A Novel Hybrid Retinal Blood Vessel Segmentation Algorithm for Enlarging the Measuring Range of Dual-Wavelength Retinal Oximetry
Abstract
1. Introduction
2. Dual-Wavelength Retinal Oximetry
3. The Proposed Retinal Vessel Segmentation Algorithm
3.1. Image Pre-Processing
3.1.1. Median Filter
3.1.2. High- and Low-Clarity Region Extraction
- Mask extraction of the fundus image
- 2.
- Clarity value calculation based on block
- 3.
- Region segmentation based on clarity histogram
3.2. Vessel Segmentation in the High-Clarity Region
3.2.1. Gaussian Filtering
3.2.2. Matched Filter
3.2.3. Morphological Segmentation
3.3. Vessel Segmentation in the Low-Clarity Region
3.3.1. Guided Filtering
3.3.2. Dynamic Threshold Segmentation
3.4. Post-Processing
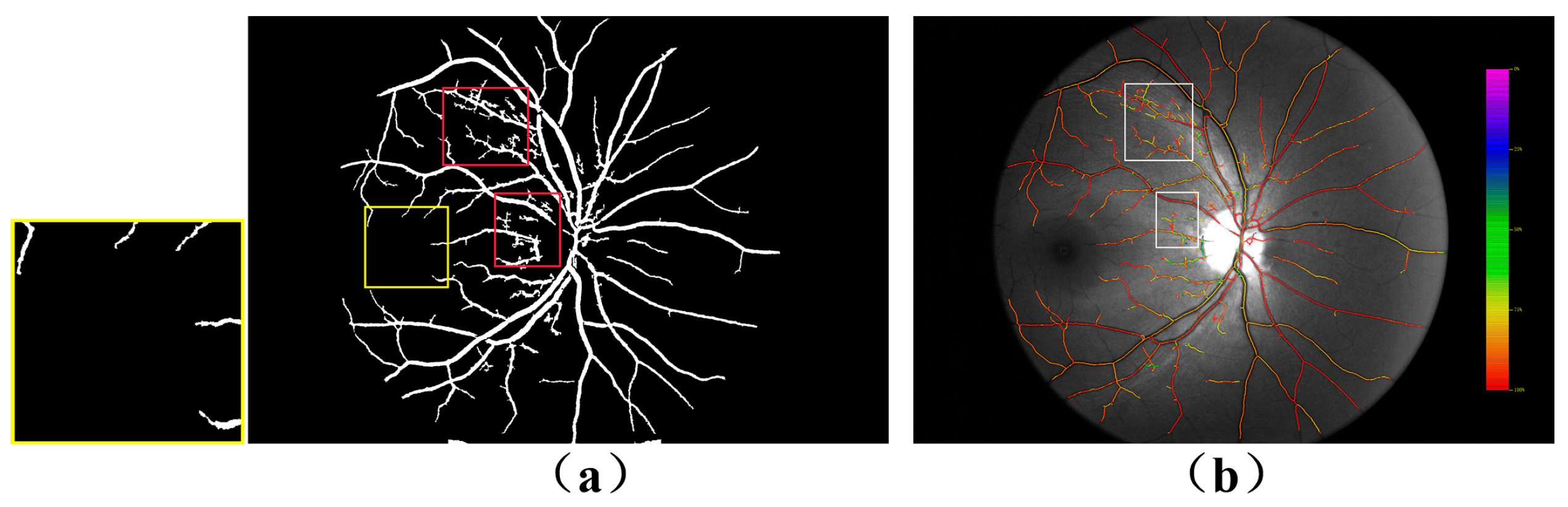
4. Analysis of the Results and Comparisons
4.1. Comparison of Dual-Wavelength Retinal Image Segmentation Results
4.2. Evaluation of the Effect of the Segmentation Algorithm on the Calculation of SO2
4.3. Evaluation of Time Complexity
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hardarson, S.H.; Harris, A.; Karlsson, R.A.; Halldorsson, G.H.; Kagemann, L.; Rechtman, E.; Zoega, G.M.; Eysteinsson, T.; Benediktsson, J.A.; Thorsteinsson, A.; et al. Automatic retinal oximetry. Investig. Ophthalmol. Vis. Sci. 2006, 47, 5011–5016. [Google Scholar] [CrossRef]
- Bojinova, R.I.; Schorderet, D.F.; Valmaggia, C.; Türksever, C.; Schoetzau, A.; Todorova, M.G. Higher retinal vessel oxygen saturation: Investigating its relationship with macular oedema in retinitis pigmentosa patients. Eye 2018, 32, 1209–1219. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, T.; Hirooka, K.; Nakano, Y.; Nitta, E.; Ukegawa, K.; Tsujikawa, A. Oxygen venular saturation correlates with a functional loss in primary open-angle glaucoma and normal-tension glaucoma patients. Acta Ophthalmol. 2018, 96, e304–e308. [Google Scholar] [CrossRef] [PubMed]
- Blindbaek, S.L.; Peto, T.; Grauslund, J. Correlation between Diabetic Retinopathy Severity and Oxygen Metabolism in Patients with Diabetic Macular Edema during Treatment with Intravitreal Aflibercept. Ophthalmic Res. 2020, 63, 106–113. [Google Scholar] [CrossRef]
- Geirsdottir, A.; Hardarson, S.H.; Olafsdottir, O.B.; Stefánsson, E. Retinal oxygen metabolism in exudative age-related macular degeneration. Acta Ophthalmol. 2014, 92, 27–33. [Google Scholar] [CrossRef]
- Brown, J.M.; Campbell, J.P.; Beers, A.; Chang, K.; Ostmo, S.; Chan, R.V.P.; Dy, J.; Erdogmus, D.; Ioannidis, S.; Kalpathy-Cramer, J.; et al. Automated diagnosis of plus disease in retinopathy of prematurity using deep convolutional neural networks. JAMA Ophthalmol. 2018, 136, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Hayreh, S.S.; Zimmerman, M.B. Fundus changes in central retinal vein occlusion. Retina 2015, 35, 29–42. [Google Scholar] [CrossRef]
- Hickam, J.B.; Sieker, H.O.; Frayser, R. Studies of retinal circulation and A-V oxygen difference in man. Trans. Am. Clin. Clim. Assoc. 1959, 71, 34–44. [Google Scholar]
- Harris, A.; Dinn, R.B.; Kagemann, L.; Rechtman, E. A review of methods for human retinal oximetry. Ophthalmic Surg. Lasers Imaging. 2003, 34, 152–164. [Google Scholar] [CrossRef]
