Maximizing Contents of Phytochemicals Obtained from Dried Sour Cherries by Ultrasound-Assisted Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Reagents
2.3. Preliminary Ultrasound-Assisted Extraction (UAE) and Screening of Variables
2.4. Experimental Design and Statistical Analysis
2.5. Analyses
2.5.1. Yield
2.5.2. Total Phenolic Content
2.5.3. Total Flavonoid Content
2.5.4. Total Anthocyanin Content
2.5.5. DPPH Assay
2.5.6. FRAP Assay
2.5.7. ABTS Assay
3. Results and Discussion
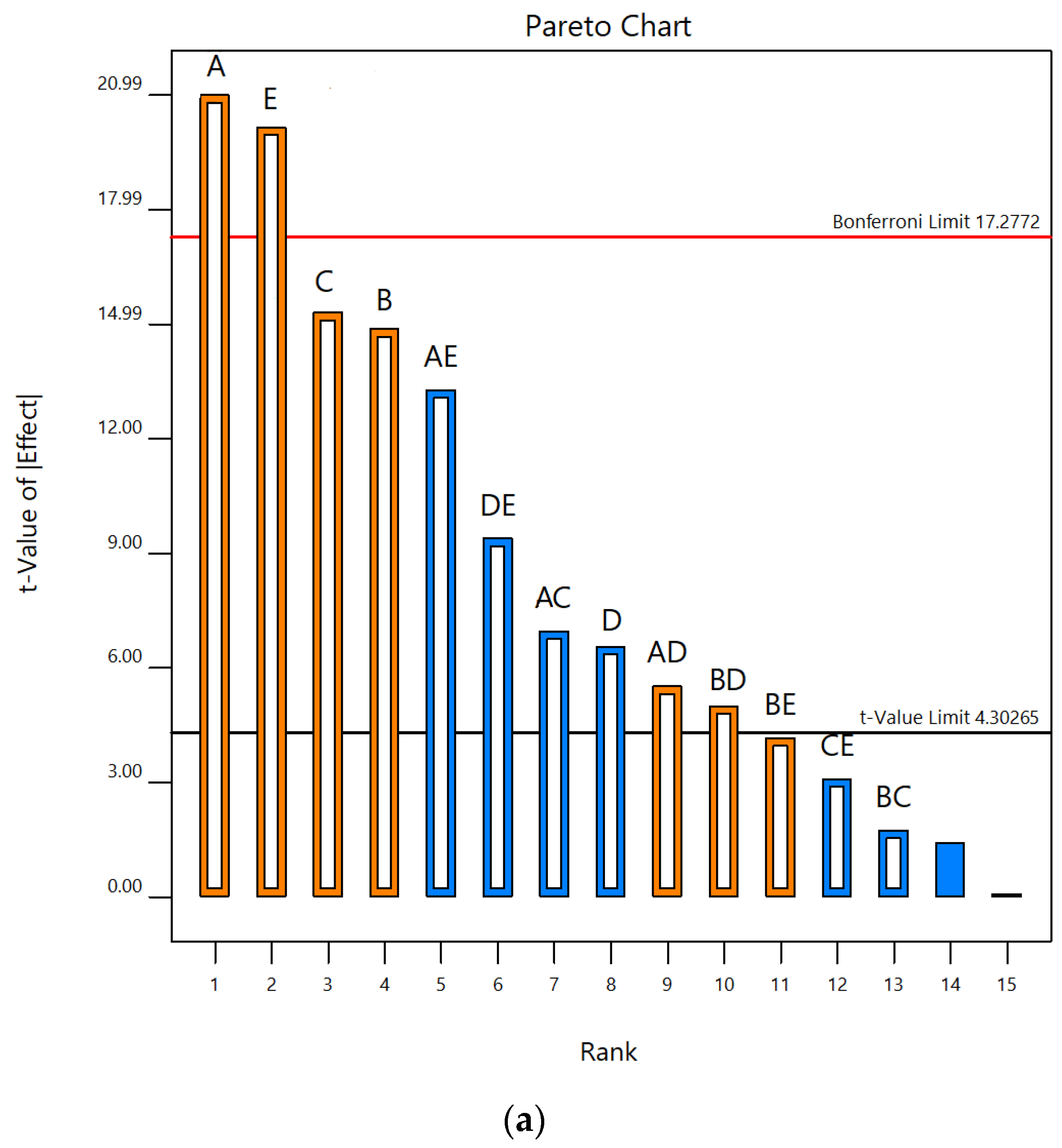
3.1. Screening of UAE Factors
3.2. Defining the Influence of Process Parameters Extraction Process Modeling
3.3. Effects of Investigated Extraction Parameters of Yield, Total Phenolics, Flavonoid and Monomeric Anthocyanins Content and Antioxidant Activity
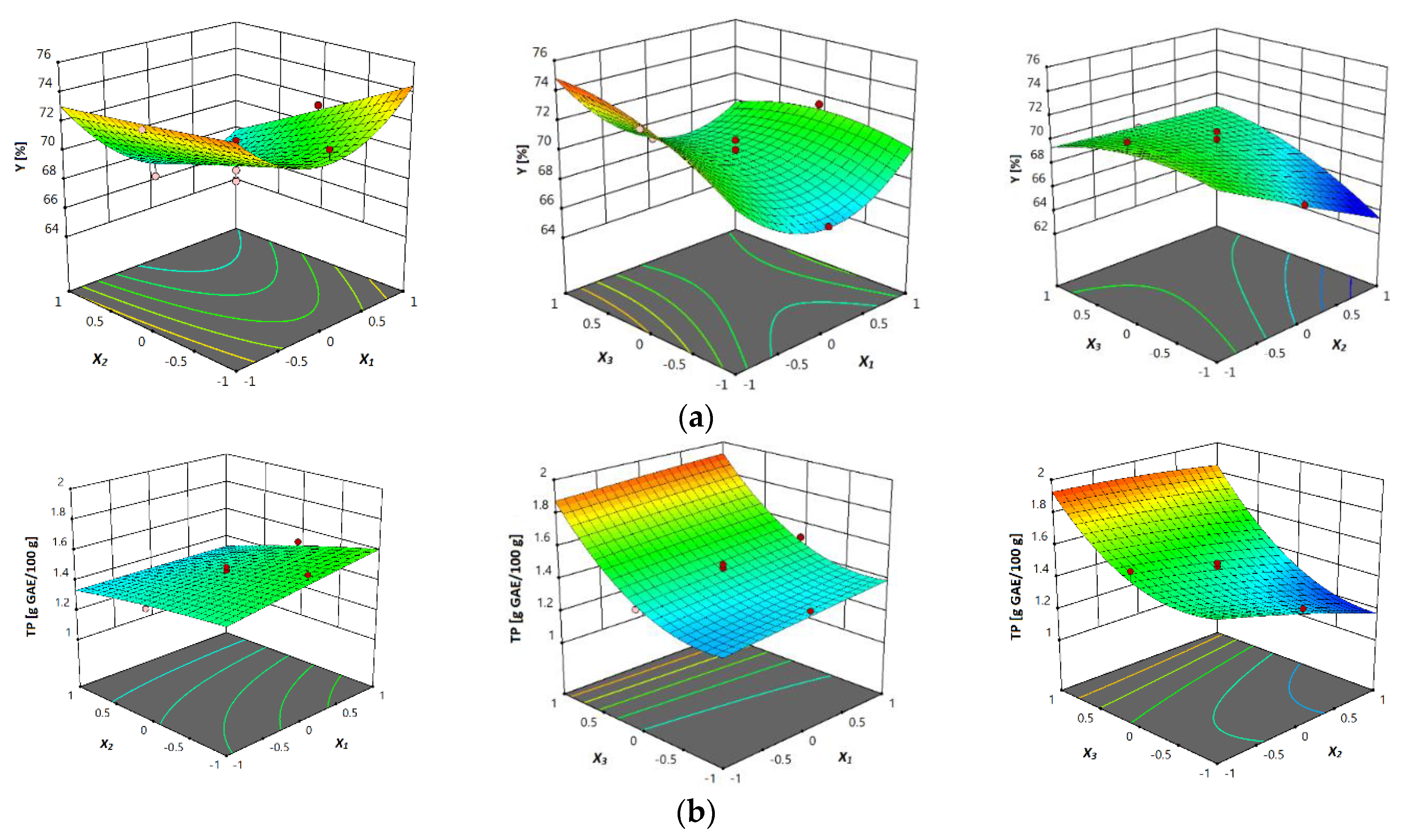
3.3.1. Total Extraction Yield (Y)
3.3.2. Total Phenols Content (TPC)
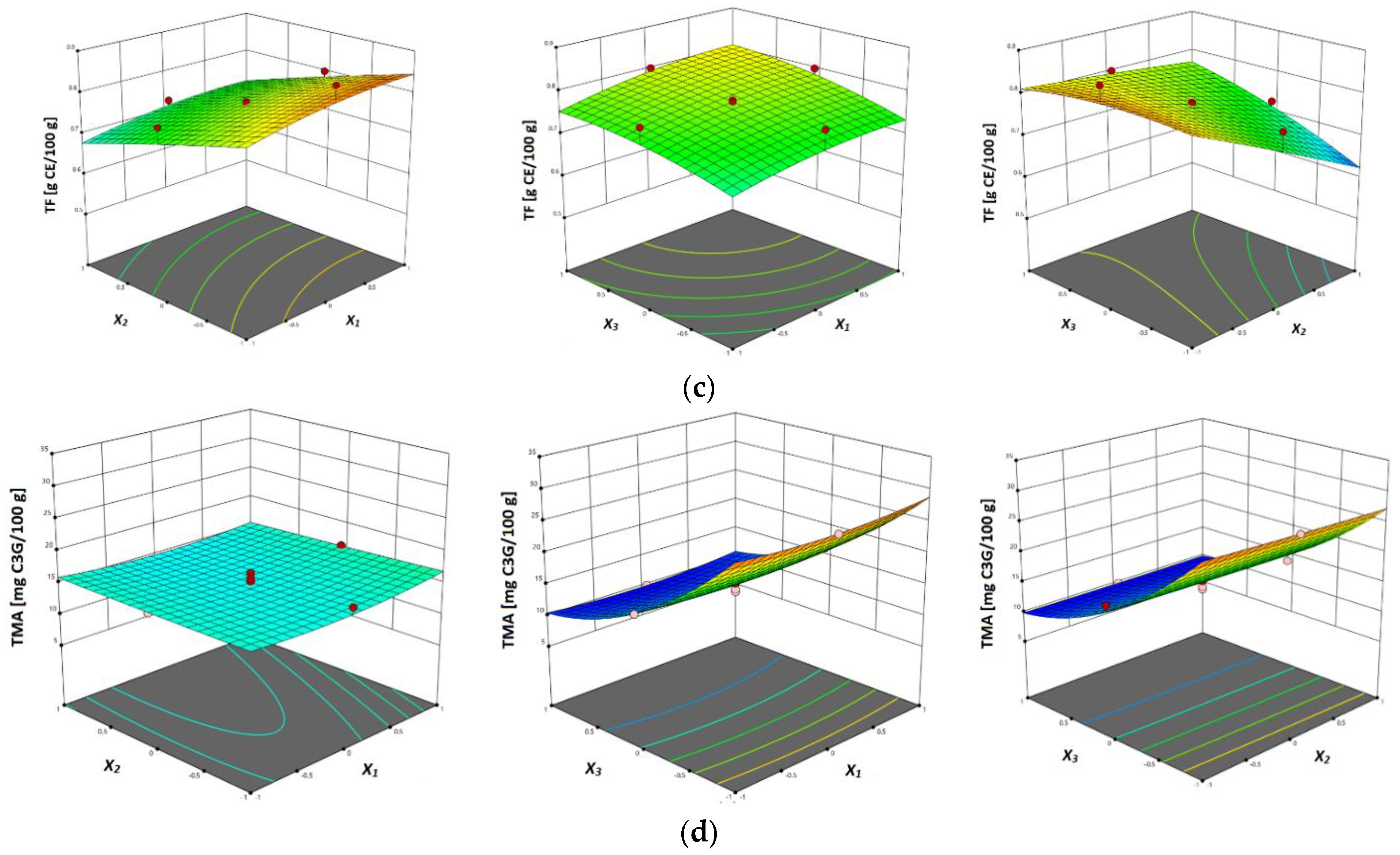
3.3.3. Total Flavonoids Content (TFC)
3.3.4. Total Monomeric Anthocyanins Content (TMAC)
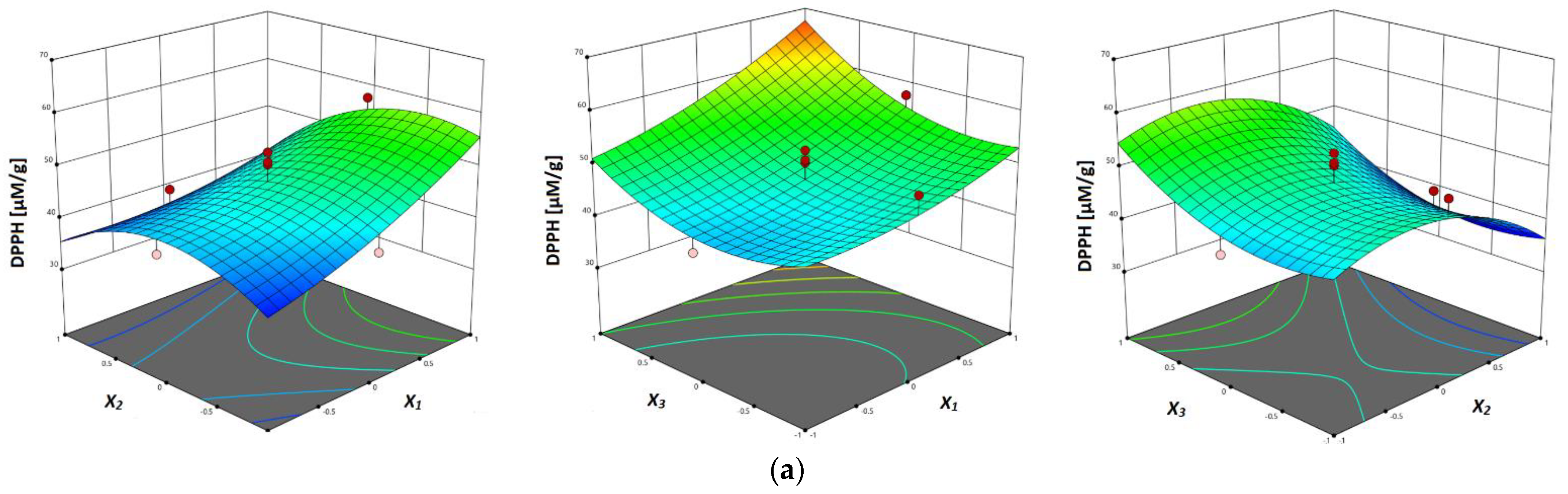
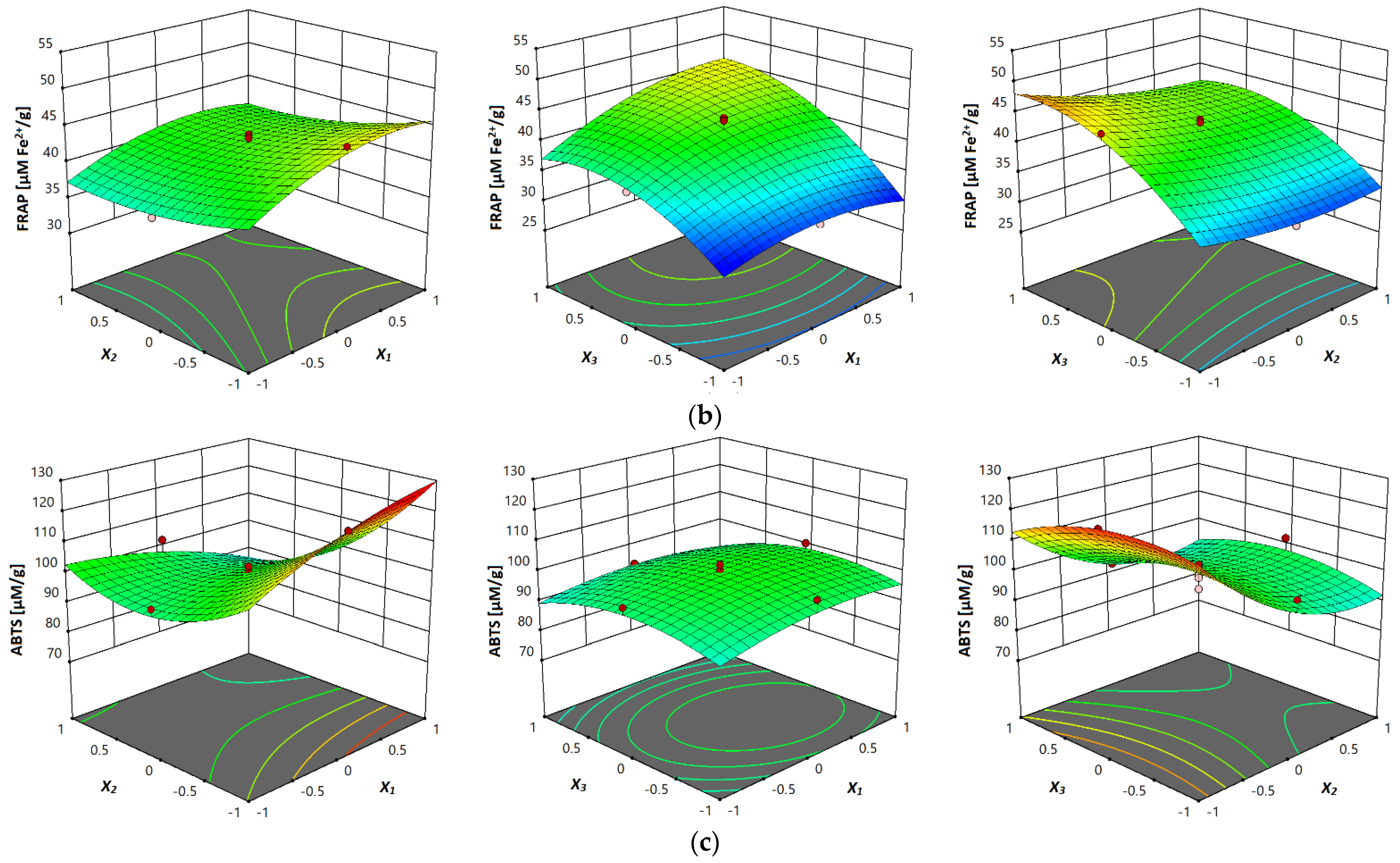
3.3.5. Antioxidant Activity (DPPH, FRAP and ABTS Assay)
3.4. Process Optimization and Experimental Verification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- Lukač Bulatović, M.; Rajić, Z.; Đoković, J. Development of Fruit Production and Processing in the Republic of Serbia; Балканская научная ассoциация экoнoмистoв-аграрникoв, Serbia: 2013; Volume 1. Available online: https://ageconsearch.umn.edu/record/146744 (accessed on 13 September 2021).
- Milošević, T.; Milošević, N.; Mladenović, J. Combining fruit quality and main antioxidant attributes in the sour cherry: The role of new clonal rootstock. Sci. Hortic. 2020, 265, 109236. [Google Scholar] [CrossRef]
- Zlatic, E.; Pichler, A.; Vidrih, R.; Hribar, J.; Piližota, V.; Kopjar, M. Volatile profile of sour cherry puree as affected by sucrose and trehalose. Int. J. Food Prop. 2018, 20, S3237–S3245. [Google Scholar] [CrossRef]
- Tepić Horecki, A.; Vakula, A.; Pavlić, B.; Jokanović, M.; Malbaša, R.; Vitas, J.; Jaćimović, V.; Šumić, Z. Comparative drying of cornelian cherries: Kinetics modeling and physico-chemical properties. J. Food Process. Preserv. 2018, 42, e13562. [Google Scholar] [CrossRef]
