysis of biofluid spots by coated blade spray mass spectrometry, a new approach to rapid screening. Sci. Rep. 2017, 7, 16104. [Google Scholar] [CrossRef]
- Gómez-Ríos, G.A.; Tascon, M.; Pawliszyn, J. Coated blade spray: Shifting the paradigm of direct sample introduction to MS. Bioanalysis 2018, 10, 257–271. [Google Scholar] [CrossRef]
- Pereira, I.; Rodrigues, M.F.; Chaves, A.R.; Vaz, B.G. Molecularly imprinted polymer (MIP) membrane assisted direct spray ionization mass spectrometry for agrochemicals screening in foodstuffs. Talanta 2018, 178, 507–514. [Google Scholar] [CrossRef]
- Espy, R.D.; Teunissen, S.F.; Manicke, N.E.; Ren, Y.; Ouyang, Z.; van Asten, A.; Cooks, R.G. Paper Spray and Extraction Spray Mass Spectrometry for the Direct and Simultaneous Quantification of Eight Drugs of Abuse in Whole Blood. Anal. Chem. 2014, 86, 7712–7718. [Google Scholar] [CrossRef]
- Hu, B.; So, P.-K.; Chen, H.; Yao, Z.-P. Electrospray Ionization Using Wooden Tips. Anal. Chem. 2011, 83, 8201–8207. [Google Scholar] [CrossRef]
- Hu, B.; So, P.-K.; Yang, Y.; Deng, J.; Choi, Y.-C.; Luan, T.; Yao, Z.-P. Surface-Modified Wooden-Tip Electrospray Ionization Mass Spectrometry for Enhanced Detection of Analytes in Complex Samples. Anal. Chem. 2018, 90, 1759–1766. [Google Scholar] [CrossRef]
- Hu, B.; Yao, Z. Detection of native proteins using solid-substrate electrospray ionization mass spectrometry with nonpolar solvents. Anal. Chim. Acta 2018, 1004, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Deng, J.; Yu, Y.; Yang, Y.; Wang, X.; Liu, H.; Luan, T. Coupling liquid-phase microextraction with paper spray for rapid analysis of malachite green, crystal violet and their metabolites in complex samples using mass spectrometry. Anal. Methods 2016, 8, 6651–6656. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Q.; Ding, Y. Paper-based solid-phase microextraction for analysis of 8-hydroxy-2’-deoxyguanosine in urine sample by CE-LIF. Electrophoresis 2017, 38, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Saraji, M.; Farajmand, B. Chemically modified cellulose paper as a thin film microextraction phase. J. Chromatogr. A 2013, 1314, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Gómez, J.; Lucena, R.; Cárdenas, S. Paper supported polystyrene membranes for thin film microextraction. Microchem. J. 2017, 133, 90–95. [Google Scholar] [CrossRef]
- Ríos-Gómez, J.; García-Valverde, M.T.; López-Lorente, Á.I.; Toledo-Neira, C.; Lucena, R.; Cárdenas, S. Polymeric ionic liquid immobilized onto paper as sorptive phase in microextraction. Anal. Chim. Acta 2019. [Google Scholar] [CrossRef]
- Hashemian, Z.; Khayamian, T.; Saraji, M. Anticodeine aptamer immobilized on a Whatman cellulose paper for thin-film microextraction of codeine from urine followed by electrospray ionization ion mobility spectrometry. Anal. Bioanal. Chem. 2014, 407. [Google Scholar] [CrossRef]
- Kim, E.-A.; Lim, Y.Y. Effective preconcentration of volatile organic compounds from aqueous solutions with polydimethylsiloxane-coated filter paper. Microchem. J. 2019, 145, 979–987. [Google Scholar] [CrossRef]
