Abstract
In recent years, oral tobacco-derived nicotine (OTDN) pouches have emerged as a new oral tobacco product category. They are available in a variety of flavors and do not contain cut or ground tobacco leaf. The on!® nicotine pouches fall within this category of OTDN products and are currently marketed in seven (7) flavors with five (5) different nicotine levels. Evaluation of the nicotine release from these products is valuable for product assessment and product-to-product comparisons. In this work, we characterized the in vitro release profiles of nicotine from the 35 varieties of on!® nicotine pouches using a fit-for-purpose dissolution method, employing the U.S. Pharmacopeia flow-through cell dissolution apparatus 4 (USP-4). The nicotine release profiles were compared using the FDA’s Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms. The cumulative release profiles of nicotine show a dose dependent response for all nicotine levels. The on!® nicotine pouches exhibit equivalent percent nicotine release rates for each flavor variant across all nicotine levels. Furthermore, the nicotine release profiles from on!® nicotine pouches were compared to a variety of other commercially available OTDN pouches and traditional pouched smokeless tobacco products. The percent nicotine release rates were found to be dependent on the product characteristics, showing similarities and differences in the nicotine release profiles between the on!® nicotine pouches and other compared products.
1. Introduction
Over recent years, oral tobacco products have provided alternatives to smoking cigarettes [1,2]. The use of oral tobacco products is considered by many to have potentially reduced risks of harm compared to smoking cigarettes [3,4,5]. Oral tobacco products exist in two major categories: traditional smokeless tobacco and modern oral nicotine products. Typically, traditional smokeless tobacco products come in three different types, including chewing tobacco (loose leaf, plug, or twist), snuff (finely ground tobacco that can be dry, moist, or packaged in pouches (e.g., snus)), and dissolvable (finely ground tobacco pressed into shapes such as tablets and sticks) products [6]. While traditional smokeless tobacco products contain tobacco leaves, modern oral nicotine products are tobacco leaf-free that contain tobacco-derived nicotine and food grade ingredients [7]. In the last decade, modern oral tobacco-derived nicotine (OTDN) products have been commercialized in various solid forms, including lozenges, gums, and dissolving tablets [8,9,10]. More recently, nicotine pouches have emerged as a new category of OTDN products. These products are pre-portioned pouches similar to snus but replace the tobacco leaf with non-tobacco filler and tobacco-derived nicotine.
The scientific evidence regarding the long-term health effects of OTDN pouches has not yet been established; however, the vast body of literature on other oral tobacco products, such as moist smokeless tobacco products, suggests that nicotine pouches will pose significantly lower risks than cigarettes [11]. Based on our review of statements from authoritative bodies regarding the long-term health effects of nicotine and available scientific literature on nicotine replacement therapy (NRT) as well as moist smokeless tobacco products, we believe that OTDN pouch products are not risk free and can lead to dependence. Nicotine, while not benign, has substantially lower health risks compared to smoking cigarettes [12,13].
Tobacco product manufacturers are currently selling OTDN pouch products under different brand names such as ZYN®, Velo, and on!® [7]. These products come in a variety of flavors and different nicotine contents per pouch. The on!® nicotine pouch products, for example, are currently marketed with seven flavor variants (e.g., Citrus, Wintergreen, Mint, Coffee, Berry, Cinnamon, and Original) and 5 different nicotine levels (1.5, 2, 3.5, 4, and 8 mg per pouch), providing an overall portfolio of 35 combinations of flavor variants and nicotine levels. These products are consumed by placing the pouch between the gum and upper lip, allowing for the dissolution of nicotine to occur in the saliva before being absorbed in the oral cavity and entering the bloodstream [14].
The market for oral nicotine pouches has been increasing in recent years as adult tobacco consumers are looking for alternatives to more traditional tobacco products, such as cigarettes [4]. Therefore, research evaluating the release of nicotine from these pouches is needed for product characterization and product-to-product comparisons.