- Ciccoritti, R.; Paliotta, M.; Centioni, L.; Mencarelli, F.; Carbone, K. The effect of genotype and drying condition on the bioactive compounds of sour cherry pomace. Eur. Food Res. Technol. 2018, 244, 635–645. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Görgüç, A.; Karaaslan, M.; Vardin, H.; Ersus Bilek, S.; Uygun, Ö.; Bircan, C. Sour cherry by-products: Compositions, functional properties and recovery potentials—A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3549–3563. [Google Scholar] [CrossRef]
- Šumić, Z.; Vakula, A.; Tepić, A.; Čakarević, J.; Vitas, J.; Pavlić, B. Modeling and optimization of red currants vacuum drying process by response surface methodology (RSM). Food Chem. 2016, 203, 465–475. [Google Scholar] [CrossRef]
- Vakula, A.; Tepić Horecki, A.; Pavlić, B.; Jokanović, M.; Ognjanov, V.; Milović, M.; Teslić, N.; Parpinello, G.; Decleer, M.; Šumić, Z. Application of different techniques on stone fruit (Prunus spp.) drying and assessment of physical, chemical and biological properties: Characterization of dried fruit properties. J. Food Process. Preserv. 2021, 45, e15158. [Google Scholar] [CrossRef]
- Attila, H.; Nóra, P.; Anna, B.; Éva, B. Health effects of sour cherries with unique polyphenolic composition in their fruits. Orv. Hetil. 2018, 159, 720–725. [Google Scholar] [CrossRef] [Green Version]
- Nemes, A.; Szollosi, E.; Stündl, L.; Biró, A.; Homoki, J.R.; Szarvas, M.M.; Balogh, P.; Cziáky, Z.; Remenyik, J. Determination of flavonoid and proanthocyanidin profile of Hungarian sour cherry. Molecules 2018, 23, 3278. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, N.N.; Khomich, L.M.; Perova, I.B.; Eller, K.I. Sour cherry juice nutritional profile. Vopr. Pitan. 2018, 87, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, K.; Choi, Y.H.; Jeon, J.Y.; Jo, I.H. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from grape (Vitis vinifera) seeds. J. Agric. Food Chem. 2009, 57, 4988–4994. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aslam Maan, A.; Khan, M.K.I.; Nadeem, M.; Khalil, A.A.; Din, A.; Aadil, R.M. An inclusive overview of advanced thermal and nonthermal extraction techniques for bioactive compounds in food and food-related matrices. Food Rev. Int. 2020, 1–31. [Google Scholar] [CrossRef]
