Bronchodilator Secondary Metabolites from Rhazya stricta Decne Aerial Parts
Abstract
1. Introduction
2. Materials and Methods
2.1. General
2.2. Plant Materials
2.3. Extraction and Isolation
2.4. Bronchodilator Effect
2.4.1. Chemicals
2.4.2. Animals
2.4.3. Guinea-Pig Trachea
3. Results
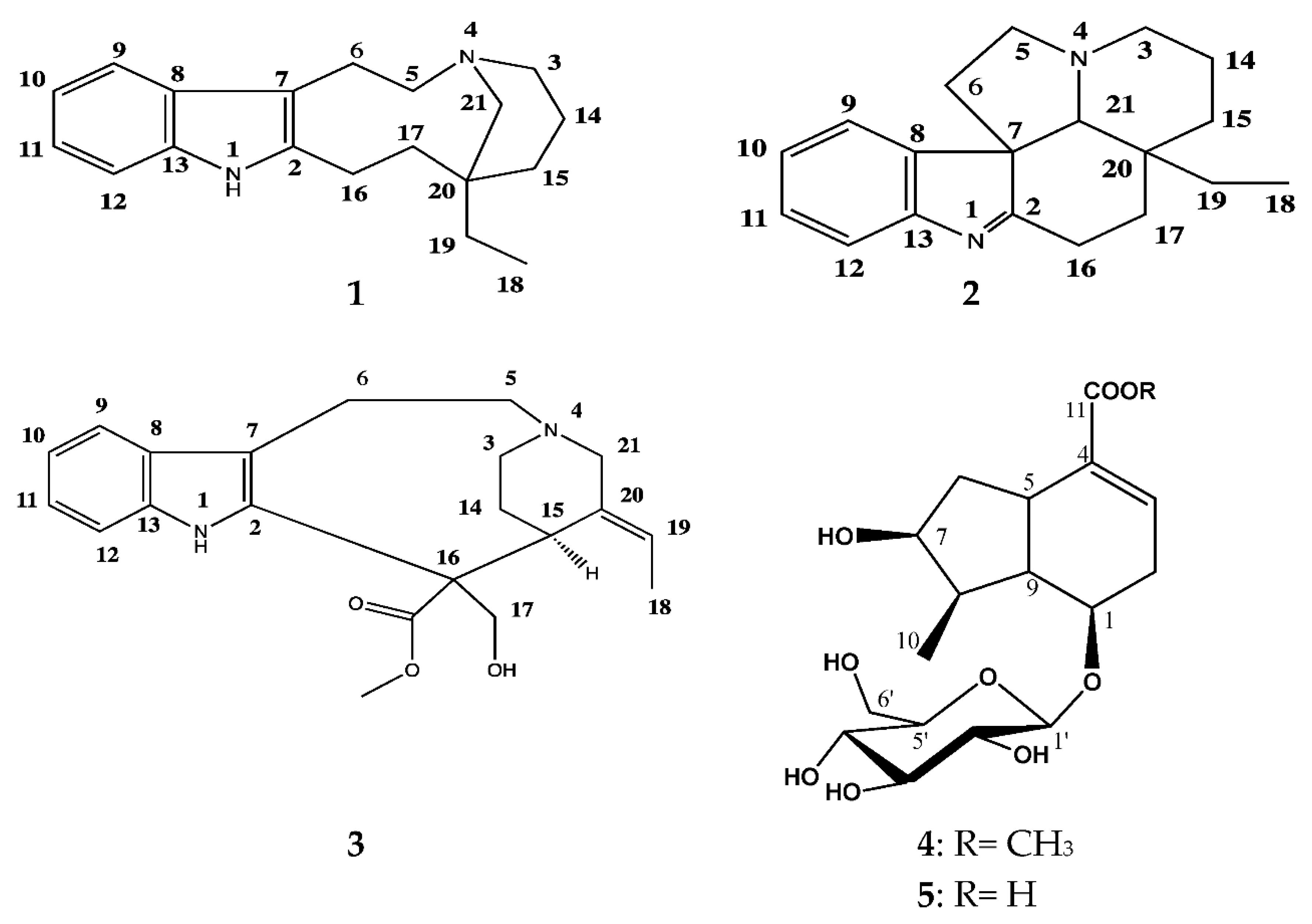
3.1. Identification of the Isolated Compounds 1–5
3.1.1. (−)-Quebrachamine (1)
3.1.2. (+)-Eburenine (2)
3.1.3. (+)-Stemmadenine (3)
3.1.4. Loganic Acid (4)
3.1.5. Loganine (5)
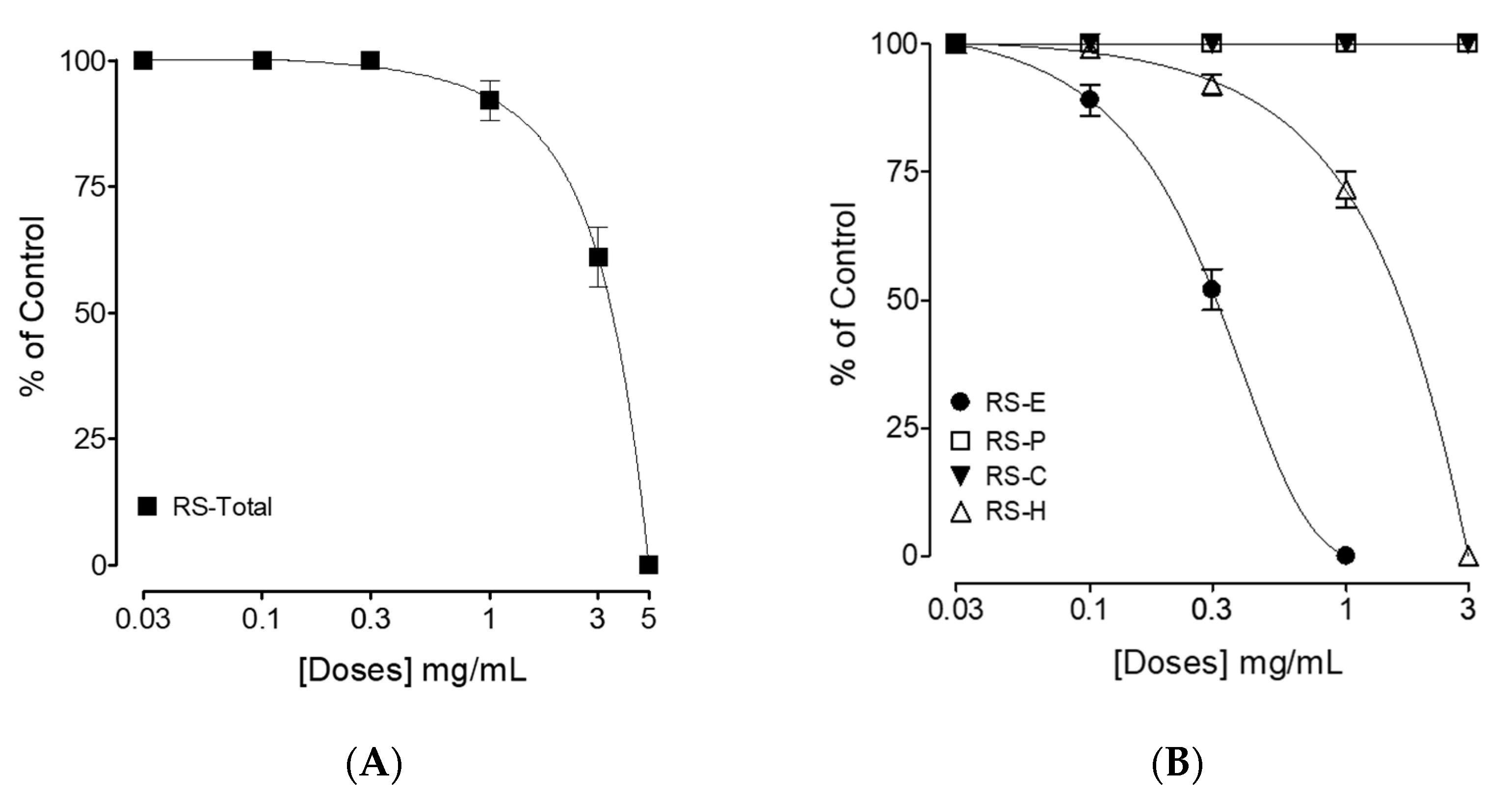
3.2. Bronchodilator Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bukhari, N.A.; Al-Otaibi, R.A.; Ibhrahim, M.M. Phytochemical and taxonomic evaluation of Rhazya stricta in Saudi Arabia. Saudi J. Biol. Sci. 2017, 24, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Gilani, S.A.; Kikuchi, A.; Shinwari, Z.K.; Khattak, Z.I.; Watanabe, K.N. Phytochemical, pharmacological and ethnobotanical studies of Rhazya stricta Decne. Phytother. Res. 2007, 21, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Dymock, W.; Warden, C.J.H.; Hooper, D. Pharmacographia Indica; Kegan Paul, Trench, Trubner & Co.: London, UK, 1890. [Google Scholar]
- Read, M. Plants of Dhofar (The Southern Region of Oman, Traditional Economic and Medicinal Uses) Anthony G. Miller and Miranda Morris The Office of the Adviser for Conservation of the Environment, Diwan of Royal Court, Sultanate of Oman. 1988, 361pp., HB. In UK, available from Holmes McDougall Ltd., Allander House, 137–141 Leith Walk, Edinburgh EH6 8NS. £37.50 inc postage. Oryx 2009, 24, 232. [Google Scholar]
- Ahmad, H.; Bhatti, G.R.; Latif, A. Medicinal flora of the thar desert: An overview of problems and their feasible solutions. Zonas Áridas 2006, 8, 73–84. [Google Scholar]
- Ahmed, M. Checklist of Medicinal Flora of Tehsil Isakhel, District Mianwali-Pakistan. Ethnobot. Leafl. 2006, 2006, 10. [Google Scholar]
- Yahya, M.A. Saudi Plants: A Phytochemical and Biological Approach; King Abdulaziz City for Science and Technology: Riyadh, Saudi Arabia, 1990. [Google Scholar]