Dissolution testing is commonly used by the pharmaceutical industry to assess product quality, demonstrate equivalency in constituent release, guide formulation design, and develop in vivo/in vitro correlation (IVIVC) [15,16,17,18,19]. Dissolution testing measures in vitro drug release as a function of time, which may reflect the reproducibility of the manufacturing process and, in some cases, relates to the active ingredient’s in vivo release [20,21,22,23]. Despite the numerous well-established and standardized methods described in the pharmacopoeias, only a few dissolution methods have been developed for the comparison of OTDN, using a variety of dissolution apparatus and analytical methods [24,25,26,27]. Recently, we developed and validated a fit-for-purpose method for the dissolution testing of nicotine from a variety of traditional smokeless tobacco products using a USP-4 flow-through cell apparatus. This method quantitatively determines the nicotine release into artificial saliva from a variety of smokeless tobacco products selectively and precisely. This discriminatory dissolution methodology was successfully applied to study the dissolution release profiles from a variety of traditional reference and commercial smokeless tobacco products. We demonstrated the ability of this method to be used as an important tool for tobacco product assessment and product-to-product comparisons, and also that the nicotine release profile is dependent on the form and cut of the studied traditional smokeless tobacco products [28].
In this study, we built on our initial findings and expanded the scope of our validated method to include oral nicotine pouch products, on!®. We characterized the dissolution release of nicotine from 35 on!® nicotine pouch products across the seven flavors and five nicotine levels by comparing the cumulative and percent of total nicotine release profiles. We further calculated the difference factor (f1) and similarity factor (f2) using a methodology referenced in the Guidance for Industry from FDA’s Center for Drug Evaluation and Research (CDER) [29,30]. Furthermore, the nicotine release profiles from on!® nicotine pouches were compared to a variety of OTDN pouches and traditional smokeless tobacco products to better understand the nicotine release rates within and across product categories.
2. Materials and Methods
The dissolution testing was carried out using a USP-4 flow-through cell apparatus (SOTAX, Westborough, MA, USA) following our previous methodology [28]. The determination of nicotine was performed using Acquity I-Class Ultra Performance Liquid Chromatography coupled to a Photodiode Array detector (UPLC-PDA) (Waters, Milford, MA, USA). The UPLC was fitted with a BEH C18 analytical column (2.1 × 100 mm, 1.7 µm) and a BEH C18 VanGuard pre-column (2.1 × 5 mm, 1.7 µm) (Waters, Milford, MA, USA) [28]. The artificial saliva was prepared according to the method described in the German Institute for Standardization (DIN) recipe listed in the German standard DIN V Test Method 53160-1 2002-10 [31]. The USP-4 fractions collection and UPLC solutions and standards preparation were performed following our previously published report [28].
2.1. Test Products
The 35 on!® nicotine pouch products, currently marketed with seven flavor variants (Citrus, Wintergreen, Mint, Coffee, Berry, Cinnamon, and Original) and 5 different nicotine levels (1.5, 2, 3.5, 4, and 8 mg per pouch) were provided by the manufacturer. Similarly, the Skoal® Bandits and Skoal® pouches (Wintergreen flavored traditional pouched smokeless tobacco products) were provided by the manufacturer. The ZYN® nicotine pouch products used in this study, with different flavor variants (Coffee, Wintergreen, and Cool Mint) and nicotine levels (3 and 6 mg per pouch), were purchased from retail stores.
2.2. Dissolution Fractions Collection
The USP-4 apparatus used in this study consisted of an array of seven flow-through cells, a cell holder including a water bath, a reservoir and pump for artificial saliva, and a fractions collection rack. The pump delivered a constant flow of artificial saliva (4 mL/min) through the flow-through cells. The flow-through cells were mounted vertically with a filter system that prevents the pouches from exiting the cell. The cells were immersed in the water bath, and the temperature was maintained at 37 ± 0.5 °C. A 5 mm ruby bead check valve was placed in the bottom of each sample cell, and approximately 6.6 g of 1 mm glass beads was added to the conical portion of the cell to ensure a laminar flow. Pouched products were weighed, and a single pouch of on!® (~0.265 g), ZYN® (~0.393 g), or traditional pouched smokeless tobacco product (~0.72–1.55 g), was added directly into each vessel. The cell was then filled with approximately 6.6 g of 3 mm glass beads to maintain the pouch position in the center of the flow-through cell. The dissolution testing was conducted according to the guidance issued by the FDA using 12 replicates of one product and taking a dissolution profile at a maximum of 15-min intervals [29,30]. Each replicate was dissolved into 9 fractions. The collection time was 4 min for fractions 1–5, resulting in a final collection volume of 16 mL for each fraction, and 10 min for fractions 6–9, resulting in a final collection volume of 40 mL in each fraction for a total dissolution time of 60 min.