- Baldino, L.; Scognamiglio, M.; Reverchon, E. Supercritical fluid technologies applied to the extraction of compounds of industrial interest from Cannabis sativa L. and to their pharmaceutical formulations: A review. J. Supercrit. Fluids 2020, 165, 104960. [Google Scholar] [CrossRef]
- Bonfigli, M.; Godoy, E.; Reinheimer, M.A.; Scenna, N.J. Comparison between conventional and ultrasound-assisted techniques for extraction of anthocyanins from grape pomace. Experimental results and mathematical modeling. J. Food Eng. 2017, 207, 56–72. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Rostagno, M.A.; Palma, M.; Barroso, C.G. Ultrasound-assisted extraction of soy isoflavones. J. Chromatogr. A 2003, 1012, 119–128. [Google Scholar] [CrossRef]
- Görgüç, A.; Bircan, C.; Yılmaz, F.M. Sesame bran as an unexploited by-product: Effect of enzyme and ultrasound-assisted extraction on the recovery of protein and antioxidant compounds. Food Chem. 2019, 283, 637–645. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.Y.; Xia, Y.; Guo, H.; He, X.Q.; Li, H.; Wu, D.T.; Geng, F.; Lin, F.J.; Li, H.-B.; et al. Screening and process optimization of ultrasound-assisted extraction of main antioxidants from sweet tea (Lithocarpus litseifolius [Hance] Chun). Food Biosci. 2021, 43, 101277. [Google Scholar] [CrossRef]
- Luo, M.; Zhou, D.-D.; Shang, A.; Gan, R.-Y.; Li, H.-B. Influences of microwave-assisted extraction parameters on antioxidant activity of the extract from akebia trifoliata peels. Foods 2021, 10, 1432. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Meullemiestre, A.; Turk, M.; Perino, S.; Fabiano-Tixier, A.S.; Abert-Vian, M. Review of green food processing techniques. Preservation, transformation, and extraction. Innov. Food Sci. Emerg. Technol. 2017, 41, 357–377. [Google Scholar] [CrossRef]
- Ramić, M.; Vidović, S.; Zeković, Z.; Vladić, J.; Cvejin, A.; Pavlić, B. Modeling and optimization of ultrasound-assisted extraction of polyphenolic compounds from Aronia melanocarpa by-products from filter-tea factory. Ultrason. Sonochem. 2015, 23, 360–368. [Google Scholar] [CrossRef]
- Aydar, A.Y. Utilization of response surface methodology in optimization of extraction of plant materials. In Statistical Approaches with Emphasis on Design of Experiments Applied to Chemical Processes; InTech: London, UK, 2018. [Google Scholar]