- Díaz-Liñán, M.C.; López-Lorente, A.I.; Cárdenas, S.; Lucena, R. Molecularly imprinted paper-based analytical device obtained by a polymerization-free synthesis. Sens. Actuators B Chem. 2019, 287, 138–146. [Google Scholar] [CrossRef]
- Ríos-Gómez, J.; Ferrer-Monteagudo, B.; López-Lorente, Á.I.; Lucena, R.; Luque, R.; Cárdenas, S. Efficient combined sorption/photobleaching of dyes promoted by cellulose/titania-based nanocomposite films. J. Clean. Prod. 2018, 194, 167–173. [Google Scholar] [CrossRef]
- Ríos-Gómez, J.; Fresco-Cala, B.; García-Valverde, M.; Lucena, R.; Cárdenas, S. Carbon Nanohorn Suprastructures on a Paper Support as a Sorptive Phase. Molecules 2018, 23, 1252. [Google Scholar] [CrossRef] [PubMed]
- Dotan, A. Biobased Thermosets. In Handbook of Thermoset Plastics; Elsevier: Amsterdam, The Netherlands, 2014; pp. 577–622. [Google Scholar]
- Deng, J.; Yu, T.; Yao, Y.; Peng, Q.; Luo, L.; Chen, B.; Wang, X.; Yang, Y.; Luan, T. Surface-coated wooden-tip electrospray ionization mass spectrometry for determination of trace fluoroquinolone and macrolide antibiotics in water. Anal. Chim. Acta 2017, 954, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ma, Y.; Hu, H.; Guo, P.; Miao, L.; Yang, Y.; Zhang, M. Rapid and sensitive detection of trace malachite green and its metabolite in aquatic products using molecularly imprinted polymer-coated wooden-tip electrospray ionization mass spectrometry. RSC Adv. 2017, 7, 52091–52100. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Q.; Chen, X.; Song, Y.; Wu, Q.; Yang, Y.; He, L. Sensitive analysis of trace macrolide antibiotics in complex food samples by ambient mass spectrometry with molecularly imprinted polymer-coated wooden tips. Talanta 2019, 204, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Karimiyan, H.; Hadjmohammadi, M.R.; Kunjali, K.L.; Moein, M.M.; Dutta, J.; Abdel-Rehim, M. Graphene Oxide/Polyethylene Glycol-Stick for Thin Film Microextraction of β-Blockers from Human Oral Fluid by Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2019, 24, 3664. [Google Scholar] [CrossRef]

| Technique | Raw Cork Format | Analytes | Matrix | Desorption Volume (μL) | LOQ (μg/L) | Instrumentation | Ref. |
|---|---|---|---|---|---|---|---|
| BAμE | Powder | Benzophenone, Trichlocarban and Parabens | Lagoon water | 250 | 1.6–20 | LC-DAD | [30] |
| BAμE | Powder | Cancer biomarkers | Urine | 100 | 250–300 | LC-DAD | [31] |
| DPX | Powder | 10 Pharmaceuticals | Urine | 85 | 5–10 | LC-DAD | [32] |
| TF-SPME | Powder | Endocrine Disruptors Compounds | River water | 300 | 0.8–15 | LC-DAD | [33] |
| RDSE | Powder | Parabens | River water | 3000 | 0.8 | LC-MS/MS | [34] |
| DPX | Powder | Pb and Cd | Water | 300 | 0.3–4.1 | HR-CS GFAAS | [35] |
| DPX | Powder | Parabens and UV filters | Lake water | 100 | 2.0–4.3 | LC-DAD | [36] |
| RDSE | Laminar | Hormones | Wastewater | 5000 | 0.01–0.062 | GC-MS | [37] |
| RDSE | Laminar | 20 Pesticides, PAHs and UV filters | River water | 1000 | 0.3–4.8 | GC-MS | [38] |
| Technique | Material | Analytes | Matrix | LOD | Instrumentation | Ref. |
|---|---|---|---|---|---|---|
| In-pipette-tip SPE | Cotton fibers modified with 4-formylphenylboronic acid | Nucleosides | Urine | 5.1 and 6.1 ng/mL | LC-UV | [46] |