2.3. Quantitative Analysis of Nicotine
Upon collection of all 9 fractions from each sample replicate, 0.1 mL of each dissolution fraction was added to an autosampler vial, followed by the addition of 0.1 mL of ethyl benzoate as an internal standard (1 mg/mL) and 0.8 mL of artificial saliva. The nicotine concentration in µg/mL was quantitated in all fractions collected from the 12 replicates following the analytical UPLC-PDA method described previously [28]. The concentration of the nicotine based on sample pouch (nicotine amount (mg) released), was determined using the calculated concentration of nicotine (µg/mL), weight of the sample analyzed, and volume of the dissolution fraction.
2.4. Cumulative and Percent of Total Release Profiles
The cumulative concentrations of nicotine (nicotine amount (mg) released) from each tested product were calculated by summing the averaged nicotine released for each fraction time point from all 12 replicates. The sum of the averaged cumulative nicotine amount corresponds to the total amount of nicotine released up to each time point. The percentage relative to the total nicotine released at each time point (rate) was then calculated and plotted to provide the total release profile. The relative percentage to the total nicotine released was calculated by dividing the amount of nicotine released up to each time point for each fraction by the cumulative amount released in 60 min.
2.5. F1 and F2 Calculations
The difference factor (f1) and similarity factor (f2) were calculated by adopting a methodology referenced in the Guidance for Industry from FDA’s Center for Drug Evaluation and Research (CDER) [29,30]. These two factors can be calculated mathematically by the following equations [32,33]:
Rt and Tt are the cumulative percentage dissolved at each of the selected n time points of the two products. The factor f1 is proportional to the average difference between the two profiles, whereas factor f2 is inversely proportional to the average squared difference between the two profiles, with emphasis on the larger difference among all the time points. Following the FDA’s guidance document, at least 12 replicates should be used for each profile determination. The dissolution measurements of the two products should also be made under identical test conditions. For curves (kinetic release profiles) to be considered equivalent, f1 values should be close to 0 and f2 values should be close to 100. Generally, f1 values up to 15 (0–15) and f2 values of 50 or greater (50–100) demonstrate equivalence of the two curves, reflecting a similar performance of the two products.
3. Results and Discussion
Previously, we developed and validated a dissolution method to quantitatively evaluate the rate of nicotine release from traditional smokeless tobacco products using USP-4 flow-through cell dissolution apparatus and UPLC-PDA. We based our approach on consensus methodology already existing in the field of pharmaceutical products, including the choice of apparatus, dissolution medium, and the analytical method used for the nicotine quantitation [26,34,35]. We described approaches for product-to-product comparisons between various nicotine-containing traditional loose and pouched traditional smokeless tobacco products [28]. Here, we expanded this methodology to measure the rate of nicotine release for the on!® nicotine pouches portfolio, consisting of 35 products (7 flavors at 5 different nicotine levels).
3.1. Method Validation
Our USP-4 flow-through cell/UPLC-PDA method was initially validated to study the dissolution release of nicotine from loose and pouched traditional smokeless tobacco products. To study the nicotine release profile from on!® nicotine pouch products, we conducted a supplemental validation to expand the scope of our original method. The supplemental validated elements of the method were accuracy, precision, specificity, and fraction stability. Accuracy of the analytical method was measured by calculating the recovery from two fortification levels in pooled fractions collected from 1.5 mg and 8 mg on!® nicotine pouch products of all flavor variants. Dissolution fractions from the beginning (fractions 1–5) and end (fractions 6–9) of the collection were combined into two pools: pool #1 (fractions 1–5) and pool #2 (fractions 6–9). The fortification levels were 50 and 200 μg/mL for pool #1 and 10 and 50 μg/mL for pool #2. Three replicates of each fortified sample were analyzed to determine accuracy. To determine the % recovery, the measured nicotine value from the unfortified samples was subtracted from each of the fortified samples. The corresponding results were divided by the fortified amounts to determine % recovery. All fortification levels and matrix types had calculated nicotine recovery values between 85 and 107%. Intra-day precision was determined by analyzing 3 replicates each of on!® Mint 1.5 mg and 8 mg pouches within a single day and was found to be <3% Relative Standard Deviation (RSD). Intermediate precision was measured by analyzing 3 replicates each of the same product over the course of three days (n = 9) and was found to be <4% RSD. The specificity of the method was validated by examining the chromatograms in all fractions and artificial saliva (used as a blank). The chromatograms were free of matrix interference, showing the ability of the method to quantitate nicotine in this sample matrix. Finally, the stability of the dissolution fractions was assessed over a period of 14 days in amber glass bottles with a screw cap at 0–4 °C. An initial analysis was made for time zero (day 1) and compared to the latter time points. The day 1 fractions were prepared and analyzed immediately in triplicate after dissolution. The average concentration of the aged samples (triplicates) on each day was calculated and compared to the concentrations of day 1 samples. The percent change from the initial measurement was calculated for all aged samples and was found to be less than 5% after 14 days of storage in the above conditions.
