Exact Solutions to Cancer Laser Ablation Modeling
Abstract
1. Introduction
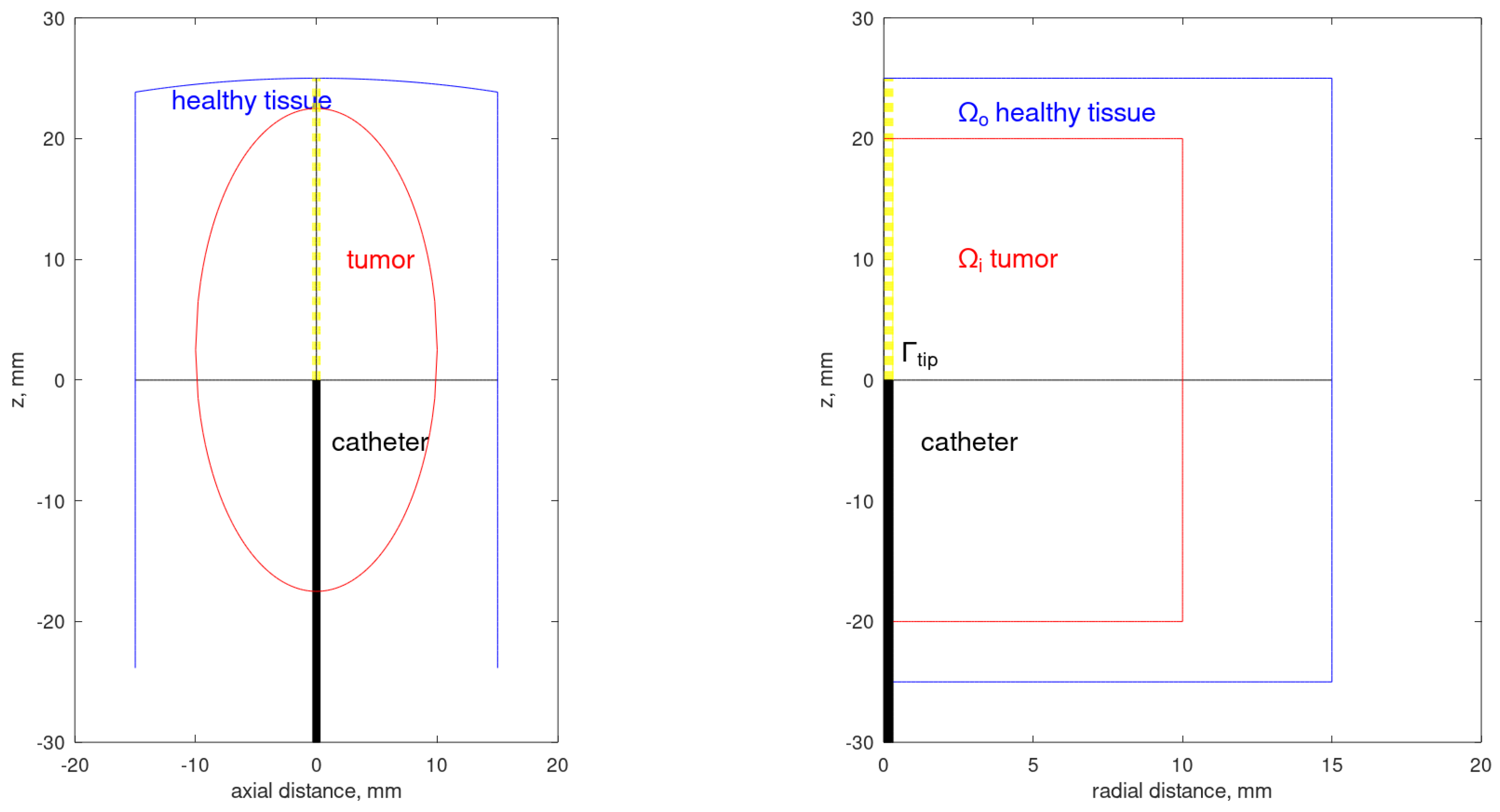
2. Mathematical Formulation
2.1. Photon Transport
2.2. Heat Transfer
2.3. Thermal Damage to the Tissue
3. Analytical Solutions
- Radiative transfer: , , , and in Section 2.1;
- Heat transfer: , , , and in Section 2.2.