- Vakula, A.; Šumić, Z.; Zeković, Z.; Tepić Horecki, A.; Pavlić, B. Screening, influence analysis and optimization of ultrasound-assisted extraction parameters of cornelian cherries (Cornus mas L.). J. Food Process. Preserv. 2019, 43, e14226. [Google Scholar] [CrossRef]
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 2018, 194, 40–47. [Google Scholar] [CrossRef]
- Zeković, Z.; Pintać, D.; Majkić, T.; Vidović, S.; Mimica-Dukić, N.; Teslić, N.; Versari, A.; Pavlić, B. Utilization of sage by-products as raw material for antioxidants recovery—Ultrasound versus microwave-assisted extraction. Ind. Crops Prod. 2017, 99, 49–59. [Google Scholar] [CrossRef]
- Šumić, Z.; Tepić, A.; Vidović, S.; Jokić, S.; Malbaša, R. Optimization of frozen sour cherries vacuum drying process. Food Chem. 2013, 136, 55–63. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Harborne, J.B. Methods of Plant Analysis; Springer: Dordrecht, The Netherlands, 1984. [Google Scholar]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 1. extraction and determination of total anthocyanin in Cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kumar, K.; Srivastav, S.; Sharanagat, V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: A review. Ultrason. Sonochem. 2021, 70, 105325. [Google Scholar] [CrossRef]
- Zou, T.-B.; Wang, M.; Gan, R.Y.; Ling, W.H. Optimization of ultrasound-assisted extraction of anthocyanins from mulberry, using response surface methodology. Int. J. Mol. Sci. 2011, 12, 3006–3017. [Google Scholar] [CrossRef]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Dumitraşcu, L.; Enachi, E.; Stănciuc, N.; Aprodu, I. Optimization of ultrasound assisted extraction of phenolic compounds from cornelian cherry fruits using response surface methodology. CYTA J. Food 2019, 17, 814–823. [Google Scholar] [CrossRef]
- Chen, X.Q.; Li, Z.H.; Wang, Z.J.; Liu, L.L.; Sun, T.T.; Ma, J.Z.; Zhang, Y. Ultrasound-assisted extraction of total anthocyanins from Rubia sylvatica Nakai fruit and radical scavenging activity of the extract. Ind. Crops Prod. 2020, 150, 112420. [Google Scholar] [CrossRef]