- Bashir, A.K.; Abdalla, A.A.; Hassan, E.S.; Wasfi, I.A.; Amiri, M.A.; Crabb, T.A. Alkaloids with antimicrobial activity from the root of Rhazya stricta Decn. growing in United Arab Emirates. Arab. Gulf J. Sci. Res. 1994, 12, 119–131. [Google Scholar]
- Tanira, M.; Ali, B.; Bashir, A.; Chandranath, I. Some pharmacologic and toxicologic studies on rhazya stricta decne in rats, mice and rabbits. Gen. Pharmacol. Vasc. Syst. 1996, 27, 1261–1267. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; El-Sayed, A.; Handy, G.A.; Cordell, G.A. Catharanthus Alkaloids XXXVII. 16-Epi-Z-Isositsirikine, a Monomeric Indole Alkaloid with Antineoplastic Activity from Catharanthus roseus and Rhazya stricta. J. Nat. Prod. 1983, 46, 409–413. [Google Scholar] [CrossRef]
- Atta ur, R.; Zaman, K. 1,2-dehydroaspidospermidine-N-oxide, an alkaloid from Rhazya stricta. Phytochemistry 1986, 25, 1779–1780. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Handy, G.A.; Funayama, S.; Cordell, G.A. Anticancer Indole Alkaloids of Rhazya stricta. J. Nat. Prod. 1981, 44, 696–700. [Google Scholar] [CrossRef]
- Banerji, A.; Majumder, P.; Chatterjee, A. Occurrence of geissoschizine and other minor biogenetically related alkaloids in Rhazya stricta. Phytochemistry 1970, 9, 1491–1493. [Google Scholar] [CrossRef]
- Walls, F.; Collera, O.; Sandoval, A.L. Alkaloids from stemmadenia species-I: The alkaloids of S. Donnell-Smithii and S. Galeottiana. Tetrahedron 1958, 2, 173–182. [Google Scholar] [CrossRef]
- Mariee, N.K.; Khalil, A.A.; Nasser, A.A.; Al-Hiti, M.M.; Ali, W.M. Isolation of the Antimicrobial Alkaloid Stemmadenine from Iraqi Rhazya stricta. J. Nat. Prod. 1988, 51, 186–187. [Google Scholar] [CrossRef] [PubMed]
