Substrate-Assisted Laser-Induced Breakdown Spectroscopy Combined with Variable Selection and Extreme Learning Machine for Quantitative Determination of Fenthion in Soybean Oil
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Setup
2.2. Sample Preparation
2.3. Methods and Theories
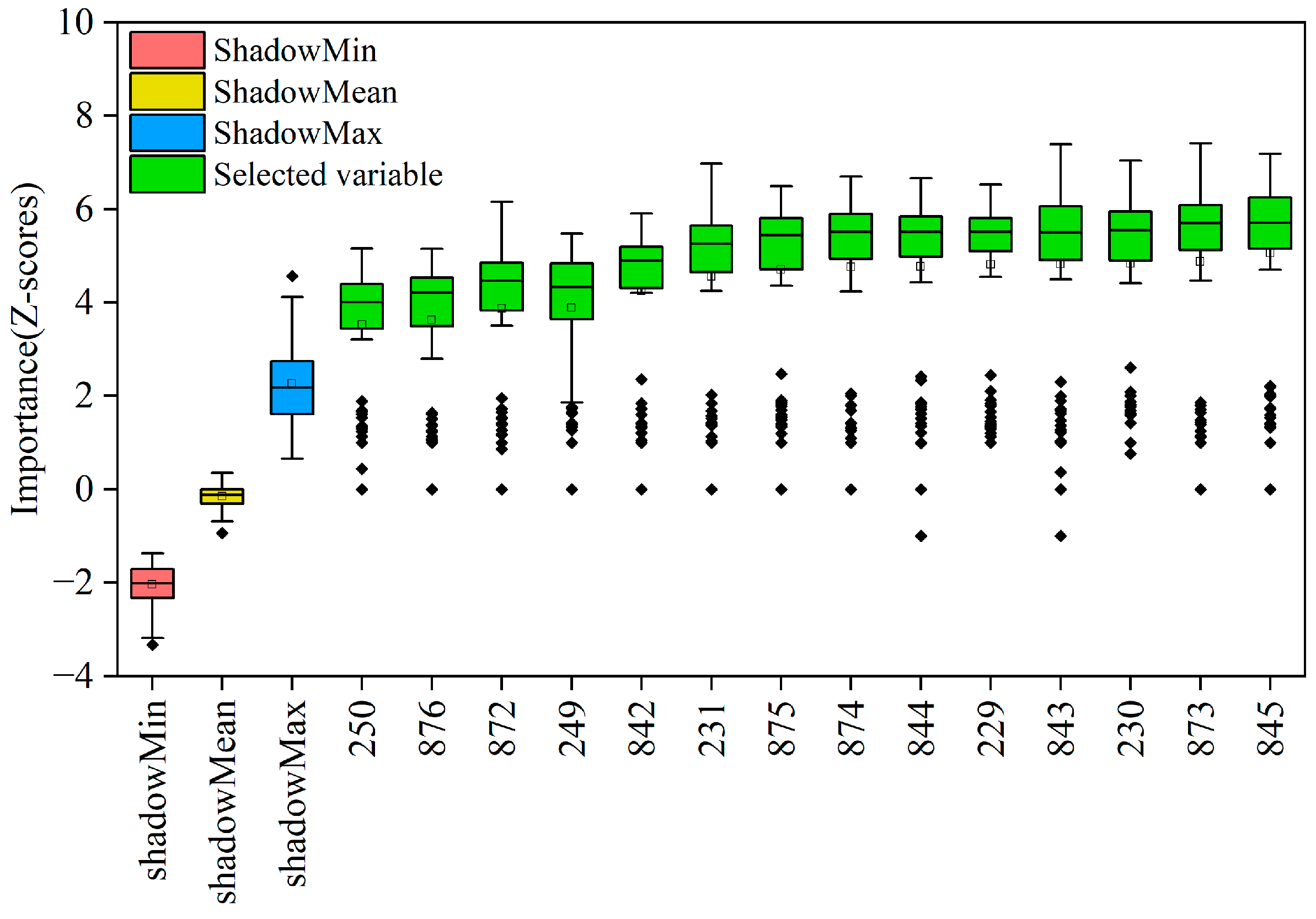
2.3.1. Boruta Algorithm
2.3.2. Extreme Learning Machine
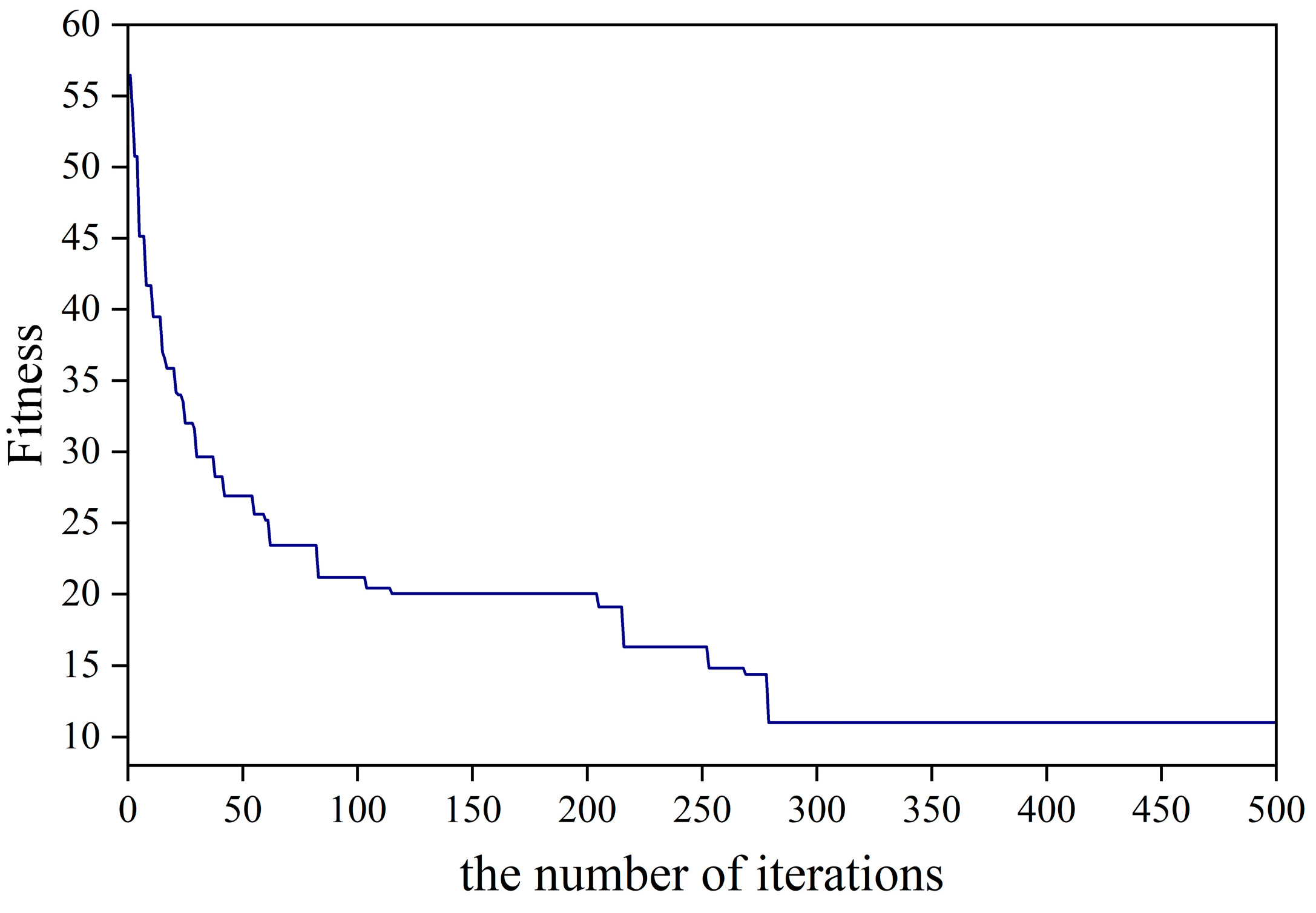
2.3.3. Genetic Algorithm
- (1)
- Determining the number of neurons in the input, hidden, and output layers of ELM; setting the maximal evolutionary generation; randomly generating the input weights and hidden layer thresholds of ELM and binary encoding them to generate the initial population.
- (2)
- Calculating the fitness function. The fitness function (Fitness) is used to evaluate the degree of merit of the randomly generated initial population. Each individual in the population contains the ELM model’s initial weights and implied layer threshold. The fitness value of each individual within the population is calculated individually. The smaller the fitness value, the better the individual.
- (3)
- Using selection, crossover, and variation, the superior individuals within a population are optimized, resulting in a new population. Then, verify whether the evolutionary condition is met; if not, return to recalculate; and if met, terminate the operation, select the optimal population individuals, determine them as the optimal initial weights and implied layer thresholds, and complete the optimization of the ELM model.