- Esmaeilzadeh Kenari, R.; Dehghan, B. Optimization of ultrasound-assisted solvent extraction of hemp (Cannabis sativa L.) seed oil using RSM: Evaluation of oxidative stability and physicochemical properties of oil. Food Sci. Nutr. 2020, 8, 4976–4986. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.; Riar, C.S.; Jindal, N. Optimization of ultrasound assisted extraction of polyphenols from Meghalayan cherry fruit (Prunus nepalensis) using response surface methodology (RSM) and artificial neural network (ANN) approach. J. Food Meas. Charact. 2021, 15, 119–133. [Google Scholar] [CrossRef]
- Mrkonjić, Ž.; Rakić, D.; Kaplan, M.; Teslić, N.; Zeković, Z.; Pavlić, B. Pressurized-liquid extraction as an efficient method for valorization of thymus serpyllum herbal dust towards sustainable production of antioxidants. Molecules 2021, 26, 2548. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef]
- Ciric, A.; Krajnc, B.; Heath, D.; Ogrinc, N. Response surface methodology and artificial neural network approach for the optimization of ultrasound-assisted extraction of polyphenols from garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef] [PubMed]
- Sasikumar, R.; Das, D.; Jaiswal, A.K. Effects of extraction methods and solvents on the bioactive compounds, antioxidant activity, and storage stability of anthocyanin rich blood fruit (Haematocarpus validus) extracts. J. Food Process. Preserv. 2021, 45, e15401. [Google Scholar] [CrossRef]

| Run | Factors | Responses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | Extraction Time (min) | Ethanol Concentration (%) | Ultrasonic Power (W/L) | Liquid–Solid Ratio (mL/g) | TP (g GAE/100 g) | TMA (mg C3G/100 g) | ||||||
| Coded | Natural | Coded | Natural | Coded | Natural | Coded | Natural | Coded | Natural | |||
| 1 | −1 | 40 | 1 | 40 | 1 | 60 | 1 | 60 | −1 | 10 | 1.3131 | 24.4973 |
| 2 | −1 | 40 | −1 | 20 | 1 | 60 | −1 | 30 | −1 | 10 | 1.2311 | 19.8717 |
| 3 | −1 | 40 | 1 | 40 | −1 | 40 | −1 | 30 | −1 | 10 | 1.0402 | 21.0907 |
| 4 | −1 | 40 | −1 | 20 | −1 | 40 | −1 | 30 | 1 | 20 | 1.3864 | 28.7221 |
| 5 | −1 | 40 | −1 | 20 | −1 | 40 | 1 | 60 | −1 | 10 | 0.8650 | 16.7824 |
| 6 | −1 | 40 | −1 | 20 | 1 | 60 | 1 | 60 | 1 | 20 | 1.3280 | 23.5788 |
| 7 | 1 | 60 | −1 | 20 | −1 | 40 | −1 | 30 | −1 | 10 | 1.3378 | 30.0580 |
| 8 | 1 | 60 | −1 | 20 | 1 | 60 | 1 | 60 | −1 | 10 | 1.5118 | 23.7625 |
| 9 | 1 | 60 | 1 | 40 | −1 | 40 | −1 | 30 | 1 | 20 | 1.6491 | 30.7927 |