- Beach, J. Pathway to Retinal Oximetry. Transl. Vis. Sci. Technol. 2014, 3, 2. [Google Scholar] [CrossRef]
- Linsenmeier, R.A.; Zhang, H.F. Retinal oxygen: From animals to humans. Prog. Retin. Eye Res. 2017, 58, 115–151. [Google Scholar] [CrossRef]
- MacKenzie, L.E.; Harvey, A.R.; McNaught, A.I. Spectroscopic oximetry in the eye: A review. Expert Rev. Ophthalmol. 2017, 12, 345–356. [Google Scholar] [CrossRef]
- Yap, Z.L.; Verma, S.; Lee, Y.F.; Ong, C.; Mohla, A.; Perera, S.A. Glaucoma related retinal oximetry: A technology update. Clin. Ophthalmol. 2018, 12, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Eliasdottir, T.S. Retinal oximetry and systemic arterial oxygen levels. Acta Ophthalmol. 2018, A113, 1–44. [Google Scholar] [CrossRef] [PubMed]
- Shughoury, A.; Mathew, S.; Arciero, J.; Wurster, P.; Adjei, S.; Ciulla, T.; Siesky, B.; Harris, A. Retinal oximetry in glaucoma: Investigations and findings reviewed. Acta Ophthalmol. 2020, 98, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Hubnerova, P.; Mlcak, P.; Sinova, I.; Karhanova, M.; Sin, M. Current use of the automatic retinal oximetry. Czech Slovak Ophthalmol. 2020, 76, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.K.; Knight, D.; Lando, L.; Chao, D.L. Advances in retinal oximetry. Transl. Vis. Sci. Technol. 2021, 10, 5. [Google Scholar] [CrossRef]
- Martin, H.; Walthard, V. Retinal vessel oximetry-calibration, compensation for vessel diameter and fundus pigmentation, and reproducibility. J. Biomed. Opt. 2008, 13, 054015. [Google Scholar]
- Palsson, O.; Geirsdottir, A.; Hardarson, S.H.; Olafsdottir, O.B.; Kristjansdottir, J.V.; Stefansson, E. Retinal oximetry images must be standardized: A methodological analysis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1729–1733. [Google Scholar] [CrossRef]
- Geirsdottir, A.; Palsson, O.; Hardarson, S.H.; Olafsdottir, O.B.; Kristjansdottir, J.V.; Stefánsson, E. Retinal vessel oxygen saturation in healthy individuals. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5433–5442. [Google Scholar] [CrossRef]
- Robert, A.K.; Olof, B.O.; Vedis, H. Automation improves repeatability of retinal oximetry measurements. PLoS ONE 2021, 16, e0260120. [Google Scholar]
- Olafsdottir, O.B.; Vandewalle, E.; Pinto, L.A.; Geirsdottir, A.; De Clerck, E.; Stalmans, P.; Gottfredsdottir, M.S.; Kristjansdottir, J.V.; Van Calster, J.; Zeyen, T.; et al. Retinal oxygen metabolism in healthy subjects and glaucoma patients. Br. J. Ophthalmol. 2014, 98, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Beach, J.M.; Schwenzer, K.J.; Srinivas, S.; Kim, D.; Tiedeman, J.S. Oximetry of retinal vessels by dual-wavelength imaging: Calibration and influence of pigmentation. J. Appl. Physiol. 1999, 86, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.H.; Denninghoff, K.R.; Lompado, A. Retinal vessel oximetry: Toward absolute calibration. In Proceedings of the International Symposium on Biomedical Optics, San Jose, CA, USA, 7–12 May 2000; Volume 3908, pp. 217–226. [Google Scholar]
- Nabili, A.; Bardakci, D.; Helling, K.; Matyas, C.; Muro, S.; Ramella-Roman, J.C. Calibration of an eye oximetry with a dynamic eye phantom. In Proceedings of the SPIE, BiOS, San Jose, CA, USA, 21 February 2008; Volume 6870. [Google Scholar]