- Tanira, M.O.M.; Ali, B.H.; Bashir, A.K.; Wasfi, I.A.; Chandranath, I. Evaluation of the Relaxant Activity of some United Arab Emirates Plants on Intestinal Smooth Muscle. J. Pharm. Pharmacol. 1996, 48, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Benchikha, N.; Chelalba, I.; Debbeche, H.; Messaoudi, M.; Begaa, S.; Larkem, I.; Amara, D.G.; Rebiai, A.; Simal-Gandara, J.; Sawicka, B.; et al. Lobularia libyca: Phytochemical Profiling, Antioxidant and Antimicrobial Activity Using In Vitro and In Silico Studies. Molecules 2022, 27, 3744. [Google Scholar] [CrossRef] [PubMed]
- Benchikha, N.; Messaoudi, M.; Larkem, I.; Ouakouak, H.; Rebiai, A.; Boubekeur, S.; Ferhat, M.A.; Benarfa, A.; Begaa, S.; Benmohamed, M.; et al. Evaluation of Possible Antioxidant, Anti-Hyperglycaemic, Anti-Alzheimer and Anti-Inflammatory Effects of Teucrium polium Aerial Parts (Lamiaceae). Life 2022, 12, 1579. [Google Scholar] [CrossRef]
- Rodrigo, G.J.; Rodrigo, C. The role of anticholinergics in acute asthma treatment: An evidence-based evaluation. Chest 2002, 121, 1977–1987. [Google Scholar] [CrossRef]
- Gross, N.J.; Skorodin, M.S. Anticholinergic, Antimuscarinic Bronchodilators. Am. Rev. Respir. Dis. 1984, 129, 856–870. [Google Scholar] [CrossRef]
- Hansel, T.T.; Barnes, P.J. An Atlas of Chronic Obstructive Pulmonary Disease, 1st ed.; The Parthenon Publishing Group Inc.: New York, NY, USA, 2004. [Google Scholar]
- Ward, A.J.; Mckenniff, M.; Evans, J.M.; Page, C.P.; Costello, J.F. Theophylline-an immunomodulatory role in asthma? Am. Rev. Respir. Dis. 1993, 147, 518–523. [Google Scholar] [CrossRef]
- Page, C.; Cotter, T.; Kilfeather, S.; Sullivan, P.; Spina, D.; Costello, J. Effect of chronic theophylline treatment on the methacholine dose-response curve in allergic asthmatic subjects. Eur. Respir. J. 1998, 12, 24–29. [Google Scholar] [CrossRef]
- Atta ur, R.; Zaman, K.; Perveen, S.; Muzaffar, A.; Choudhary, M.I.; Pervin, A. Alkaloids from Rhazya stricta. Phytochemistry 1991, 30, 1285–1293. [Google Scholar] [CrossRef]
- Deutsch, H.F.; Evenson, M.A.; Drescher, P.; Sparwasser, C.; Madsen, P.O. Isolation and biological activity of aspidospermine and quebrachamine from an Aspidosperma tree source. J. Pharm. Biomed. Anal. 1994, 12, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.A.D.; Ferreira, D.T.; Pinto, J.P.; Faccione, M.; Braz-Filho, R. New Alkaloid from Aspidosperma Polyneuron Roots. Nat. Prod. Commun. 2008, 3, 1934578X0800300212. [Google Scholar] [CrossRef]