| 10 | 1 | 60 | −1 | 20 | 1 | 60 | −1 | 30 | 1 | 20 | 1.5705 | 32.5294 |
| 11 | 1 | 60 | 1 | 40 | −1 | 40 | 1 | 60 | −1 | 10 | 1.5410 | 28.5551 |
| 12 | 1 | 60 | 1 | 40 | 1 | 60 | −1 | 30 | −1 | 10 | 1.5029 | 27.8370 |
| 13 | 1 | 60 | 1 | 40 | 1 | 60 | 1 | 60 | 1 | 20 | 1.6289 | 30.9263 |
| 14 | −1 | 40 | 1 | 40 | −1 | 40 | 1 | 60 | 1 | 20 | 1.3931 | 30.5924 |
| 15 | 1 | 60 | −1 | 20 | −1 | 40 | 1 | 60 | 1 | 20 | 1.3347 | 29.8576 |
| 16 | −1 | 40 | 1 | 40 | 1 | 60 | −1 | 30 | 1 | 20 | 1.7502 | 32.9970 |
| Independent Variables | Responses | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Run | Temperature (°C) | Ethanol Concentration (%) | Liquid–Solid Ratio (mL/g) | Y (%) | TP (g GAE/100 g) | TF (g CE/100 g) | TMA (mg C3G/100 g) | DPPH (µM/g) | FRAP (µM Fe2+/g) | ABTS (µM/g) |
| 1 | 40 | 40 | 10 | 71.96 | 1.39 | 0.77 | 27.75 | 40.17 | 31.72 | 109.33 |
| 2 | 40 | 80 | 30 | 75.66 | 1.87 | 0.72 | 10.50 | 44.30 | 37.31 | 101.18 |
| 3 | 40 | 80 | 10 | 69.03 | 1.20 | 0.60 | 29.22 | 36.65 | 30.19 | 91.07 |
| 4 | 40 | 60 | 20 | 73.30 | 1.39 | 0.78 | 15.73 | 40.53 | 36.94 | 98.26 |
| 5 | 40 | 40 | 30 | 74.37 | 1.88 | 0.78 | 10.62 | 47.33 | 39.91 | 96.90 |
| 6 | 60 | 40 | 20 | 72.04 | 1.60 | 0.87 | 16.55 | 40.89 | 46.23 | 122.42 |
| 7 | 60 | 60 | 20 | 70.10 | 1.44 | 0.75 | 16.62 | 40.78 | 43.93 | 99.02 |
| 8 | 60 | 60 | 20 | 68.70 | 1.49 | 0.78 | 15.71 | 42.10 | 40.47 | 102.26 |
| 9 | 60 | 60 | 30 | 69.00 | 1.87 | 0.80 | 9.80 | 53.19 | 42.50 | 93.19 |
| 10 | 60 | 80 | 20 | 66.24 | 1.27 | 0.72 | 13.81 | 38.97 | 38.87 | 102.07 |
| 11 | 60 | 60 | 20 | 68.00 | 1.47 | 0.73 | 14.33 | 50.44 | 43.39 | 100.54 |
| 12 | 60 | 60 | 20 | 67.92 | 1.43 | 0.78 | 15.31 | 50.96 | 42.03 | 94.13 |
| 13 | 60 | 60 | 20 | 70.74 | 1.43 | 0.74 | 13.91 | 52.78 | 43.62 | 97.88 |
| 14 | 60 | 60 | 10 | 67.07 | 1.38 | 0.77 | 27.67 | 51.00 | 31.80 | 100.76 |
| 15 | 80 | 80 | 10 | 64.69 | 1.16 | 0.61 | 27.60 | 37.02 | 31.37 | 83.84 |
| 16 | 80 | 80 | 30 | 69.27 | 1.87 | 0.80 | 11.47 | 52.08 | 43.78 | 76.93 |
| 17 | 80 | 40 | 30 | 70.59 | 1.96 | 0.81 | 10.35 | 69.69 | 51.33 | 123.45 |
| 18 | 80 | 60 | 20 | 71.44 | 1.51 | 0.80 | 16.21 | 57.58 | 40.81 | 100.48 |
| 19 | 80 | 40 | 10 | 75.57 | 1.57 | 0.84 | 30.17 | 52.31 | 31.80 | 125.22 |