2.3.4. Model Evaluation Metrics
3. Results and Discussion
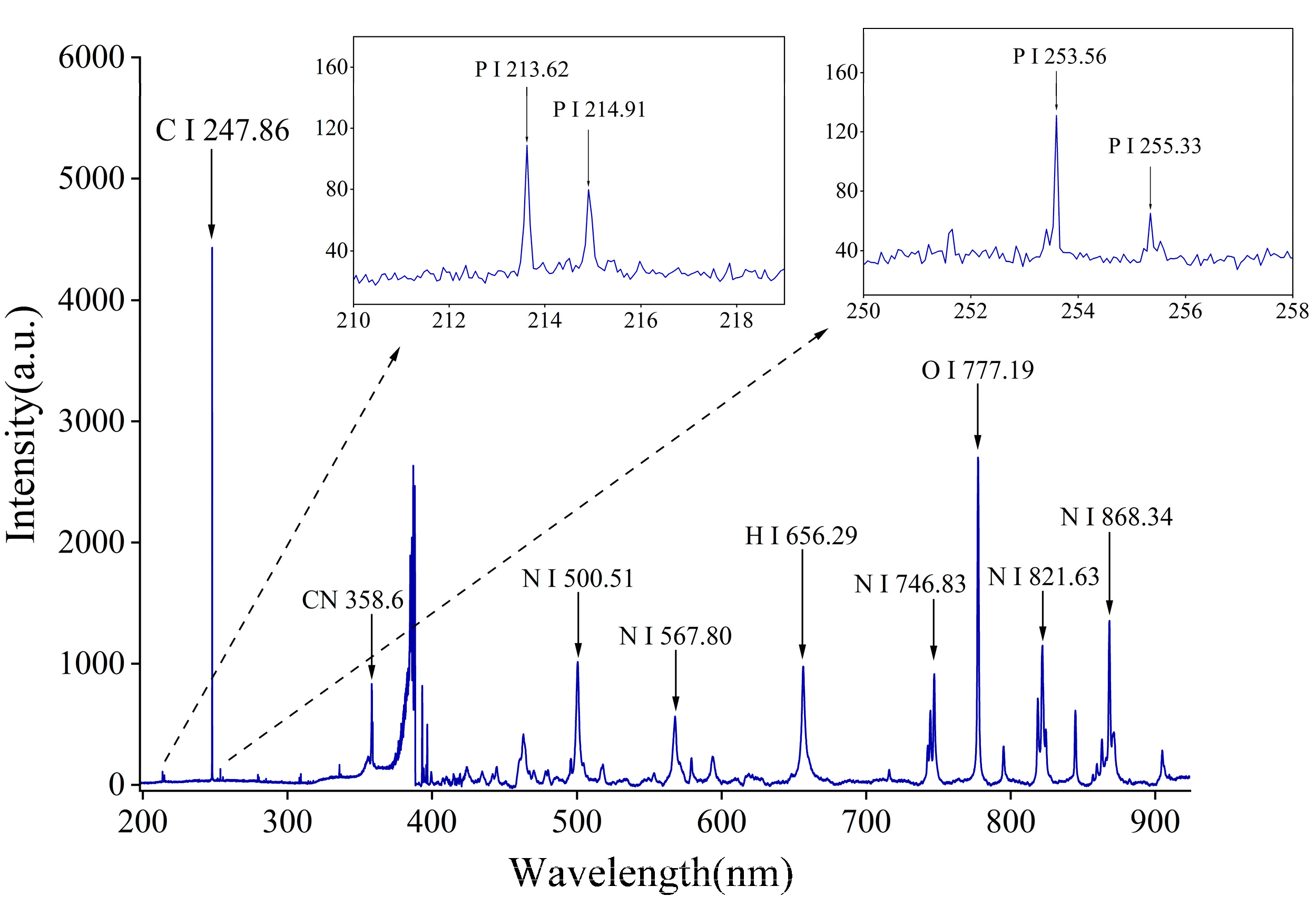
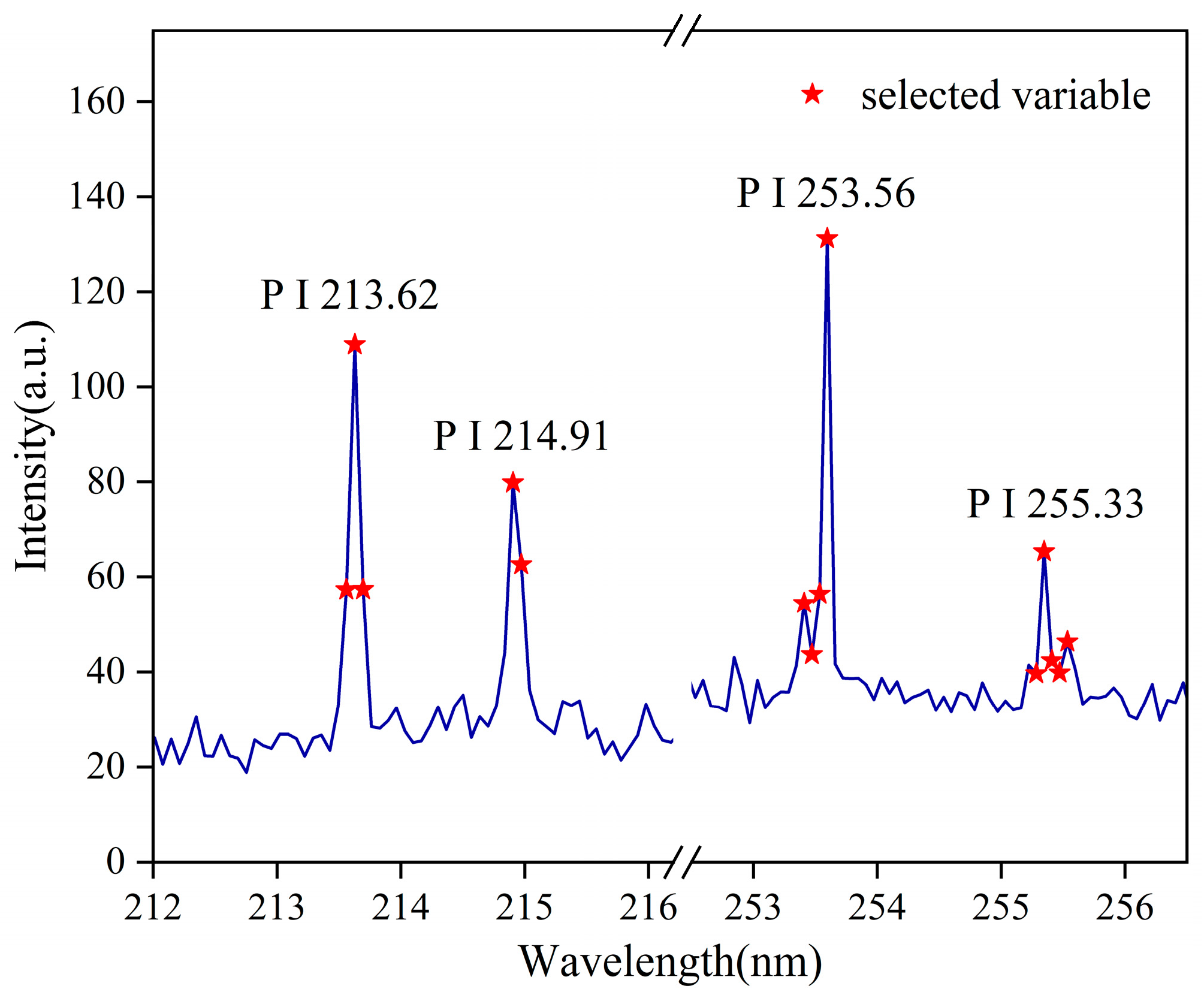
3.1. Analysis of Elemental Spectral Lines by LIBS
3.2. Experimental Parameter Optimization
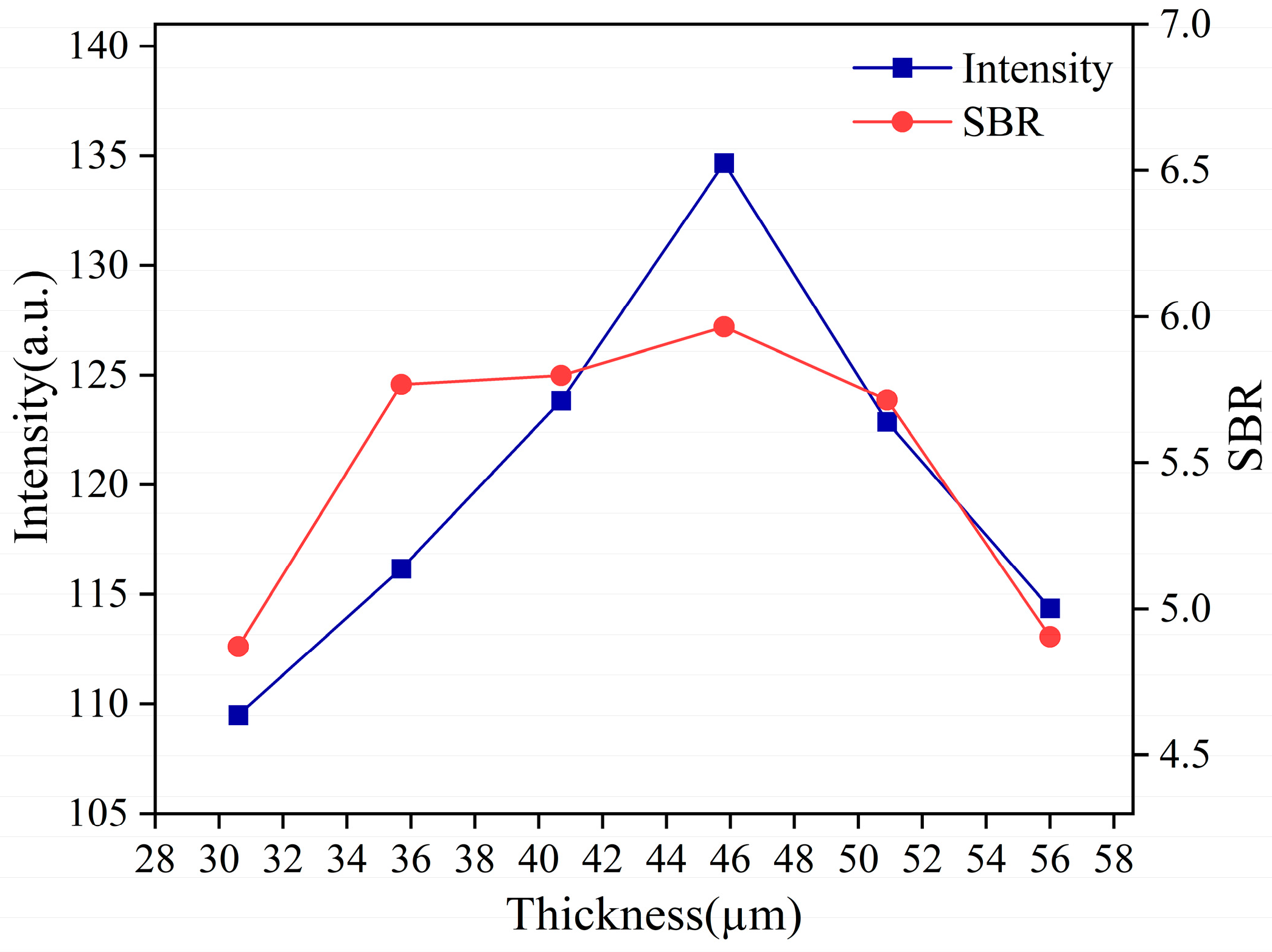
3.2.1. The Average Oil Film Thickness Optimization
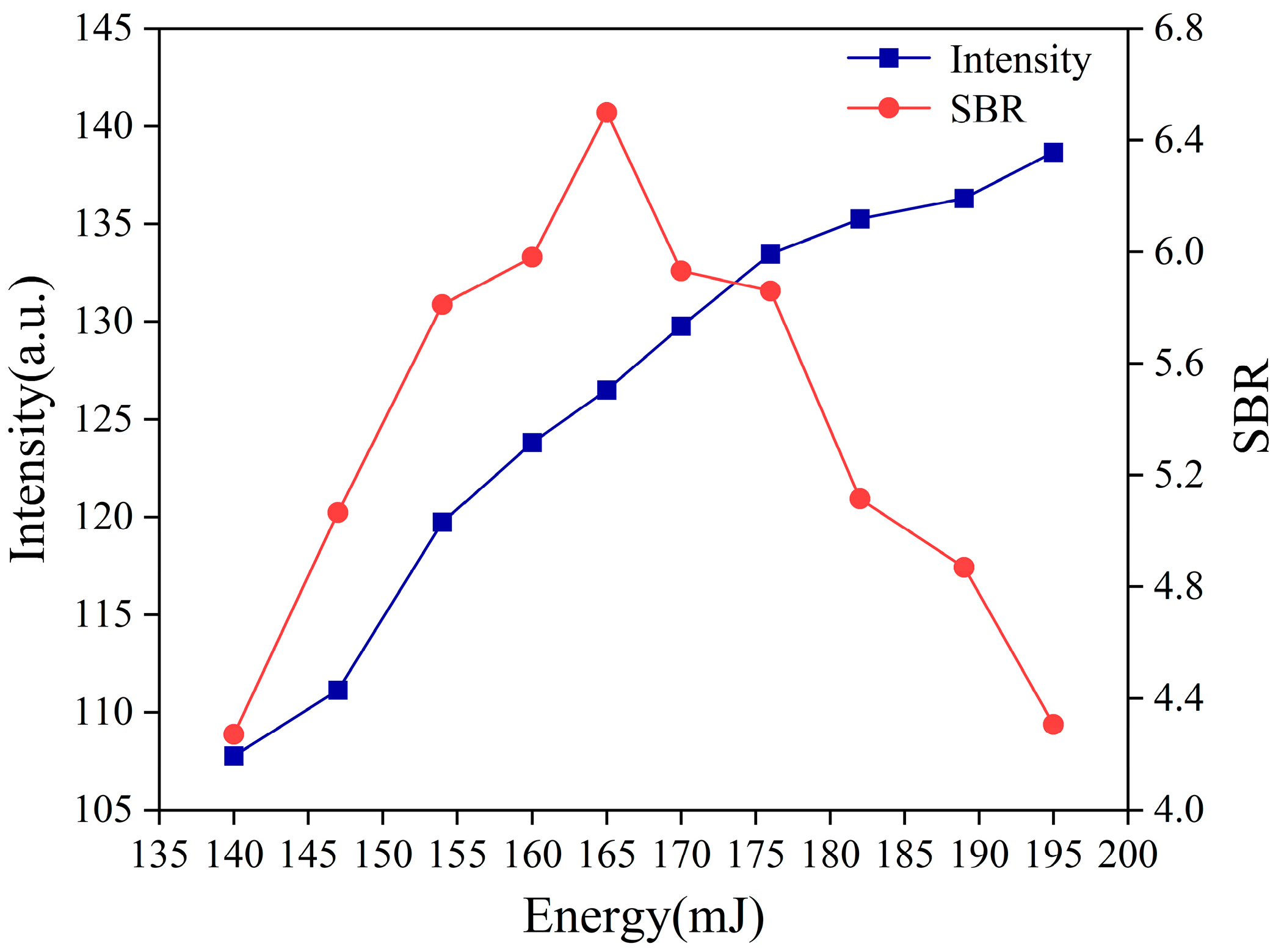
3.2.2. The Laser Energy Optimization
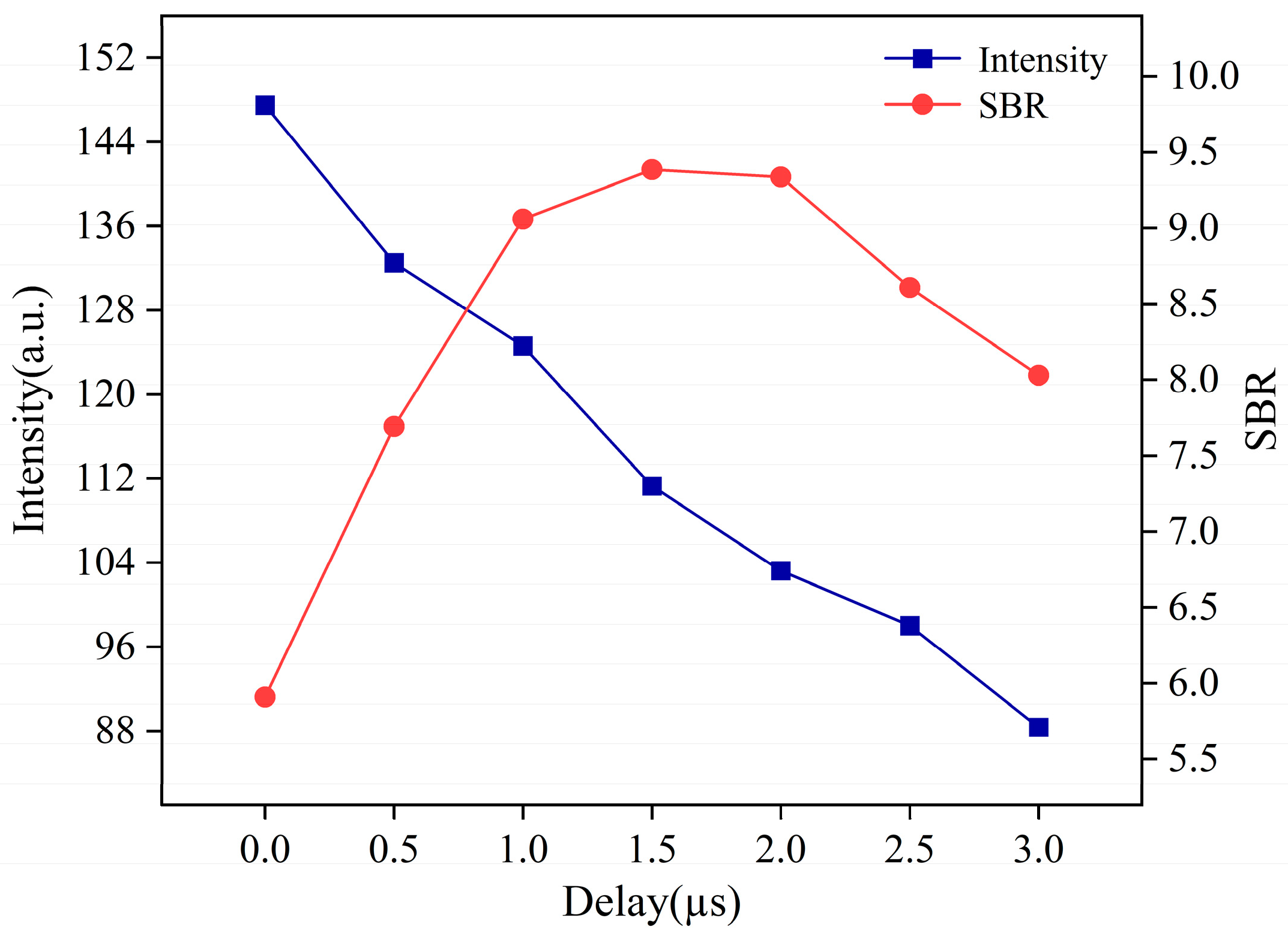
3.2.3. The Delay Time Optimization
3.3. Quantitative Analysis
3.3.1. Analysis of Prediction Results Based on the Full-Spectrum ELM Model
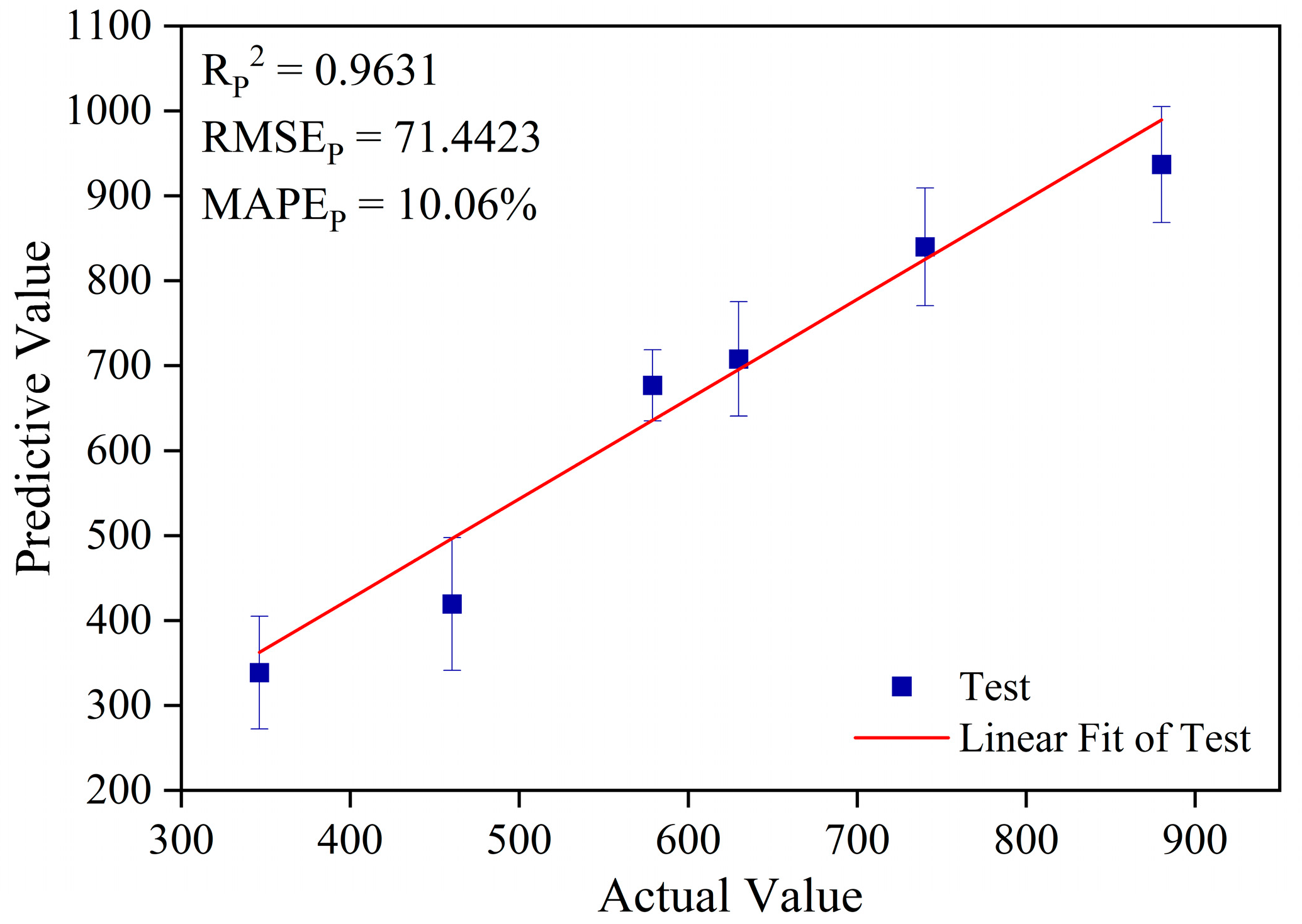
3.3.2. Analysis of Prediction Results Based on the Boruta-ELM Model
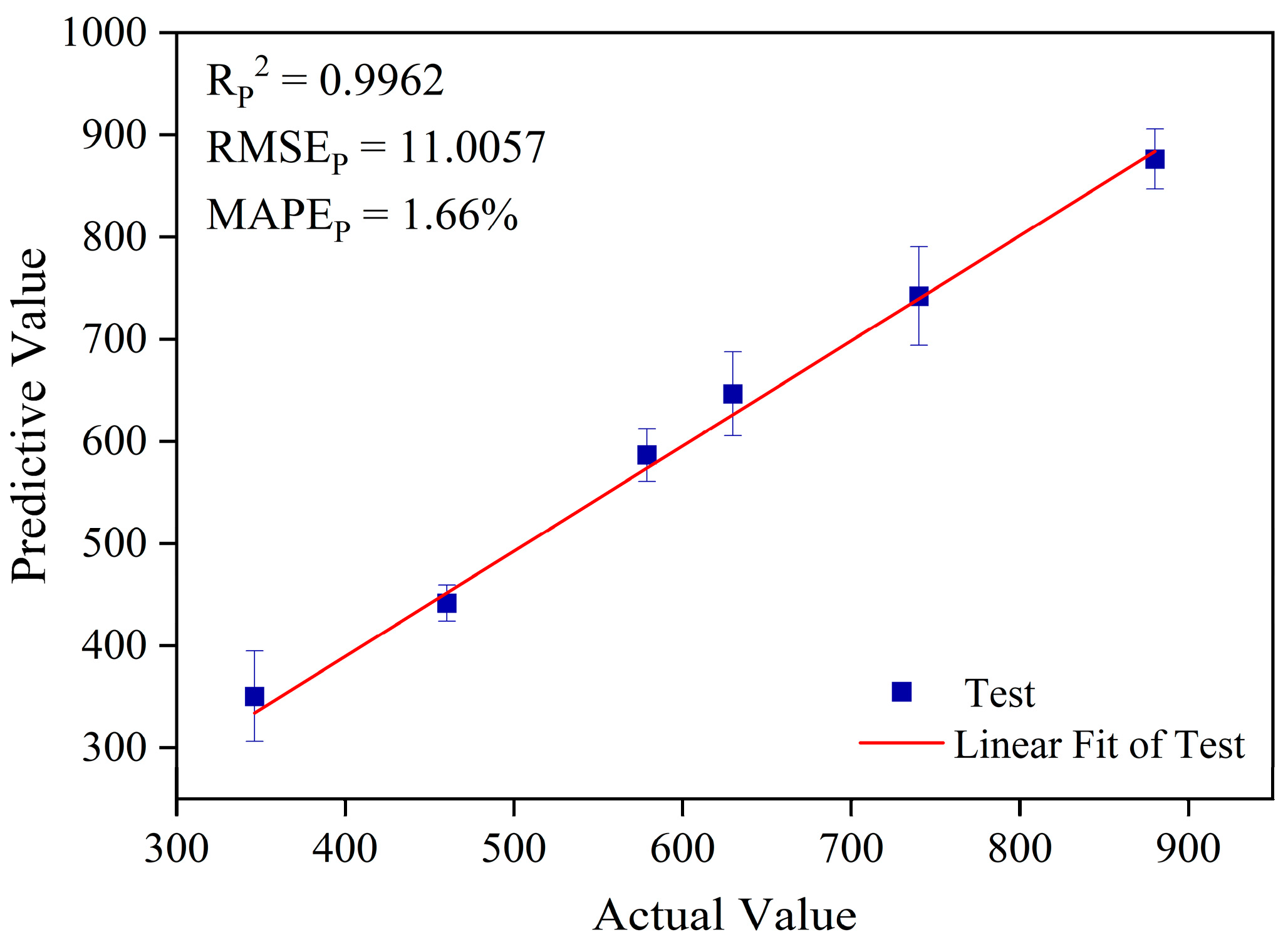
3.3.3. Analysis of Prediction Results Based on the GA-Boruta-ELM Model
3.3.4. Comparison of the Prediction Performance of Different Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, J.; Li, J.; Gao, Y.; He, X.; Hao, G. Nano-based smart formulations: A potential solution to the hazardous effects of pesticide on the environment. J. Hazard. Mater. 2023, 456, 131599. [Google Scholar] [CrossRef]
- Yu, Z.; Lu, T.; Qian, H. Pesticide interference and additional effects on plant microbiomes. Sci. Total Environ. 2023, 888, 164149. [Google Scholar] [CrossRef]
- Kitamura, S.; Kadota, T.; Yoshida, M.; Jinno, N.; Ohta, S. Whole-body metabolism of the organophosphorus pesticide, fenthion, in goldfish, Carassius auratus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2000, 126, 259–266. [Google Scholar] [CrossRef]