4. Results
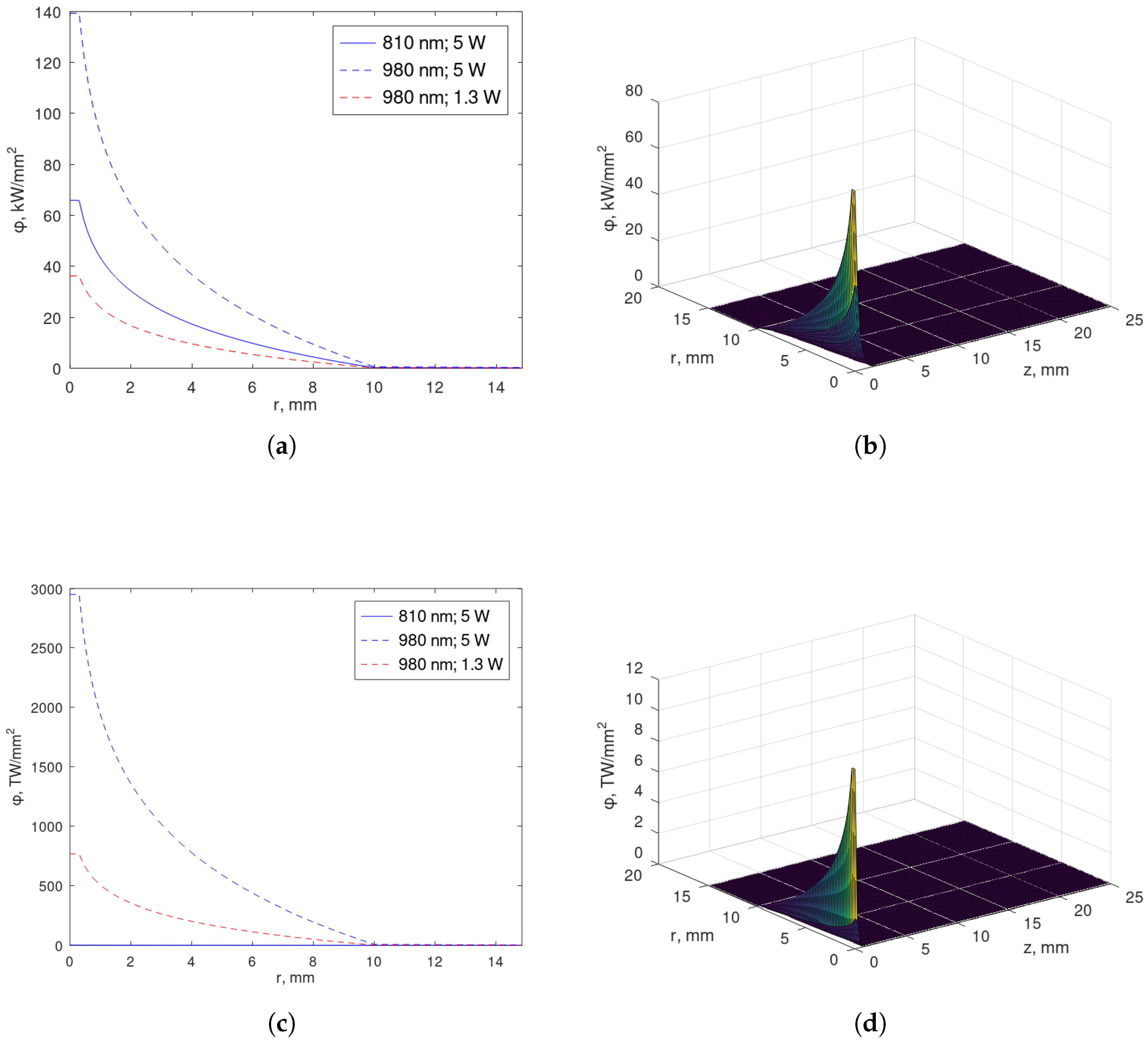
4.1. Exact Solution for the Fluence Rate
- Case
- . The fluence rate , as given in (22) and (23), verifies the initial condition . The source S, as defined in (2)–(4), is dependent on the optical parameters, and the optical parameters are tissue-dependent. Then, S is a discontinuous function on the tumor–healthy interface, , in both breast and prostate tissues. Indeed, S can be neglected for , as mentioned in Section 4.2. Hence, we consistently consider if .For and , by the interface continuity condition at and the boundary condition (5) at , we may extend asThe existence of and is shown in Appendices Appendix A.1 and Appendix A.2, respectively, and and are in accordance with Appendix B. In noting that the fluence rate verifies the initial condition , then . This means that for , and we might consistently consider for either or if . As we do not expect a zero fluence rate at , we assume discontinuous in time.The correspondent parameters are and , with defined in (A2), and and , with defined in (A6). In particular, we havewhere superscripts (i) and (o) stand for inner and outer regions, respectively.For , the continuity of the fluence rate and the requirement of (17) being satisfied imply the symmetry of is relative to .
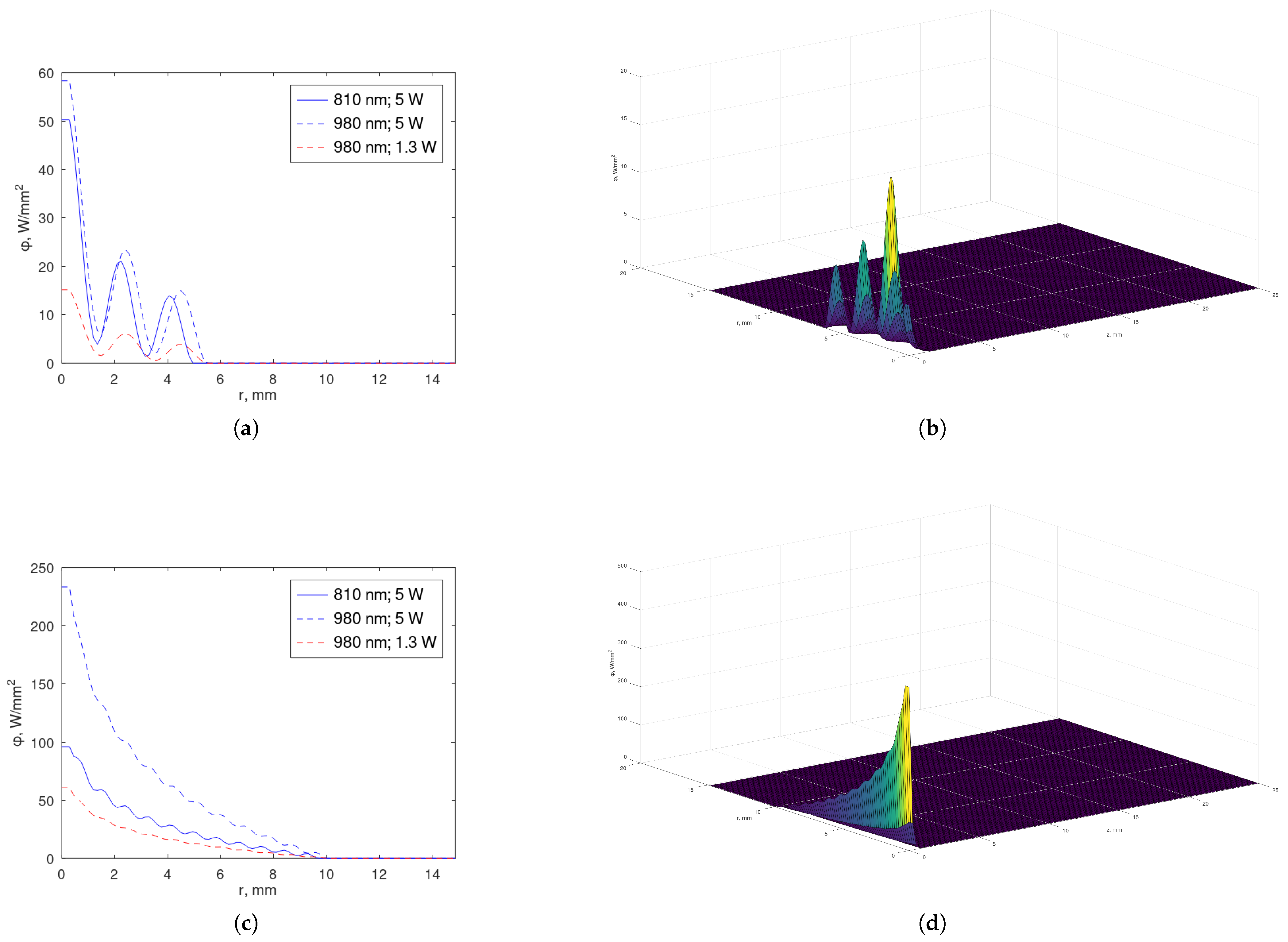
- Case
- . In this one pulse-to-pulse interval , we seek a solution such that satisfies the homogeneous PDE (1) and the initial condition . Moreover, the solution should decrease in time, with the time parameter defined in (17). The principle of superposition now guarantees that the complete solution is constructed as (20), taking into account.For and , we consider the Fourier–Bessel seriesInitial constant , a denotes the radius correspondent to the region under study of the multidomain, and .For and , the function may be given bywhereif parameters are determined by (17), that is, the sequence of parameters verifies for o = outer, orif .Constant is determined by the Robin boundary condition (5) on function at , taking (7) into account.For the remaining multidomain, we may proceed analogously considering the principle of superposition [45].
4.2. Profiles
4.3. Temperature T and Tissue Damage
- If ,
- If ,
5. Discussion
6. Conclusions
- Although the validity of the diffusion approximation of the radiative transfer equation is achieved under the criteron , the exact solution for the fluence rate depends on the choice of the pulse width. Different pulse widths affect the fluence rate, with significant changes observed at pulse widths of 10 ps or more. This emphasizes the importance of diffusion approximation and the unsteady-state fluence rate, contributing to the understanding of biothermophysical problems.
- If the laser tip is fixed at the center of the tumor, its action is local. Then, a moving tip, as used in EVLA [31], should be the object of study in future research in order to find out if a better performance will be provided.
- The exact solutions are of the exponential type as functions of time. The increasing behavior during the temporal pulse width has greater contribution than the decreasing behavior during the subsequent period (the pulse-to-pulse interval). At pulse widths of order , we may conclude that a further study of the time-dependent searchlight problem is a priority.