| SPE under stirring | Graphene-coated cotton fibers | Multiclass pesticide residues | Water | Low µg/L | GC-MS | [48] |
| D-µSPE | Carbon fibers | Chlorophenols | Urine | 0.1–0.9 µg/L | GC-MS | [62] |
| D-µSPE | Magnetic cellulose-based carbon fibers | Bisphenol analogues | Environmental samples | 0.56–0.83 ng/mL | LC-UV | [63] |
| D-µSPE | Carbon fibers | Pollutants of different polarities | Water | Low ng/mL | HS-GC-MS | [64] |
| Magnetic D-µSPE | Fe3O4@CCNs@MIPs | Fluoroquinolones | Egg | 3.6–18.4 ng g−1 | LC-DAD | [73] |
| Technique | Material | Analytes | Matrix | LOD | Instrumentation | Ref. |
|---|---|---|---|---|---|---|
| HILIC-SPE | Raw pollen | Plant growth regulators | Fruit and vegetables | 0.01–1.1 µg/kg | UHPLC-MS/MS | [78] |
| NP-SPE | Raw pollen | Trans-resveratrol | Peanut oil | 2.7 ng/g | LC-UV | [79] |
| Magnetic SPE | Sporollenin modified with cyclodextrin | Non-steroidal autoinflammatory drugs | Water | 0.16–0.37 ng/mL | LC-UV | [80] |
| Magnetic SPE | Sporollenin modified with CNPrTEOS | Non-steroidal autoinflammatory drugs | Water | 0.21–0.51 µg/L | LC-UV | [81] |
| Technique | Material | Analytes | Matrix | LOD | Instrumentation | Ref. |
|---|---|---|---|---|---|---|
| Dispersive SPE | Banana peel | Cadmium | Environmental and industrial wastewaters | 1.7 µg/L | FAAS | [87] |
| SPE | Banana peel | Copper, Lead | River water | -- | FAAS | [88] |
| SPE | Descurainia Sophia seeds | Cadmium | Water rice flour | 1.0 µg/L | FAAS | [89] |
| SPE | Garlic peel | Quinolone antibiotics | Water | 0.65–0.85 µg/L | LC-DAD | [92] |
| SPE | Banana peel | Carbamate pesticides | Cucumber watermelon | 0.05–0.20 ng/g | LC-DAD | [94] |
| SPME | Pomelo peel | Benzene homologues | Water Soil | 0.05–0.18 ng/L 0.11–0.18 ng/Kg | GC-MS | [95] |
| Magnetic-SPE | Pomelo peel | Parabens Fluoroquinolones | Water | 0.011–0.053 µg/L 0.012–0.46 µg/L | LC-DAD | [97] |
| Magnetic-SPE | Pomelo peel | Triazoles fungicides | Fruits | 0.12–0.55 µg/Kg | GC-MS | [98] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafra, G.; García-Valverde, M.T.; Millán-Santiago, J.; Carasek, E.; Lucena, R.; Cárdenas, S. Returning to Nature for the Design of Sorptive Phases in Solid-Phase Microextraction. Separations 2020, 7, 2. https://doi.org/10.3390/separations7010002
Mafra G, García-Valverde MT, Millán-Santiago J, Carasek E, Lucena R, Cárdenas S. Returning to Nature for the Design of Sorptive Phases in Solid-Phase Microextraction. Separations. 2020; 7(1):2. https://doi.org/10.3390/separations7010002
Chicago/Turabian StyleMafra, Gabriela, María Teresa García-Valverde, Jaime Millán-Santiago, Eduardo Carasek, Rafael Lucena, and Soledad Cárdenas. 2020. "Returning to Nature for the Design of Sorptive Phases in Solid-Phase Microextraction" Separations 7, no. 1: 2. https://doi.org/10.3390/separations7010002
APA StyleMafra, G., García-Valverde, M. T., Millán-Santiago, J., Carasek, E., Lucena, R., & Cárdenas, S. (2020). Returning to Nature for the Design of Sorptive Phases in Solid-Phase Microextraction. Separations, 7(1), 2. https://doi.org/10.3390/separations7010002