- Doemoetoerova, M.; Matisova, E. Fast gas chromatography for pesticide residues analysis. J. Chromatogr. A 2008, 1207, 1–16. [Google Scholar] [CrossRef]
- Presta, M.A.; Kolberg, D.I.S.; Wickert, C.; Pizzutti, I.R.; Adaime, M.B.; Zanella, R. High Resolution Gel Permeation Chromatography Followed by GC-ECD for the Determination of Pesticide Residues in Soybeans. Chromatographia 2009, 69, 237–241. [Google Scholar] [CrossRef]
- Li, L.; Zhou, S.; Jin, L.; Zhang, C.; Liu, W. Enantiomeric separation of organophosphorus pesticides by high-performance liquid chromatography, gas chromatography and capillary electrophoresis and their applications to environmental fate and toxicity assays. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 1264–1276. [Google Scholar] [CrossRef]
- Reyes-Garces, N.; Myers, C. Analysis of the California list of pesticides, mycotoxins, and cannabinoids in chocolate using liquid chromatography and low-pressure gas chromatography-based platforms. J. Sep. Sci. 2021, 44, 2564–2576. [Google Scholar] [CrossRef]
- Ali, I.; Suhail, M.; Alharbi, O.M.L.; Hussain, I. Advances in sample preparation in chromatography for organic environmental pollutants analyses. J. Liq. Chromatogr. Relat. Technol. 2019, 42, 137–160. [Google Scholar] [CrossRef]
- Castro, R.; Natera, R.; Duran, E.; Garcia-Barroso, C. Application of solid phase extraction techniques to analyse volatile compounds in wines and other enological products. Eur. Food Res. Technol. 2008, 228, 1–18. [Google Scholar] [CrossRef]