| Source | Sum of Squares | DF | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| Yield | |||||
| Model | 153.21 | 9 | 17.02 | 12.23 | 0.0005 |
| Residual | 12.53 | 9 | 1.39 | ||
| Lack of fit | 6.08 | 5 | 1.22 | 0.75 | 0.6253 |
| R2 = 0.9244 | |||||
| CV = 1.68% | |||||
| Total phenols content | |||||
| Model | 1.048 | 9 | 0.1165 | 53.87 | 9.4 × 10−7 |
| Residual | 0.0195 | 9 | 0.0022 | ||
| Lack of fit | 0.0016 | 5 | 0.0032 | 3.9134 | 0.1053 |
| R2 = 0.9818 | |||||
| CV = 3.03% | |||||
| Total flavonoids content | |||||
| Model | 0.0696 | 9 | 0.0077 | 5.7993 | 0.0076 |
| Residual | 0.0120 | 9 | 0.0013 | ||
| Lack of fit | 0.0101 | 5 | 0.0020 | 4.3134 | 0.0908 |
| R2 = 0.8529 | |||||
| CV = 4.80% | |||||
| Total monomeric anthocyanins | |||||
| Model | 891.49 | 9 | 99.06 | 79.97 | 1.65 × 10−7 |
| Residual | 11.1481 | 9 | 1.2387 | ||
| Lack of fit | 4.4464 | 5 | 1.2893 | 1.0969 | 0.4776 |
| R2 = 0.9877 | |||||
| CV = 6.34% | |||||
| DPPH | |||||
| Model | 1058.70 | 9 | 117.63 | 5.2163 | 0.0109 |
| Residual | 202.96 | 9 | 22.55 | ||
| Lack of fit | 80.22 | 5 | 16.0448 | 0.5229 | 0.7533 |
| R2 = 0.8391 | |||||
| CV = 10.04% | |||||
| FRAP | |||||
| Model | 589.1 | 9 | 65.45 | 21.26 | 5.02 × 10−5 |
| Residual | 27.21 | 9 | 3.0787 | ||
| Lack of fit | 19.43 | 5 | 3.8865 | 1.8784 | 0.2806 |
| R2 = 0.9551 | |||||
| CV = 4.46% | |||||
| ABTS | |||||
| Model | 2545.6 | 9 | 282.8 | 13.04 | 0.0004 |
| Residual | 195.2 | 9 | 21.68 | ||
| Lack of fit | 157.4 | 5 | 31.4853 | 3.3389 | 0.1330 |
| R2 = 0.9288 | |||||
| CV = 4.61% | |||||
| Responses | |||||||
|---|---|---|---|---|---|---|---|
| Term | Y | TP | TF | TMA | DPPH | FRAP | ABTS |
| Linear | |||||||
| x1 | 0.0076 * | 0.0416 * | 0.0890 | 0.5861 | 0.0032 * | 0.0025 * | 0.3940 |
| x2 | 0.0005 * | 6.63 × 10−5 * | 0.0005 * | 0.4407 | 0.0223 * | 0.0066 * | 1.65 × 10−5 * |
| x3 | 0.0196 * | 1.66 × 10−8 * | 0.0225 * | 1.06 × 10−9 * | 0.0093 * | 2.49 × 10−6 * | 0.2390 |
| Interaction | |||||||
| x12 | 0.0115 * | 0.0472 * | 0.9443 | 0.3960 | 0.0814 | 0.4594 | 0.0003 * |
| x13 | 0.0198 * | 0.5813 | 0.7175 | 0.9753 | 0.2219 | 0.0085 * | 0.6403 |
| x23 | 0.0026 * | 0.0037 * | 0.0111 * | 0.5207 | 0.8950 | 0.1336 | 0.2192 |
| Quadratic | |||||||
| x11 | 0.0013 * | 0.9392 | 0.5751 | 0.2324 | 0.4869 | 0.0552 | 0.2955 |