3.2. Nicotine Release from on!® Pouches
Following method validation for oral nicotine pouches, we characterized the in vitro release profiles of nicotine from the 35 varieties of on!® nicotine pouch products. As an example of the release profiles measured, Figure 1 shows the cumulative release profiles (Figure 1A) and percent of total release (Figure 1B) of nicotine from on!® Mint pouches at five different nicotine levels (1.5, 2, 3.5, 4, and 8 mg per pouch). As expected, the cumulative nicotine released from the on!® pouches increases as the nicotine content of the product increases. The percent of total release profiles of nicotine from the on!® Mint pouches at various nicotine levels were equivalent (Figure 1B). More rapid nicotine dissolution was observed for all five products with a total percent release of ~80% in the profile region between zero and 20 min. The total percent of release for all products (>95%) was achieved within 40 min before the nicotine dissolution profiles reached a plateau. Despite differences in total nicotine content, on!® Mint pouches at various nicotine levels exhibit similar kinetic profiles. Similar observations were seen for the other flavor variants of on!® nicotine pouches including Citrus, Wintergreen, Coffee, Berry, Cinnamon, and Original across all five nicotine levels (Table 1).
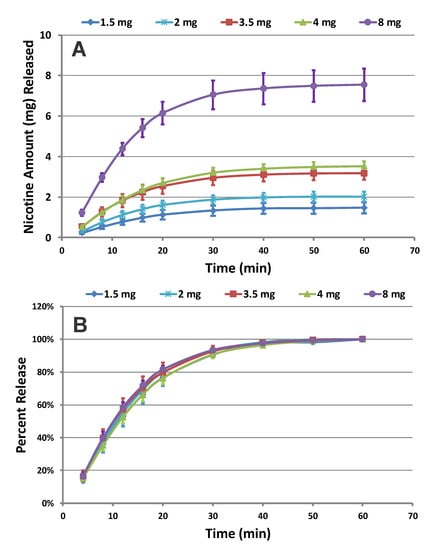
Figure 1.
(A) Cumulative release and (B) percent of total dissolution release profiles of nicotine collected from Mint on!® pouches across all nicotine levels (n = 12) (Error Bars ± 1 SD).

Table 1.
f1 and f2 values for on!® nicotine pouch comparisons across all nicotine levels for each flavor. The on!® 4 mg pouches for each flavor were used as the reference products for all comparisons.
To further confirm the above observations, we analyzed the nicotine release profiles by calculating the difference factor (f1) and similarity factor (f2) by adopting a methodology referenced in the Guidance for Industry from FDA’s Center for Drug Evaluation and Research (CDER) [29,30]. Table 1 shows the f1 and f2 values obtained by using the 4 mg on!® nicotine pouches as the reference products for all flavor variants. In this study, we have chosen the on!® 4 mg as a comparator as it represents the mid-range nicotine concentration of all products. The f1 and f2 values for the 35 on!® nicotine pouches at different nicotine strengths and within each flavor variant demonstrate equivalency of the products with calculated f1 lower than 15 and f2 higher than 50. These data indicate that the total amount of nicotine content in on!® pouches does not affect the nicotine release profile.
To assess the influence of the flavor on the nicotine release rate, we evaluated the nicotine release profiles from all flavored on!® pouch products at each nicotine level. As an example, Figure 2 shows the cumulative release (Figure 2A) and percent of total release profiles (Figure 2B) of nicotine from the 3.5 mg on!® pouches with seven different flavor variants. The cumulative nicotine release profiles show that similar amounts of nicotine are released from the pouches (Figure 2B). The overlapping percent of total release profiles of nicotine indicate equivalency between the seven flavor variants of on!® pouch products at the 3.5 mg nicotine level. Moreover, the calculated f1 and f2 values demonstrated equivalency between these products (Table 2) using the on!® Mint nicotine pouches as a comparator. This shows that the flavor in the 3.5 mg on!® nicotine pouches do not influence the release profile of nicotine under our experimental conditions.