- Cobzac, S.C.; Gocan, S. Sample preparation for high performance liquid chromatography: Recent progress. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1157–1267. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, S.; Tian, H.; Sun, B. Research progress in the use of liquid-liquid extraction for food flavour analysis. Trends Food Sci. Technol. 2023, 132, 138–149. [Google Scholar] [CrossRef]
- Ding, Y.; Zhang, W.; Zhao, X.; Zhang, L.; Yan, F. A hybrid random forest method fusing wavelet transform and variable importance for the quantitative analysis of K in potassic salt ore using laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2020, 35, 1131–1138. [Google Scholar] [CrossRef]
- Deng, F.; Ding, Y.; Chen, Y.; Zhu, S. Quantitative analysis of the content of nitrogen and sulfur in coal based on laser-induced breakdown spectroscopy: Effects of variable selection. Plasma Sci. Technol. 2020, 22, 074005. [Google Scholar] [CrossRef]
- Qiao, S.; Ding, Y.; Tian, D.; Yao, L.; Yang, G. A Review of Laser-Induced Breakdown Spectroscopy for Analysis of Geological Materials. Appl. Spectrosc. Rev. 2015, 50, 1–26. [Google Scholar] [CrossRef]
- Fichet, P.; Mauchien, P.; Wagner, J.-F.; Moulin, C. Quantitative elemental determination in water and oil by laser induced breakdown spectroscopy. Anal. Chim. Acta 2001, 429, 269–278. [Google Scholar] [CrossRef]