- Chen, H.; Liu, G.; Zhang, S.; Zhang, S.; Shen, S.; Luo, Y.; Li, J.; Roberts, C.; Sun, M.; Xu, R. Fundus-simulating phantom for calibration of retinal vessel oximetry devices. Appl. Opt. 2019, 58, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Chen, H.; Liu, G.; Liu, G.l.; Shen, S.W.; Wu, Q.; Hu, C.; Li, J.L.; Dong, E.B.; Xu, R.X. Design of a portable phantom device to simulate tissue oxugenation and blood perfusion. Appl. Opt. 2018, 57, 3938–3946. [Google Scholar] [CrossRef]
- Hirsch, K.; Cubbidge, R.P.; Heitmar, R. Dual wavelength retinal vessel oximetry—Influence of fundus pigmentation. Eye 2022, 12, 1–6. [Google Scholar] [CrossRef]
- Garhöfer, G.; Bata, A.M.; Popa-Cherecheanu, A.; Hommer, A.; Vass, C.; Resch, H.; Schmidl, D.; Werkmeister, R.M.; Schmetterer, L. Retinal Oxygen Extraction in Patients with Primary Open-Angle Glaucoma. Int. J. Mol. Sci. 2022, 23, 10152. [Google Scholar] [CrossRef]
- Heitmar, R.; Blann, A.D. Oxygen saturation in retinal vessels and their correlation with endothelial microparticles in diabetes mellitus and/or cardiovascular disease. Microvasc. Res. 2022, 142, 104336. [Google Scholar] [CrossRef]
- Chen, K.; Mao, J.B.; Liu, H.; Wang, X.N.; Dou, P.; Lu, Y.; Sun, M.Z.; Shen, L.J.; Liu, L. Screening of idiopathic epiretinal membrane using fundus images combined with blood oxygen saturation and vascular morphological features. Int. Ophthalmol. 2023, 43, 1215–1228. [Google Scholar] [CrossRef]
- Xian, Y.; Dai, Y.; Gao, C.; Du, R. Dual-wavelength retinal images denoising algorithm for improving the accuracy of oxygen saturation calculation. J. Biomed. Opt. 2017, 22, 016004. [Google Scholar] [CrossRef]
- Xian, Y.; Wang, C.; Dai, Y. Image registration based on camera calibration for dual-wavelength retinal oximetry. IEEE Access 2019, 7, 128498–128509. [Google Scholar] [CrossRef]
- Zhang, J.; Dashtbozorg, B.; Bekkers, E.; Pluim, J.P.; Duits, R.; Haar Romeny, B.M. Robust retinal vessel segmentation via locally adaptive derivative frames in orientation scores. IEEE Trans. Med. Imaging 2016, 35, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Lu, J.; Wei, C.; Huang, H.; Cai, X.; Chen, X. A hierarchical image matting model for blood vessel segmentation in fundus images. IEEE Trans. Image Process. 2018, 28, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Zou, B.; Chen, Z.; Liu, Q. Improved multi-scale line detection method for retinal blood vessel segmentation. IET Image Process. 2018, 12, 1450–1457. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, X.; Cheng, K.T. Joint segment-level and pixel-wise losses for deep learning based retinal vessel segmentation. IEEE Trans. Biomed. Eng. 2018, 65, 1912–1923. [Google Scholar] [CrossRef]
- Roy, S.; Mitra, A.; Roy, S.; Setua, S.K. Blood vessel segmentation of retinal image using clifford matched filter and clifford convolution. Multimed. Tools Appl. 2019, 78, 34839–34865. [Google Scholar] [CrossRef]
- Shukla, A.K.; Pandey, R.K.; Pachori, R.B. A fractional filter based efficient algorithm for retinal blood vessel segmentation. Biomed. Signal Process. Control 2020, 59, 101883. [Google Scholar] [CrossRef]