- The treatment should have a moving focal point with a short exposure time to preserve the healthy tissue.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Arrhenius factor []; |
| c | light velocity ( ); |
| specific heat capacity per unit mass []; | |
| D | diffusion coefficient []; |
| E | planar irradiance []; |
| activation energy for the irreversible damage reaction []; | |
| g | scattering anisotropy coefficient [dimensionless]; |
| k | thermal conductivity []; |
| n | relative refractive index [dimensionless]; |
| maximum optical power output by the laser []; | |
| q | absorbed optical power density []; |
| R | universal gas constant ( ); |
| S | source of scattered photons []; |
| T | temperature []; |
| w | volumetric flow []; |
| wavelength []; | |
| fluence rate []; | |
| absorption coefficient []; | |
| scattering coefficient []; | |
| reduced scattering coefficient []; | |
| total attenuation coefficient []; | |
| transport attenuation coefficient []; | |
| light velocity in the tissue []; | |
| blood perfusion rate []; | |
| density of the tissue []. |
Appendix A. Extending Inside the Tumor
Appendix A.1. Particular Solution
Appendix A.2. General Solution
Appendix B. Extending Outside the Tumor (z < ℓ)
Appendix B.1. Case β2 < 0 (Bessel Functions)
Appendix B.2. Case β2 > 0 (Modified Bessel Functions)
References
- Voeller, R.K.; Schuessler, R.B.; Damiano, R.J., Jr. Surgical Treatment of Atrial Fibrillation. In Cardiac Surgery in the Adult; Lh, C., Ed.; McGraw-Hill: New York, NY, USA, 2008; Chapter 59; pp. 1375–1394. [Google Scholar]
- Fan, Y.; Xu, L.; Liu, S.; Li, J.; Xia, J.; Qin, X.; Li, Y.; Gao, T.; Tang, X. The state-of-the-art and perspectives of Laser Ablation for tumor treatment. Cyborg Bionic Syst. 2024, 5, 0062. [Google Scholar] [CrossRef] [PubMed]
- Loiola, B.R.; Orlande, H.R.B.; Dulikravich, G.S. Thermal damage during ablation of biological tissues. Numer. Heat Transf. Part A Appl. 2018, 73, 685–701. [Google Scholar] [CrossRef]
- Lazarovich, A.; Viswanath, V.; Dahmen, A.S.; Sidana, A. A narrative clinical trials review in the realm of focal therapy for localized prostate cancer. Transl. Cancer Res. 2024, 13, 6529–6539. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Bischof, J. Quantification of temperature and injury response in thermal therapy and cryosurgery. Crit. Rev. Biomed. Eng. 2003, 31, 355–421. [Google Scholar] [CrossRef]
- Consiglieri, L.; dos Santos, I.; Haemmerich, D. Theoretical analysis of the heat convection coefficient in large vessels and the significance for thermal ablative therapies. Phys. Med. Biol. 2003, 48, 4125–4134. [Google Scholar] [CrossRef]
- Consiglieri, L. Continuum models for the cooling effect of blood flow on thermal ablation techniques. Int. J. Thermophys. 2012, 33, 864–884. [Google Scholar] [CrossRef]
- Pupo, A.; González, M.; Cabrales, L.; Reys, J.; Oria, E.; Nava, J.; Jiménez, R.; Sanchéz, F.; Ciria, H.; Cabrales, J. 3d current density in tumors and surrounding healthy tissues generated by a system of straight electrode arrays. Math. Comput. Simul. 2017, 138, 49–64. [Google Scholar] [CrossRef]
- González-Suárez, A.; Pérez, J.J.; Irastorza, R.M.; D’Avila, A.; Berjano, E. Computer modeling of radiofrequency cardiac ablation: 30 years of bioengineering research. Comput. Methods Programs Biomed. 2022, 214, 106546. [Google Scholar] [CrossRef]