- Yaroshchyk, P.; Morrison, R.J.S.; Body, D.; Chadwick, B.L. Quantitative determination of wear metals in engine oils using laser-induced breakdown spectroscopy: A comparison between liquid jets and static liquids. Spectrochim. Acta Part B 2005, 60, 986–992. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, R.; Zhang, W.; Yi, R.; Tang, S.; Guo, L.; Hao, Z.; Li, X.; Lu, Y.; Zeng, X. High-sensitivity determination of cadmium and lead in rice using laser-induced breakdown spectroscopy. Food Chem. 2019, 272, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Lu, J.; Zheng, J.; Dong, M. Analyzing unburned carbon in fly ash using laser-induced breakdown spectroscopy with multivariate calibration method. J. Anal. At. Spectrom. 2012, 27, 473–478. [Google Scholar] [CrossRef]
- Nicolodelli, G.; Marangoni, B.S.; Cabral, J.S.; Villas-Boas, P.R.; Senesi, G.S.; dos Santos, C.H.; Romano, R.A.; Segnini, A.; Lucas, Y.; Montes, C.R.; et al. Quantification of total carbon in soil using laser-induced breakdown spectroscopy: A method to correct interference lines. Appl. Opt. 2014, 53, 2170–2176. [Google Scholar] [CrossRef]
- Kumari, R.; Kumar, R.; Rai, A.; Rai, A.K. Evaluation of Na and K in anti-diabetic ayurvedic medicine using LIBS. Lasers Med. Sci. 2022, 37, 513–522. [Google Scholar] [CrossRef]
- Kim, G.; Kwak, J.; Choi, J.; Park, K. Detection of Nutrient Elements and Contamination by Pesticides in Spinach and Rice Samples Using Laser-Induced Breakdown Spectroscopy (LIBS). J. Agric. Food Chem. 2012, 60, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Dong, D.; Zhao, X.; Jiao, L.; Han, P.; Lang, Y. Detection of pesticide residues on fruit surfaces using laser induced breakdown spectroscopy. RSC Adv. 2015, 5, 79956–79963. [Google Scholar] [CrossRef]
- Zhao, X.; Zhao, C.; Du, X.; Dong, D. Detecting and Mapping Harmful Chemicals in Fruit and Vegetables Using Nanoparticle-Enhanced Laser-Induced Breakdown Spectroscopy. Sci. Rep. 2019, 9, 906. [Google Scholar] [CrossRef]
- Xiu, J.; Motto-Ros, V.; Panczer, G.; Zheng, R.; Yu, J. Feasibility of wear metal analysis in oils with parts per million and sub-parts per million sensitivities using laser-induced breakdown spectroscopy of thin oil layer on metallic target. Spectrochim. Acta Part B 2014, 91, 24–30. [Google Scholar] [CrossRef]
- Zheng, L.; Cao, F.; Xiu, J.; Bai, X.; Motto-Ros, V.; Gilon, N.; Zeng, H.; Yu, J. On the performance of laser-induced breakdown spectroscopy for direct determination of trace metals in lubricating oils. Spectrochim. Acta Part B 2014, 99, 1–8. [Google Scholar] [CrossRef]
- Ma, S.; Tang, Y.; Ma, Y.; Chu, Y.; Chen, F.; Hu, Z.; Zhu, Z.; Guo, L.; Zeng, X.; Lu, Y. Determination of trace heavy metal elements in aqueous solution using surface-enhanced laser-induced breakdown spectroscopy. Opt. Express 2019, 27, 15091–15099. [Google Scholar] [CrossRef] [PubMed]
- Kursa, M.B.; Rudnicki, W.R. Feature Selection with the Boruta Package. J. Stat. Softw. 2010, 36, 1–13. [Google Scholar] [CrossRef]