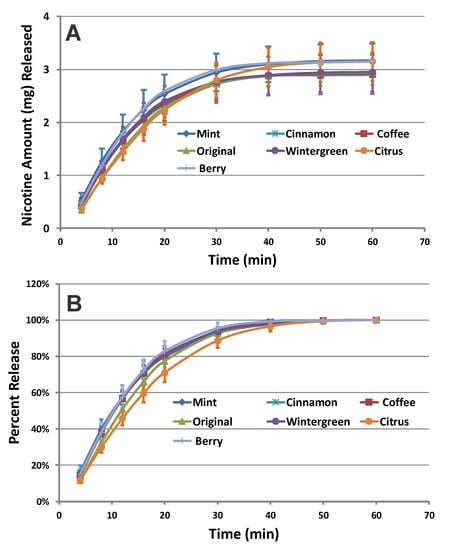
Figure 2.
(A) Cumulative release and (B) percent of total dissolution release profiles of nicotine collected from all flavored on!® pouches at 3.5 mg nicotine level (n = 12) (Error Bars ± 1 SD).

Table 2.
f1 and f2 values for on!® 3.5 mg nicotine pouch comparisons across all seven flavors. Mint on!® 3.5 mg nicotine pouches were used as a reference product for all comparisons.
3.3. Comparison with Smokeless Tobacco and Other OTDN Pouch Products
To better understand the release rates of on!® nicotine pouches and how they compare to traditional smokeless tobacco products and other OTDN pouch products, we compared the nicotine release profiles of on!® nicotine pouches to Skoal® Bandits and Skoal® pouches (commercially available traditional pouched smokeless tobacco products) and ZYN® nicotine pouches, another OTDN pouch products.
The on!® nicotine pouches are free of tobacco and do not have the same matrix content as the traditional pouched smokeless tobacco products. In addition, they are smaller in size and have a lower amount of nicotine per pouch compared to traditional pouched smokeless tobacco. Figure 3A shows the cumulative release profiles of nicotine from the Wintergreen flavored on!® 3.5 mg compared to Wintergreen flavored traditional smokeless tobacco pouch products, Skoal® Bandits and Skoal® pouches. The pouch weights for each product are 0.263 g, 0.72 g, and 1.55 g for on!®, Skoal® Bandits, and Skoal® pouches, respectively. The amount of total nicotine released (nicotine amount (mg) released) from Skoal® Bandits and Skoal® pouches as compared to the on!® product is attributed to the differences in nicotine concentration per pouch (Figure 3A). Despite the different pouch weight, nicotine concentration per pouch, and pouch composition, the percent nicotine released at each collection time point for the on!® and Skoal® Bandits pouches were found to be equivalent, as indicated by the overlapping release profiles (Figure 3B). However, the rate of nicotine release from the Skoal® pouches was found to be slower than on!® and Skoal® Bandits pouches (Figure 3B). In the profile region between zero and 20 min, a rapid dissolution was observed for on!® and Skoal® Bandits pouches, with a total percent release of 80% nicotine, whereas only 65% of the nicotine was released for Skoal® pouches. These observations were confirmed by calculating the f1 and f2 values. The calculated f1 and f2 values were 8.1 and 61.0 when comparing on!® to Skoal® Bandits, indicating equivalency between these products, and 21.1 and 46.0 when comparing on!® to Skoal® pouches, showing a difference in the nicotine release rates between these two products. These data illustrate that on!® nicotine pouches show similar or faster nicotine release profiles than the traditional pouched smokeless products tested here.
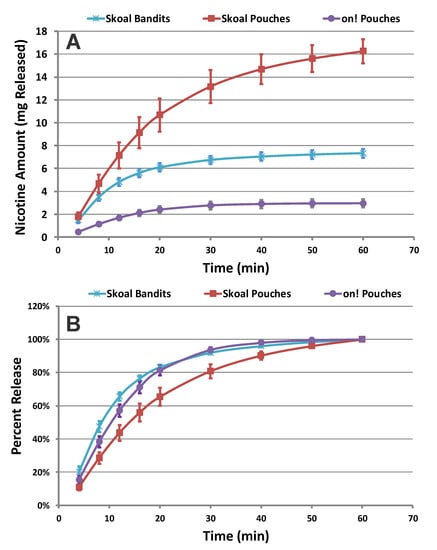
Figure 3.