- Chiang, J.; Birla, S.; Bedoya, M.; Jones, D.; Subbiah, J.; Brace, C.L. Modeling and validation of microwave ablations with internal vaporization. IEEE Trans. Biomed. Eng. 2015, 62, 657–663. [Google Scholar] [CrossRef]
- Wang, J.; Wu, S.; Wu, Z.; Gao, H.; Huang, S. Influences of blood flow parameters on temperature distribution during liver tumor microwave ablation. Front. Biosci.-Landmark 2021, 26, 504–516. [Google Scholar] [CrossRef]
- Franco, W.; Liu, J.; Wang, G.X.; Nelson, J.S.; Aguilar, G. Radial and temporal variations in surface heat transfer during cryogen spray cooling. Phys. Med. Biol. 2005, 50, 387. [Google Scholar] [CrossRef] [PubMed]
- Boronyak, S.M.; Merryman, W.D. Development of a simultaneous cryo-anchoring and radiofrequency ablation catheter for percutaneous treatment of mitral valve prolapse. Ann. Biomed. Eng. 2012, 40, 1971–1981. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Rai, K.N. Three phase bio-heat transfer model in three-dimensional space for multiprobe cryosurgery. J. Therm. Anal. Calorim. 2022, 147, 14491–14507. [Google Scholar] [CrossRef]
- Ho, C.S.; Ju, K.C.; Cheng, T.Y.; Chen, Y.Y.; Lin, W.L. Thermal therapy for breast tumors by using a cylindrical ultrasound phased array with multifocus pattern scanning: A preliminary numerical study. Phys. Med. Biol. 2007, 52, 4585–4599. [Google Scholar] [CrossRef]
- Mordon, S.; Buys, B.; Brunetaud, J.; Moschetto, Y. New directions in medical laser concept: Role of laser-tissue interaction modelization and feedback control. Lasers Med. Sci. 1989, 4, 317–327. [Google Scholar] [CrossRef]
- Vogel, A.; Venugopalan, V. Pulsed Laser Ablation of Soft Biological Tissues. In Optical-Thermal Response of Laser-Irradiated Tissue; Welch, A.J., van Gemert, M.J., Eds.; Springer Science+Business Media B.V.: Dordrecht, The Netherlands, 2011; Chapter 14; pp. 551–615. [Google Scholar] [CrossRef]
- Putzer, M.; Rogério da Silva, G.; Michael, K.; Schröder, N.; Schudeleit, T.; Bambach, M.; Wegener, K. Geometrical modeling of ultrashort pulse laser ablation with redeposition and analysis of the influence of spot size and shape. Mater. Des. 2024, 246, 113357. [Google Scholar] [CrossRef]
- Vita, E.D.; Landro, M.D.; Massaroni, C.; Iadicicco, A.; Saccomandi, P.; Schena, E.; Campopiano, S. Fiber optic sensors-based thermal analysis of perfusion-mediated tissue cooling in liver undergoing laser ablation. IEEE Trans. Biomed. Eng. 2021, 68, 1066–1073. [Google Scholar] [CrossRef]
- Schena, E.; Saccomandi, P.; Fong, Y. Laser Ablation for cancer: Past, Present and Future. J. Funct. Biomater. 2017, 8, 19. [Google Scholar] [CrossRef]
- Star, W.M. Diffusion Theory of Light Transport. In Optical-Thermal Response of Laser-Irradiated Tissue; Welch, A.J., van Gemert, M.J., Eds.; Springer: New York, NY, USA, 1995; Chapter 6; pp. 131–206. [Google Scholar]
- Liu, H.; Boas, D.; Zhang, Y.; Yodh, A.; Chance, B. Determination of optical properties and blood oxygenation in tissue using continuous NIR light. Phys. Med. Biol. 1995, 40, 1983–1993. [Google Scholar] [CrossRef]
- Oden, J.T.; Diller, K.R.; Bajaj, C.; Browne, J.C.; Hazle, J.; Babuška, I.; Bass, J.; Biduat, L.; Demkowicz, L.; Elliott, A.; et al. Dynamic data-driven finite element models for laser treatment of cancer. Numer. Methods Partial Differ. Equ. 2007, 23, 904–922. [Google Scholar] [CrossRef]