- Agjee, N.e.H.; Ismail, R.; Mutanga, O. Identifying relevant hyperspectral bands using Boruta: A temporal analysis of water hyacinth biocontrol. J. Appl. Remote Sens. 2016, 10, 042002. [Google Scholar] [CrossRef]
- Huang, G.-B.; Zhu, Q.-Y.; Siew, C.-K. Extreme learning machine: Theory and applications. Neurocomputing 2006, 70, 489–501. [Google Scholar] [CrossRef]
- Ding, S.; Xu, X.; Nie, R. Extreme learning machine and its applications. Neural. Comput. Appl. 2014, 25, 549–556. [Google Scholar] [CrossRef]
- Sun, J.; Liu, Q.; Wang, Y.; Wang, L.; Song, X.; Zhao, X. Five-Year Prognosis Model of Esophageal Cancer Based on Genetic Algorithm Improved Deep Neural Network. IRBM 2023, 44, 100748. [Google Scholar] [CrossRef]
- Qin, Y.; Song, K.; Zhang, N.; Wang, M.; Zhang, M.; Peng, B. Robust NIR quantitative model using MIC-SPA variable selection and GA-ELM. Infrared Phys. Technol. 2023, 128, 104534. [Google Scholar] [CrossRef]



| Sample Number | Fenthion Content/(µg/g) | Sample Number | Fenthion Content/(µg/g) |
|---|---|---|---|
| 1 | 307.8 | 10 | 674.2 |
| 2 # | 346.2 | 11 # | 740.2 |
| 3 | 357.3 | 12 | 760.5 |
| 4 | 430.5 | 13 | 827.6 |
| 5 # | 460.3 | 14 | 873.2 |
| 6 | 501.2 | 15 # | 880.1 |
| 7 # | 578.8 | 16 | 909.1 |
| 8 | 627.1 | 17 | 942.7 |
| 9 # | 629.8 | 18 | 984.4 |
| Model | Number of Variables | RP2 | RMSEP | MAPEP |
|---|---|---|---|---|
| Full-spectrum ELM | 8190 | 0.8417 | 167.2986 | 26.46% |
| Boruta-ELM | 14 | 0.9631 | 71.4423 | 10.06% |
| GA-Boruta-ELM | 14 | 0.9962 | 11.0057 | 1.66% |
| Model | RCV2 | RMSECV | MAPECV |