(A) Cumulative release and (B) percent of total dissolution release profiles of nicotine collected from Wintergreen flavored on!® 3.5 mg, Skoal® Bandits, and Skoal® pouches (n = 12) (Error Bars ± 1 SD).
We also compared the performance of on!® to ZYN® pouches, a product marketed by Swedish Match North America. For this comparison, we selected the 3.5 mg and 8 mg on!® Mint, Wintergreen, and Coffee and 3 mg and 6 mg ZYN® Cool Mint, Wintergreen, and Coffee nicotine pouch products. As an example, Figure 4A shows the cumulative release profiles of nicotine from the 3.5 mg Mint on!® and 3 mg Cool Mint ZYN® pouches. Figure 4B shows the cumulative release profiles of nicotine from the 3.5 mg Wintergreen on!® and 3 mg Wintergreen ZYN® pouches. As expected, similar amounts of nicotine were released from both the on!® and the ZYN® products. The total release profiles of nicotine from the 3.5 mg on!® demonstrated a slightly slower release rate than the 3 mg ZYN® pouches (Figure 4C,D). However, when we calculate the f1 and f2 values, comparing on!® Mint to ZYN® Cool Mint, the nicotine release rate demonstrated equivalency, with f1 and f2 values of 9.2 and 60.1, respectively. In contrast, the on!® Wintergreen to ZYN® Wintergreen comparison resulted in a difference in the nicotine release rate with f1 and f2 values of 16.1 and 48.7, respectively. This observation was also seen when comparing other flavor variants and nicotine levels between on!® and ZYN® pouches (Table 3), indicating that the nicotine release profile and performance of these products could be similar but not necessarily statistically equivalent. Any measured and calculated differences between products within this OTDN category could be associated with inherent product characteristics (e.g., pouch paper and ingredients).
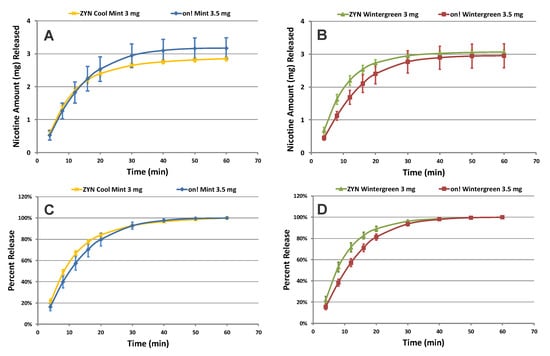
Figure 4.
(A,B) Cumulative release and (C,D) percent of total dissolution release profiles of nicotine collected from Mint and Wintergreen on!® 3.5 mg and Cool Mint and Wintergreen ZYN® 3 mg pouches (n = 12) (Error Bars ± 1 SD).

Table 3.
f1 and f2 values for on!® and ZYN® nicotine pouch comparisons.
4. Conclusions
In this report, we evaluated the release profile of nicotine from 35 on!® nicotine pouch products, which are currently marketed in seven flavor variants with five different nicotine levels. Our data show similar nicotine release profiles among the thirty-five (35) on!® products. Factor of difference (f1) and factor of similarity (f2) calculations confirmed similar product performance for all products. Nicotine release rate was not dependent on flavor and nicotine levels. Furthermore, we showed similarities and differences in the nicotine release rate from on!® nicotine pouches when compared to a few selected traditional pouched moist smokeless tobacco and non-traditional ZYN® pouch products. We believe that the data presented will provide useful information for product characterization and product-to-product comparisons. In addition, the dissolution data provided herein could be used to support clinical studies and establish future in vitro/in vivo (IVIV) correlations.
Author Contributions
Conceptualization, F.A.; validation, F.A., N.M. and C.S.; formal analysis, F.A.; investigation, F.A., N.M. and C.S.; resources, J.H.M.; data curation; writing—original draft preparation, F.A.; writing—review and editing, F.A., J.H.M. and T.L.D.; visualization, F.A.; supervision, F.A., J.H.M. and T.L.D.; project administration, F.A., T.L.D.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are openly available in [repository name e.g., FigShare] at [doi], reference number [reference number].