- Sandell, J.L.; Zhu, T.C. A review of in-vivo optical properties of human tissues and its impact on PDT. J. Biophotonics 2011, 4, 773–787. [Google Scholar] [CrossRef] [PubMed]
- Whiting, P.; Dowden, J.; Kapadia, P.; Davis, M. A one-dimensional mathematical model of laser induced thermal ablation of biological tissue. Lasers Med. Sci. 1992, 7, 357–368. [Google Scholar] [CrossRef]
- Durkee, J.J.; Antich, P.; Lee, C. Exact solutions to the multiregion time-dependent bioheat equation. I: Solution development. Phys. Med. Biol. 1990, 35, 847–867. [Google Scholar] [CrossRef]
- Consiglieri, L. An analytical solution for a bio-heat transfer problem. Int. J. Bio-Sci. Bio-Technol. 2013, 5, 267–278. [Google Scholar] [CrossRef]
- Consiglieri, L. Analytical solutions in the modeling of the local RF ablation. J. Mech. Med. Biol. 2016, 16, 1650071. [Google Scholar] [CrossRef]
- Liu, J.; Chen, X.; Xu, L. New thermal wave aspects on burn evaluation of skin subjected to instantaneous heating. IEEE Trans. Biomed. Eng. 1999, 46, 420–428. [Google Scholar]
- Paul, A.; Bandaru, N.; Narasimhan, A.; Das, S. Subsurface tumor ablation with near-infrared radiation using intratumoral and intravenous injection of nanoparticles. Int. J. Micro-Nano Scale Transp. 2014, 5, 69–80. [Google Scholar] [CrossRef]
- Consiglieri, L. Analytical solutions in the modelling of the endovenous laser ablation. Rev. Acad. Colomb. Cienc. Exactas Fis. Nat. 2024, 48, 254–270. [Google Scholar] [CrossRef]
- Banerjee, A.; Ogale, A.; Das, C.; Mitra, K.; Subramanian, C. Temperature distribution in different materials due to short pulse laser irradiation. Heat Transfer Eng. 2005, 26, 41–49. [Google Scholar] [CrossRef]
- D’Alessandro, G.; Tavakolian, P.; Sfarra, S. A review of techniques and bio-heat transfer models supporting infrared thermal imaging for diagnosis of malignancy. Appl. Sci. 2024, 14, 1603. [Google Scholar] [CrossRef]
- Manenti, G.; Perretta, T.; Nezzo, M.; Fraioli, F.; Carreri, B.; Gigliotti, P.; Micillo, A.; Malizia, A.; Di Giovanni, D.; Ryan, C.; et al. Transperineal Laser Ablation (TPLA) treatment of focal low-intermediate risk prostate cancer. Cancers 2024, 16, 1404. [Google Scholar] [CrossRef] [PubMed]
- Niemz, M. Laser-Tissue Interactions: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Marqa, M.F.; Colli, P.; Nevoux, P.; Mordon, S.; Betrouni, N. Focal Laser Ablation of prostate cancer: Numerical simulation of temperature and damage distribution. BioMed. Eng. OnLine 2011, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Prahl, S.A. The diffusion approximation in three dimensions. In Optical-Thermal Response of Laser-Irradiated Tissue; Welch, A.J., Van Gemert, M.J.C., Eds.; Springer: New York, NY, USA, 1995; Chapter 7; pp. 207–231. [Google Scholar]
- Jacques, S. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Capart, A.; Ikegaya, S.; Okada, E.; Machida, M.; Hoshi, Y. Experimental tests of indicators for the degree of validness of the diffusion approximation. J. Phys. Commun. 2021, 5, 025012. [Google Scholar] [CrossRef]
- Rylander, M.; Feng, Y.; Bass, J.; Diller, K. Thermally induced injury and heat shock protein expression in cells and tissues. Ann. N. Y. Acad. Sci. 2005, 1066, 222–242. [Google Scholar] [CrossRef]
- Guo, Z.; Kim, K. Ultrafast laser radiation transfer in heterogeneous tissues with the discrete ordinate method. Appl. Opt. 2003, 42, 2897–2905. [Google Scholar] [CrossRef]