|---|---|---|---|
| Full-spectrum ELM | 0.8249 | 184.1496 | 28.41% |
| Boruta-ELM | 0.9718 | 65.7268 | 9.16% |
| GA-Boruta-ELM | 0.9975 | 10.2618 | 1.49% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Wang, Y.; Chen, J.; Chen, W.; Hu, A.; Shu, Y.; Zhao, M. Substrate-Assisted Laser-Induced Breakdown Spectroscopy Combined with Variable Selection and Extreme Learning Machine for Quantitative Determination of Fenthion in Soybean Oil. Photonics 2024, 11, 129. https://doi.org/10.3390/photonics11020129
Ding Y, Wang Y, Chen J, Chen W, Hu A, Shu Y, Zhao M. Substrate-Assisted Laser-Induced Breakdown Spectroscopy Combined with Variable Selection and Extreme Learning Machine for Quantitative Determination of Fenthion in Soybean Oil. Photonics. 2024; 11(2):129. https://doi.org/10.3390/photonics11020129
Chicago/Turabian StyleDing, Yu, Yufeng Wang, Jing Chen, Wenjie Chen, Ao Hu, Yan Shu, and Meiling Zhao. 2024. "Substrate-Assisted Laser-Induced Breakdown Spectroscopy Combined with Variable Selection and Extreme Learning Machine for Quantitative Determination of Fenthion in Soybean Oil" Photonics 11, no. 2: 129. https://doi.org/10.3390/photonics11020129
APA StyleDing, Y., Wang, Y., Chen, J., Chen, W., Hu, A., Shu, Y., & Zhao, M. (2024). Substrate-Assisted Laser-Induced Breakdown Spectroscopy Combined with Variable Selection and Extreme Learning Machine for Quantitative Determination of Fenthion in Soybean Oil. Photonics, 11(2), 129. https://doi.org/10.3390/photonics11020129