| x22 | 0.9352 | 0.6443 | 0.9194 | 0.9206 | 0.0367 * | 0.2378 | 0.0072 * |
| x33 | 0.1771 | 0.0002 * | 0.5224 | 0.0004 * | 0.1079 | 0.0040* | 0.0814 |
| Response | Predictive model equation | ||||||
| Y | Y = 69.09 − 1.28x1 − 1.96x2 + 1.06x3 − 1.32x12 − 1.18x13 + 1.72x23 + 3.29x12 + 0.060x22 − 1.045x32 | ||||||
| TP | TP = 1.452 + 0.035x1 − 0.102x2 + 0.276x3 − 0.0378x12 − 0.009x13 + 0.064x23 − 0.002x12 − 0.013x22 + 0.176x32 | ||||||
| TF | TF = 0.776 + 0.022x1 − 0.061x2 + 0.032x3 − 0.001x11 + 0.005x13 + 0.041x23 − 0.013x12 − 0.002x22 − 0.015x32 | ||||||
| TMA | TMA = 15.15 + 0.199x1 − 0.284x2 − 8.97x3 − 0.351x12 − 0.013x13 + 0.253x23 + 0.862x12 + 0.069x22 + 3.63x32 | ||||||
| DPPH | DPPH = 47.22 + 5.970x1 − 4.137x2 + 4.944x3 − 3.294x12 + 2.203x13 − 0.228x23 + 2.083x12 − 7.043x22 + 5.128x32 | ||||||
| FRAP | FRAP = 42.03 + 2.302x1 − 1.946x2 + 5.794x3 − 0.480x12 + 2.081x13 − 1.023x23 − 2.337x12 + 1.342x22 − 4.065x32 | ||||||
| ABTS | ABTS = 100.43 + 1.318x1 − 12.223x2 − 1.857x3 − 9.240x12 − 0.796x13 + 2.174x23 − 3.129x12 + 9.741x22 − 5.528x32 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milić, A.; Daničić, T.; Tepić Horecki, A.; Šumić, Z.; Bursać Kovačević, D.; Putnik, P.; Pavlić, B. Maximizing Contents of Phytochemicals Obtained from Dried Sour Cherries by Ultrasound-Assisted Extraction. Separations 2021, 8, 155. https://doi.org/10.3390/separations8090155
Milić A, Daničić T, Tepić Horecki A, Šumić Z, Bursać Kovačević D, Putnik P, Pavlić B. Maximizing Contents of Phytochemicals Obtained from Dried Sour Cherries by Ultrasound-Assisted Extraction. Separations. 2021; 8(9):155. https://doi.org/10.3390/separations8090155
Chicago/Turabian StyleMilić, Anita, Tatjana Daničić, Aleksandra Tepić Horecki, Zdravko Šumić, Danijela Bursać Kovačević, Predrag Putnik, and Branimir Pavlić. 2021. "Maximizing Contents of Phytochemicals Obtained from Dried Sour Cherries by Ultrasound-Assisted Extraction" Separations 8, no. 9: 155. https://doi.org/10.3390/separations8090155
APA StyleMilić, A., Daničić, T., Tepić Horecki, A., Šumić, Z., Bursać Kovačević, D., Putnik, P., & Pavlić, B. (2021). Maximizing Contents of Phytochemicals Obtained from Dried Sour Cherries by Ultrasound-Assisted Extraction. Separations, 8(9), 155. https://doi.org/10.3390/separations8090155