Acknowledgments
The authors thank Mohamadi Sarkar, Jack Marshall, Christopher McFarlane, Karl Wagner, and James Skapars from Altria Client Services for the helpful discussions.
Conflicts of Interest
The authors declare no competing financial interest.
References
- Mejia, A.B.; Ling, P.M.; Glantz, S.A. Quantifying the effects of promoting smokeless tobacco as a harm reduction strategy in the USA. Tob. Control 2010, 19, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Rodu, B.; Godshall, W.T. Tobacco harm reduction: An alternative cessation strategy for inveterate smokers. Harm Reduct. J. 2006, 3, 37. [Google Scholar] [CrossRef] [PubMed]
- Hatsukami, D.K.; Lemmonds, C.; Tomar, S.L. Smokeless tobacco use: Harm reduction or induction approach? Prev. Med. 2004, 38, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Hatsukami, D.K.; Carroll, D.M. Tobacco harm reduction: Past history, current controversies and a proposed approach for the future. Prev. Med. 2020, 140, 106099. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, L.T.; Sweanor, D.T. Young or adult users of multiple tobacco/nicotine products urgently need to be informed of meaningful differences in product risks. Addict. Behav. 2018, 76, 376–381. [Google Scholar] [CrossRef]
- Centers for Disease Control and Preventions (CDC). Smokeless Tobacco: Products and Marketing. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/smokeless/products_marketing/index.htm#not-burned (accessed on 15 October 2020).
- Robichaud, M.O.; Seidenberg, A.B.; Byron, M.J. Tobacco companies introduce ‘tobacco-free’ nicotine pouches. Tob Control 2019, 29, e145–e146. [Google Scholar] [CrossRef]
- Choi, J.H.; Dresler, C.M.; Norton, M.R.; Strahs, K.R. Pharmacokinetics of a nicotine polacrilex lozenge. Nicotine Tob. Res. 2003, 5, 635–644. [Google Scholar] [CrossRef]
- West, R.; Shiffman, S. Effect of oral nicotine dosing forms on cigarette withdrawal symptoms and craving: A systematic review. Psychopharmacology 2001, 155, 115–122. [Google Scholar] [CrossRef]
- O’Connor, R.J.; Norton, K.J.; Bansal-Travers, M.; Mahoney, M.C.; Cummings, K.M.; Borland, R. US smokers’ reactions to a brief trial of oral nicotine products. Harm Reduct. J. 2011, 8, 1. [Google Scholar] [CrossRef]
- Fisher, M.T.; Tan-Torres, S.M.; Gaworski, C.L.; Black, R.A.; Sarkar, M.A. Smokeless tobacco mortality risks: An analysis of two contemporary nationally representative longitudinal mortality studies. Harm Reduct. J. 2019, 16, 27. [Google Scholar] [CrossRef]
- Gottlieb, S.; Zeller, M. A Nicotine-Focused Framework for Public Health. N. Engl. J. Med. 2017, 377, 1111–1114. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Healthcare and Excellence (NICE). Smoking: Harm Reduction. 2013. Available online: https://www.nice.org.uk/guidance/ph45/resources/smoking-harm-reduction-pdf-1996359619525 (accessed on 15 October 2020).
- Hukkanen, J.; Jacob, P.; Benowitz, N.L. Metabolism and Disposition Kinetics of Nicotine. Pharmacol. Rev. 2005, 57, 79. [Google Scholar] [CrossRef]
- Qureshi, S.A. In vitro-in vivo correlation (ivivc) and determining drug concentrations in blood from dissolution testing–a simple and practical approach. Open Drug Deliv. J. 2010, 4, 38–47. [Google Scholar] [CrossRef]
- Williams, R.L.; Foster, T.S. Dissolution; a continuing perspective. Dissolution Technol. Augysr. 2004, 6, 14. [Google Scholar] [CrossRef]
- Wang, Q.; Fotaki, N.; Mao, Y. Biorelevant dissolution: Methodology and application in drug development. Dissolution Technol. 2009, 16, 6–12. [Google Scholar] [CrossRef]
- USP. 711 Dissolution USP. Available online: https://www.usp.org/sites/default/files/usp/document/harmonization/gen-method/q01_pf_ira_33_4_2007.pdf (accessed on 15 October 2020).