- Du, H.; Zhang, X.; Song, G.; Bao, F.; Zhang, Y.; Wu, W.; Liu, P. Retinal blood vessel segmentation by using the MS-LSDNet network and geometric skeleton reconnection method. Comput. Biol. Med. 2023, 153, 106416. [Google Scholar] [CrossRef]
- Yi, S.; Wei, Y.; Zhang, G. Segmentation of retinal vessels based on MRANet. Heliyon 2023, 9, e12361. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, J.; Yang, L. Wave-Net: A lightweight deep network for retinal vessel segmentation from fundus images. Comput. Biol. Med. 2023, 152, 106341. [Google Scholar] [CrossRef]
- Narasimha-Iyer, H.; Beach, J.M.; Khoobehi, B.; Roysam, B. Automatic identification of retinal arteries and veins from dual-wavelength images using structural and functional features. IEEE Trans. Biomed. Eng. 2007, 54, 1427–1435. [Google Scholar] [CrossRef]
- Karlsson, R.A.; Hardarson, S.H. Artery and vein segmentation in retinal oximetry images using convolutional neural networks. Annu. Meet. Assoc. Res. Vis. Ophthalmol. 2019, 1502, 60. [Google Scholar]
- Dou, P.; Zhang, Y.; Zheng, R.; Ye, Y.; Mao, J.; Liu, L.; Wu, M. Retinal imaging and analysis using machine learning with information fusion of the functional and structural features based on a dual-modal fundus camera. J. Mech. Med. Biol. 2021, 21, 2150030. [Google Scholar] [CrossRef]
- Oscar, R.S.; Erick, R.E. An efficient retinal blood vessel segmentation in eye fundus images by using optimized top-hat and homomorphic filtering. Comput. Methods Programs Biomed. 2021, 201, 105949. [Google Scholar]
- Mou, L.; Chen, L.; Cheng, J.; Gu, Z.; Zhao, Y.; Liu, J. Dense dilated network with probability regularized walk for vessel detection. IEEE Trans. Med. Imaging 2019, 39, 1392–1403. [Google Scholar] [CrossRef]
- Ikram, M.K.; De Jong, F.J.; Van Dijk, E.J.; Prins, N.D.; Hofman, A.; Breteler, M.M.; De Jong, P.T. Retinal vessel diameters and cerebral small vessel disease: The rotterdam scan study. Brain 2006, 129, 182–188. [Google Scholar] [CrossRef]
- Patel, J.M.; Gamit, N.C. A review on feature extraction techniques in content based image retrieval. In Proceedings of the 2016 International Conference on Wireless Communications, Signal Processing and Networking (WiSPNET), Chennai, India, 23–25 March 2016; pp. 2259–2263. [Google Scholar]
- Kumar, S.A.; Kumar, J.S. A review on recent developments for the retinal vessel segmentation methodologies and exudate detection in fundus images using deep learning algorithms. In International Conference On Computational Vision and Bio Inspired Computing; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1363–1370. [Google Scholar]
- Iqbal, S.; Khan, T.M.; Naveed, K. Recent trends and advances in fundus image analysis: A review. Comput. Biol. Med. 2022, 151, 106277. [Google Scholar] [CrossRef]
- Khandouzi, A.; Ariafar, A.; Mashayekhpour, Z.; Pazira, M.; Baleghi, Y. Retinal Vessel Segmentation, a Review of Classic and Deep Methods. Ann. Biomed. Eng. 2022, 50, 1292–1314. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Singh, N.P. Analysis of retinal blood vessel segmentation techniques: A systematic survey. Multimed. Tools Appl. 2023, 82, 7679–7733. [Google Scholar] [CrossRef]
- Nihar, R.P.; Ajit, K.S. A Detailed Systematic Review on Retinal Image Segmentation Methods. J. Digit. Imaging Vol. 2022, 35, 1250–1270. [Google Scholar]