- Wenkert, E.; Hagaman, E.W.; Kunesch, N.; Wang, N.Y.; Zsadon, B. 13C-NMR. Spectroscopy of Naturally Occuring Substances. XLII. Conformational analysis of quebra-chamine-like indole alkaloids and related substances. Helv. Chim. Acta 1976, 59, 2711–2723. [Google Scholar] [CrossRef]
- Abdul-Hameed, Z.H.; Alarif, W.M.; Sobhi, T.R.; Abdel-Lateff, A.; Ayyad, S.-E.N.; Badria, F.A.; Saber, J. New cytotoxic indole-type alkaloids obtained from Rhazya stricta leaves. S. Afr. J. Bot. 2021, 137, 298–302. [Google Scholar] [CrossRef]
- Sandoval, A.; Walls, F.; Shoolery, J.; Wilson, J.; Budzikiewicz, H.; Djerassi, C. Alkaloid studies.: The structures of stemmadenine and condylocarpine. Tetrahedron Lett. 1962, 3, 409–414. [Google Scholar] [CrossRef]
- Achenbach, H.; Benirschke, M.; Torrenegra, R. Alkaloids and other compounds from seeds of Tabernaemontana cymosa. Phytochemistry 1997, 45, 325–335. [Google Scholar] [CrossRef]
- Perera, P.; Sandberg, F.; van Beek, T.A.; Verpoorte, R. Tertiary Indole Alkaloids of Tabernaemontana dichotoma Seeds. Planta Medica 1983, 49, 28–31. [Google Scholar] [CrossRef]
- Obaid, A.Y.; Voleti, S.; Bora, R.S.; Hajrah, N.H.; Omer, A.M.S.; Sabir, J.S.M.; Saini, K.S. Cheminformatics studies to analyze the therapeutic potential of phytochemicals from Rhazya stricta. Chem. Central J. 2017, 11, 11. [Google Scholar] [CrossRef]
- Ali, B.H.; Al-Qarawi, A.A.; Bashir, A.K.; Tanira, M.O. Phytochemistry, pharmacology and toxicity of Rhazya stricta Decne: A review. Phytother. Res. 2000, 14, 229–234. [Google Scholar] [CrossRef]
- Abdel-Kader, M.S.; Stermitz, F.R. Iridoid and other glycosides from Penstemon species. Phytochemistry 1993, 34, 1367–1371. [Google Scholar] [CrossRef]