- Jaunich, M.; Raje, S.; Kim, K.; Mitra, K.; Guo, Z. Bio-heat transfer analysis during short pulse laser irradiation of tissues. Int. J. Heat Mass Transfer 2008, 51, 5511–5521. [Google Scholar] [CrossRef]
- Tang, D.; Araki, N. On non-Fourier temperature wave and thermal relaxation time. Int. J. Thermophys. 1997, 18, 493–504. [Google Scholar] [CrossRef]
- Dutta, J.; Kundu, B. An improved analytical model for heat flow in cancerous tumours to avoid thermal injuries during hyperthermia. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2021, 235, 500–514. [Google Scholar] [CrossRef]
- Özisik, M. Heat Conduction; John Wiley & Sons, Ltd.: New York, NY, USA, 2012. [Google Scholar]
- Carp, S.; Prahl, S.; Venugopalan, V. Radiative transport in the delta-P1 approximation: Accuracy of fluence rate and optical penetration depth predictions in turbid semi-infinite media. J. Biomed. Opt. 2004, 9, 632–647. [Google Scholar] [CrossRef]
- Rylander, M.; Feng, Y.; Zimmermann, K.; Diller, K. Measurement and mathematical modeling of thermally induced injury and heat shock protein expression kinetics in normal and cancerous prostate cells. Int. J. Hyperthermia 2010, 26, 748–764. [Google Scholar] [CrossRef]
- Olver, F. Bessel functions of integer order. In Handbook of Mathematical Functions with Formulas, Graphs, and Mathematical Tables; Abramonitz, M., Stegun, I., Eds.; United States Department of Commerce: Washington, DC, USA, 1972; Chapter 9; pp. 355–389. [Google Scholar]

| [cm−1] | a [mm−1] | b | n | |||
|---|---|---|---|---|---|---|
| [nm] | 810 | 980 | 1064 | |||
| Breast tumor | 0.08 | 0.07 | 0.06 | 2.07 | 1.487 | 1.4 |
| Prostate tumor | 0.12 | 0.11 | 0.1 | 3.36 | 1.712 | 1.4 |
| Breast tissue | 0.17 | 0.2 | 0.3 | 1.68 | 1.055 | 1.35 |
| Prostate tissue | 0.6 | 0.5 | 0.4 | 3.01 | 1.549 | 1.37 |
| [nm] | 810 | 980 | 980 | 1064 |
|---|---|---|---|---|
| [W] | 5 | 5 | 1.3 | 1.3 |
| Maximum breast tumor | 198 | 214 | 56 | 77 |
| Minimum breast tumor | 9.5 | 6.8 | 1.8 | 3.9 |
| Maximum adipose breast | 4.4 | 6.3 | 1.6 | 3.1 |
| Maximum prostate tumor | 647 | 828 | 215 | 420 |
| Minimum prostate tumor | 6.6 | 2.2 | 5.7 | 2.7 |
| Maximum healthy prostate | 2.6 | 6.6 | 1.7 | 6.8 |
| Unit | Blood | Breast Tumor | Prostate Tumor | Gland | Fatty Tissue | |
|---|---|---|---|---|---|---|
| 1060.00 | 1000.00 | 999.00 | 1041.00 | 920.00 | ||
| 0.5 | 0.416 | 0.54 | 0.32 |
| [nm] | Breast | Prostate |
|---|---|---|
| 810 | 1.7 | 7.9 |
| 980 | 1.4 | 7.3 |
| 1064 | 1.7 | 1.2 |
| [nm] | Breast Tumor | Breast Tissue | Prostate Tumor | Prostate Tissue |
|---|---|---|---|---|
| 810 | 7.9 × | 1.7 × | 8.2 × | 4.2 × |
| 980 | 9.2 × | 2.4 × | 1.0 × | 4.7 × |
| 1064 | 8.9 × | 4.0 × | 1.1 × | 4.3 × |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Consiglieri, L. Exact Solutions to Cancer Laser Ablation Modeling. Photonics 2025, 12, 400. https://doi.org/10.3390/photonics12040400
Consiglieri L. Exact Solutions to Cancer Laser Ablation Modeling. Photonics. 2025; 12(4):400. https://doi.org/10.3390/photonics12040400
Chicago/Turabian StyleConsiglieri, Luisa. 2025. "Exact Solutions to Cancer Laser Ablation Modeling" Photonics 12, no. 4: 400. https://doi.org/10.3390/photonics12040400
APA StyleConsiglieri, L. (2025). Exact Solutions to Cancer Laser Ablation Modeling. Photonics, 12(4), 400. https://doi.org/10.3390/photonics12040400