- Fraz, M.M.; Remagnino, P.; Hoppe, A.; Uyyanonvara, B.; Rudnicka, A.R.; Owen, C.G.; Barman, S.A. Blood vessel segmentation methodologies in retinal images–a survey. Comput. Methods Programs Biomed. 2012, 108, 407–433. [Google Scholar] [CrossRef]
- L Srinidhi, C.; Aparna, P.; Rajan, J. Recent advancements in retinal vessel segmentation. J. Med. Syst. 2017, 41, 70. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, G.; Yang, L.; Liu, Y. GDF-Net: A multi-task symmetrical network for retinal vessel segmentation. Biomed. Signal Process. Control 2023, 81, 104426. [Google Scholar] [CrossRef]
- Iqbal, S.; Naqvi, S.S.; Khan, H.A.; Saadat, A.; Khan, T.M. G-Net Light: A Lightweight Modified Google Net for Retinal Vessel Segmentation. Photonics 2022, 9, 923. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, B.; Cui, H.; Ma, J. AMF-NET: Attention-aware multi-scale fusion network for retinal vessel segmentation. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society, Guadalajara, Mexico, 1–5 November 2021. [Google Scholar]
- Du, X.F.; Wang, J.S.; Sun, W.Z. UNet retinal blood vessel segmentation algorithm based on improved pyramid pooling method and attention mechanism. Phys. Med. Biol. 2021, 66, 175013. [Google Scholar] [CrossRef]
- Sathananthavathi, V.; Indumathi, G. Encoder enhanced atrous (EEA) unet architecture for retinal blood vessel segmentation. Cogn. Syst. Res. 2021, 67, 84–95. [Google Scholar]
- Panagiotakis, C.; Papadakis, H.; Grinias, E.; Komodakis, N.; Fragopoulou, P.; Tziritas, G. Interactive image segmentation based on synthetic graph coordinates. Pattern Recognit. 2013, 46, 2940–2952. [Google Scholar] [CrossRef]
- Zhao, Q.H.; Li, X.L.; Li, Y.; Zhao, X.M. A fuzzy clustering image segmentation algorithm based on hidden Markov random field models and Voronoi tessellation. Pattern Recognit. Lett. 2017, 85, 49–55. [Google Scholar] [CrossRef]
- Vargas-Muñoz, J.E.; Chowdhury, A.S.; Alexandre, E.B.; Galvão, F.L.; Miranda, P.A.V.; Falcão, A.X. An iterative spanning forest framework for superpixel segmentation. IEEE Trans. Image Process. 2019, 28, 3477–3489. [Google Scholar] [CrossRef] [PubMed]
- Filali, H.; Kalti, K. Image segmentation using MRF model optimized by a hybrid ACO-ICM algorithm. Soft Comput. 2021, 25, 10181–10204. [Google Scholar] [CrossRef]
- Kucybała, I.; Tabor, Z.; Ciuk, S.; Chrzan, R.; Urbanik, A.; Wojciechowski, W. A fast graph-based algorithm for automated segmentation of subcutaneous and visceral adipose tissue in 3D abdominal computed tomography images. Biocybern. Biomed. Eng. 2020, 40, 729–739. [Google Scholar] [CrossRef]
- Trombini, M.; Solarna, D.; Moser, G.; Dellepiane, S. A goal-driven unsupervised image segmentation method combining graph-based processing and Markov random fields. Pattern Recognit. 2023, 134, 109082. [Google Scholar] [CrossRef]
- Khan, T.M.; Khan, M.A.; Rehman, N.U.; Naveed, K.; Afridi, I.U.; Naqvi, S.S.; Raazak, I. Width-wise vessel bifurcation for improved retinal vessel segmentation. Biomed. Signal Process. Control 2022, 71, 103169. [Google Scholar] [CrossRef]