- Wu, M.; Wu, P.; Liu, M.; Xie, H.; Jiang, Y.; Wei, X. Iridoids from Gentiana loureirii. Phytochemistry 2009, 70, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Çelebi, B.; Recep, T.A.Ş.; Akşit, H.; Celebioglu, H.U. Effects of Loganic Acid isolated from Vinca sonerii on Surface Hydrophobicity and Auto-Aggregation of Probiotic Bacteria, Lactobacillus acidophilus and Lactobacillus rhamnosus. Erzincan Univ. J. Sci. Technol. 2020, 13, 115–122. [Google Scholar]
- Alqasoumi, S.I.; Al-Rehaily, A.J.; Abdel-Kader, M.S. Constituents of the aerial parts of Lonicera etrusca growing in Saudi Ara-bia. Nat. Prod. Sci. 2009, 15, 121–124. [Google Scholar]
- Mitsunaga, K.; Koike, K.; Fukuda, H.; Ishii, K.; Ohmoto, T. Ligustrinoside, a New Bisiridoid Glucoside from Strychnos ligustrina. Chem. Pharm. Bull. 1991, 39, 2737–2738. [Google Scholar] [CrossRef]
- Rehman, N.U.; Ansari, M.N.; Haile, T.; Karim, A.; Abujheisha, K.Y.; Ahamad, S.R.; Imam, F. Possible Tracheal Relaxant and Antimicrobial Effects of the Essential Oil of Ethiopian Thyme Species (Thymus serrulatus Hochst. ex Benth.): A Multiple Mechanistic Approach. Front. Pharmacol. 2021, 12, 615228. [Google Scholar] [CrossRef]
- Imam, F.; Rehman, N.U.; Ansari, M.N.; Qamar, W.; Afzal, M.; Al-Harbi, K.S. Effect of Roflumilast in airways disorders via dual inhibition of phosphodiesterase and Ca2+-channel. Saudi Pharm. J. 2020, 28, 698–702. [Google Scholar] [CrossRef]
- Khan, M.; Rehman, N.-U.; Khan, A.-U.; Gilani, A.H. Pharmacological basis for the medicinal use of Morus alba in gut and airways disorders. Bangladesh J. Pharmacol. 2012, 7, 289–298. [Google Scholar] [CrossRef]
- Rehman, N.U.; Ansari, M.N.; Samad, A.; Ahmad, W. In Silico and Ex Vivo Studies on the Spasmolytic Activities of Fenchone Using Isolated Guinea Pig Trachea. Molecules 2022, 27, 1360. [Google Scholar] [CrossRef]
- McCaig, D.J.; Rodger, I.W. Effects of leukotriene D4 on the mechanical and electrical properties of guinea-pig isolated trachealis. Br. J. Pharmacol. 1988, 94, 729–736. [Google Scholar] [CrossRef]