- Zieschang, L.; Klein, M.; Krämer, J.; Windbergs, M. In Vitro Performance Testing of Medicated Chewing Gums. Dissolution Technol. 2018, 25, 64–69. [Google Scholar] [CrossRef]
- Dressman, J.B.; Reppas, C. In vitro–in vivo correlations for lipophilic, poorly water-soluble drugs. Eur. J. Pharm. Sci. 2000, 11, S73–S80. [Google Scholar] [CrossRef]
- Amidon, G.L.; Lennernäs, H.; Shah, V.P.; Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef]
- Morjaria, Y.; Irwin, W.J.; Barnett, P.X.; Chan, R.S.; Conway, B.R. In Vitro Release of Nicotine From Chewing Gum Formulations. Dissolution Technol. 2004, 11, 12–15. [Google Scholar] [CrossRef]
- Delvadia, P.R.; Barr, W.H.; Karnes, H.T. A biorelevant in vitro release/permeation system for oral transmucosal dosage forms. Int. J. Pharm. 2012, 430, 104–113. [Google Scholar] [CrossRef]
- Nasr, M.M.; Reepmeyer, J.C.; Tang, Y. In vitro study of nicotine release from smokeless tobacco. J. AOAC Int. 1998, 81, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, J.; Sun, S.-H.; Xie, J.-P.; Zong, Y.-L. A novel model mouth system for evaluation of In Vitro release of nicotine from moist snuff. Chem. Cent. J. 2013, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- Cecil, T.L.; Brewer, T.M.; Holman, M.; Ashley, D.L. Food and Drug Administration. Dissolution as a Critical Comparison of Smokeless Product Performance: SE Requirements and Recommendations for the Review of Dissolution Studies. Memorandum from Cecil. 2016. Available online: https://www.fda.gov/media/124673/download (accessed on 15 October 2020).
- Kvist, C.; Andersson, S.B.; Fors, S.; Wennergren, B.; Berglund, J. Apparatus for studying in vitro drug release from medicated chewing gums. Int. J. Pharm. 1999, 189, 57–65. [Google Scholar] [CrossRef]
- Miller, J.H.; Danielson, T.; Pithawalla, Y.B.; Brown, A.P.; Wilkinson, C.; Wagner, K.; Aldeek, F. Method development and validation of dissolution testing for nicotine release from smokeless tobacco products using flow-through cell apparatus and UPLC-PDA. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1141, 122012. [Google Scholar] [CrossRef]
- Food and Drug Administration. SUPAC-IR: Immediate-Release Solid Oral Dosage Forms: Scale-Up and Post-Approval Changes: Chemistry, Manufacturing and Controls, In Vitro Dissolution Testing, and In Vivo Bioequivalence Documentation. 1995. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/supac-ir-immediate-release-solid-oral-dosage-forms-scale-and-post-approval-changes-chemistry (accessed on 15 October 2020).
- Food and Drug Administration. Guidance for Industry: Dissolution Testing of Immediate Release Solid Oral Dosage Forms. 1997. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/dissolution-testing-immediate-release-solid-oral-dosage-forms (accessed on 15 October 2020).
- DIN V Test Method 53160-1. Colorfastness to Saliva; Determination of the Colorfastness of Articles in Common Use Part 1: Resistance to Artificial Saliva. Available online: http://www.manufacturingsolutionscenter.org/colorfastness-to-saliva-testing.html (accessed on 15 October 2020).
- Shah, V.P.; Tsong, Y.; Sathe, P.; Liu, J.-P. In Vitro Dissolution Profile Comparison—Statistics and Analysis of the Similarity Factor, f2. Pharm. Res. 1998, 15, 889–896. [Google Scholar] [CrossRef]
- Chow, S.C.; Ki, F.Y. Statistical comparison between dissolution profiles of drug products. J. Biopharm. Stat. 1997, 7, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.; Maheshwari, R.; Verma, K.; Sharma, S.; Pethe, A.; Tekade, R.K. Chapter 1—Fundamentals of diffusion and dissolution: Dissolution testing of pharmaceuticals. In Drug Delivery Systems; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 1–45. [Google Scholar]
- Long, M.; Chen, Y. Chapter 14—Dissolution Testing of Solid Products. In Developing Solid Oral Dosage Forms; Qiu, Y., Chen, Y., Zhang, G.G.Z., Liu, L., Porter, W.R., Eds.; Academic Press: San Diego, CA, USA, 2009; pp. 319–340. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).