- Yan, M.; Zhou, J.; Luo, C.; Xu, T.; Xing, X. Multiscale joint optimization strategy for retinal vascular segmentation. Sensors 2022, 22, 1258. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Agrawal, S.; Mishro, P.K.; Pachori, R.B. A novel framework for retinal vessel segmentation using optimal improved frangi filter and adaptive weighted spatial FCM. Comput. Biol. Med. 2022, 147, 105770. [Google Scholar] [CrossRef]
- Mahtab, S.; Hossein, P. An active contour model using matched filter and Hessian matrix for retinalvessels segmentation. Turk. J. Electtrical Eng. Comput. Sci. 2022, 30, 20. [Google Scholar]
- Cremer, C.Z. Deep limitations? Examining expert disagreement over deep learning. Prog. Artif. Intell. 2021, 10, 449–464. [Google Scholar] [CrossRef]
- Huang, T.; Yang, G.; Tang, G. A fast two-dimensional median filtering algorithm. IEEE Trans. Acoust. Speech Signal Process. 1979, ASSP-27, 13–18. [Google Scholar] [CrossRef]
- Basu, M. Gaussian-based edge-detection methods—A survey. IEEE Trans. Syst. Man Cybern. Part C Appl. Rev. 2002, 32, 252–260. [Google Scholar] [CrossRef]
- Al-Rawi, M.; Qutaishat, M.; Arrar, M. An improved matched filter for blood vessel detection of digital retinal images. Comput. Biol. Med. 2007, 37, 262–267. [Google Scholar] [CrossRef]
- He, K.M.; Sun, J.; Tang, X.O. Guided Image Filtering. IEEE Trans. Pattern Anal. Mach. Intell. 2013, 35, 1397–1409. [Google Scholar] [CrossRef] [PubMed]
- Elahe, M.; Seyed, H.R.; Hamid, S.Z. Retinal vessel segmentation using a multi-scale medialness function. Comput. Biol. Med. 2012, 42, 50–60. [Google Scholar]
- Schweitzer, D.; Hammer, M.; Kraft, J.; Thamm, E.; Königsdörffer, E.; Strobel, J. In vivo measurement of the oxygen saturation of retinal vessels in healthy volunteers. IEEE Trans. Biomed. Eng. 1999, 46, 1454–1465. [Google Scholar] [CrossRef] [PubMed]
- Joseph, P.P.; Rachael, A.O.; Sarah, L.H.; Andrew, J.A.; Bang, V.B. Retinal oxygen saturation: Novel analysis method for the oxymap. Optometry and Vision Science. Optom. Vis. Sci. 2013, 90, 1104–1110. [Google Scholar]








| Artery | Vein | |
|---|---|---|
| Mean/% | 92.54 83.46–101.38 | 56.63 49.94–68.15 |
| SD/% | 3.15 1.28–5.12 | 3.43 1.18–6.20 |
| Artery | Vein | |
|---|---|---|
| Mean/% | 92.52 83.44–101.34 | 56.66 49.91–68.11 |
| SD/% | 3.13 1.26–5.14 | 3.45 1.19–6.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xian, Y.; Zhao, G.; Wang, C.; Chen, X.; Dai, Y. A Novel Hybrid Retinal Blood Vessel Segmentation Algorithm for Enlarging the Measuring Range of Dual-Wavelength Retinal Oximetry. Photonics 2023, 10, 722. https://doi.org/10.3390/photonics10070722
Xian Y, Zhao G, Wang C, Chen X, Dai Y. A Novel Hybrid Retinal Blood Vessel Segmentation Algorithm for Enlarging the Measuring Range of Dual-Wavelength Retinal Oximetry. Photonics. 2023; 10(7):722. https://doi.org/10.3390/photonics10070722
Chicago/Turabian StyleXian, Yongli, Guangxin Zhao, Congzheng Wang, Xuejian Chen, and Yun Dai. 2023. "A Novel Hybrid Retinal Blood Vessel Segmentation Algorithm for Enlarging the Measuring Range of Dual-Wavelength Retinal Oximetry" Photonics 10, no. 7: 722. https://doi.org/10.3390/photonics10070722
APA StyleXian, Y., Zhao, G., Wang, C., Chen, X., & Dai, Y. (2023). A Novel Hybrid Retinal Blood Vessel Segmentation Algorithm for Enlarging the Measuring Range of Dual-Wavelength Retinal Oximetry. Photonics, 10(7), 722. https://doi.org/10.3390/photonics10070722