- Gilani, A.; Rehman, N.; Mehmood, M.H.; Alkharfy, K.M. Species Differences in the Antidiarrheal and Antispasmodic Activities of Lepidium sativum and Insight into Underlying Mechanisms. Phytother. Res. 2013, 27, 1086–1094. [Google Scholar] [CrossRef]

| Pos | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| 1H ** | 13C | 1H ** | 13C | 1H ** | 13C | |
| 2 | - | 140.82 | - | 191.72 | - | 135.54 |
| 3 | 2.22 (Overl) 2.36 (m) | 55.22 | 2.17 (Overl, m) 3.19 (t, 8, 2H) | 51.69 | 2.84 (h, 6.7) 3.25 (dd, 7.7, 13.4) | 45.80 |
| 5 | 2.22 (Overl) 2.42 (m) | 53.69 | 2.65 (Overl, m) 3.19 (t, 8, 2H) | 54.26 | 3.11 (m) 3.36 (Overl) | 55.35 |
| 6 | 2.59 (dd, 7.8, 14.8) 2.73 (Overl) | 21.36 | 1.54 (Overl, m) 2.17 (Overl, m) | 35.04 | 3.36 (Overl) | 23.15 |
| 7 | - | 107.30 | - | 61.23 | - | 109.67 |
| 8 | - | 128.91 | - | 147.63 | - | 126.97 |
| 9 | 7.34 (d, 7.6) | 117.20 | 7.48 (t, 7.4, 2H) | 121.65 | 7.59 (d, 7.9) | 118.30 |
| 10 | 6.87 (t, 7.6) | 118.14 | 7.33 (t, 7.6) | 125.45 | 7.02 (t, 7.5) | 119.32 |
| 11 | 6.92 (t, 7) | 119.63 | 7.22 (t, 7.3) | 127.76 | 7.10 (t, 7.2) | 121.80 |
| 12 | 7.20 (d, 8) | 110.68 | 7.48 (t, 7.4, 2H) | 120.04 | 7.45 (d, 8) | 112.21 |
| 13 | - | 135.10 | - | 154.85 | - | 134.32 |
| 14 | 1.23 (Overl) 1.51 (m) | 22.77 | 1.54 (Overl, m) 1.85 (m) | 21.99 | 2.30 (m) 2.46 (m) | 24.85 |
| 15 | 1.57 (m) 1.80 (dd, 13.8,7) | 33.74 | 1.02 (dt, 5, 13.5) 1.42 (Overl) | 33.22 | 3.67 (Overl) | 35.13 |
| 16 | 2.73 (Overl) 2.92 (m) | 22.27 | 2.65 (Overl, m) 3.11 (dt, 5, 9) | 23.48 | - | 60.55 |
| 17 | 1.10 (Overl, m) 1.23 (Overl) | 34.78 | 1.54 (Overl, m) 2.47 (dt, 3.3, 12.6) | 27.21 | 4.14 (dd, 4.5, 10.4) 4.24 (dd, 4.5, 10.4) | 67.57 |
| 18 | 0.84 (t, 4.45) | 8.15 | 0.50 (t, 7) | 7.61 | 1.69 (d, 6) | 14.38 |
| 19 | 1.10 (Overl, m) 1.23 (Overl) | 31.83 | 0.61 (m) 1.42 (Overl) | 29.68 | 5.53 (d, 6) | 129.48 |
| 20 | - | 36.51 | - | 127.59 | ||
| 21 | 2.43 (s) | 78.29 | 2.46 (d, 14.4), 3.36 (Overl) | 53.04 | ||
| COOCH3 | - | - | - | - | 3.71 (s) | 52.74, 172.65 |
| NH | 10.64 (s) | - | - | - | 10.53 (s) | |
| OH | 5.82 (bs) | |||||
| Pos | 4 | 5 | ||
|---|---|---|---|---|
| 1H ** | 13C | 1H ** | 13C | |
| 1 | 5.26 (d, 4) | 97.53 | 5.28 (d, 4.4) | 96.37 |
| 3 | 7.38 (s) | 151.37 | 7.41 (s) | 150.85 |
| 4 | - | 114.98 | - | 112.62 |
| 5 | 3.11 (q, 8) | 32.11 | 3.12 (q, 8) | 30.73 |
| 6 | 1.67 (p, 7) 2.25 (dd, 7.7, 13.3) | 42.49 | 1.63 (m) 2.24 (dd, 8) | 41.28 |
| 7 | 4.07 (bs) | 75.08 | 4.06 (bs) | 73.70 |
| 8 | 1.87 (m) | 41.96 | 1.88 (m) | 40.70 |
| 9 | 2.04 (m) | 46.42 | 2.04 (h, 4.3) | 45.07 |
| 10 | 1.10 (d, 6.7) | 13.54 | 1.11 (d, 7) | 12.15 |
| 11 | - | 172.10 | - | 168.31 |
| 1′ | 4.70 (d, 7.8) | 99.85 | 4.68 (d, 8) | 98.64 |
| 2′ | 3.26 (t, 8.5) | 74.55 | 3.23 (t, 8.5) | 73.30 |
| 3′ | 3.46 (m) | 77.71 | 3.41 (t, 8.7) | 76.55 |
| 4′ | 3.34 (m) | 71.38 | 3.33 (m) | 70.15 |
| 5′ | 3.34 (m) | 78.00 | 3.33 (m) | 76.87 |
| 6′ | 3.72 (bd, 9) 3.92 (d, 11.6) | 62.57 | 3.68 (Overl) 3.91 (d, 11.4) | 61.35 |
| -OCH3 | - | - | 3.71 (s) | 50.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-Kader, M.S.; Rehman, N.U.; Aldosari, A.F.; Almutib, F.S.; Al Muwinea, A.I.; Saeedan, A.S. Bronchodilator Secondary Metabolites from Rhazya stricta Decne Aerial Parts. Separations 2022, 9, 412. https://doi.org/10.3390/separations9120412
Abdel-Kader MS, Rehman NU, Aldosari AF, Almutib FS, Al Muwinea AI, Saeedan AS. Bronchodilator Secondary Metabolites from Rhazya stricta Decne Aerial Parts. Separations. 2022; 9(12):412. https://doi.org/10.3390/separations9120412
Chicago/Turabian StyleAbdel-Kader, Maged S., Najeeb U. Rehman, Abdullah F. Aldosari, Fahad S. Almutib, Ali I. Al Muwinea, and Abdulaziz S. Saeedan. 2022. "Bronchodilator Secondary Metabolites from Rhazya stricta Decne Aerial Parts" Separations 9, no. 12: 412. https://doi.org/10.3390/separations9120412
APA StyleAbdel-Kader, M. S., Rehman, N. U., Aldosari, A. F., Almutib, F. S., Al Muwinea, A. I., & Saeedan, A. S. (2022). Bronchodilator Secondary Metabolites from Rhazya stricta Decne Aerial Parts. Separations, 9(12), 412. https://doi.org/10.3390/separations9120412








