Abstract
Periodontitis is an inflammatory condition of the soft and hard tooth-supporting tissues, representing the first cause of tooth loss. In addition to standard mechanical debridement (Scaling and Root Planing, SRP), further approaches have been proposed as adjuncts. The aim of the present randomized clinical trial is to compare the efficacy of ozone or photobiomodulation (PBM) therapy in addition to SRP to treat periodontal disease. According to a split-mouth design, 240 pathological sites, corresponding to 30 periodontal patients, were randomly divided according to the professional oral hygiene protocol performed at baseline (T0) and after 1 (T1), 2 (T2), 3 (T3), 4 (T4), 5 (T5), and 6 (T6) months. A total of 120 sites underwent an ozonized water administration (ozone group), whereas the other 120 sites were treated with photobiomodulation (PBM group), both in addition to SRP. At every timepoint, the following clinical indexes were assessed: Probing Pocket Depth (PPD) (measured on six sites per element), Plaque Index (PI), and Bleeding on Probing (BOP). As regards PPD, significant intergroup differences were noticed from T5, with significantly lower values in the PBM group (p < 0.05), where values further decreased at T6 (p < 0.05). Both PI and BoP generally decreased from baseline to T6 in both groups; a significant difference was found between T0 and T1 among the groups (p < 0.05), with a progressively higher reduction in the PBM group among the time frames of the study, despite intergroup comparisons not being significant (p > 0.05). Both ozone and PBM appear to be effective adjuvant treatments to SRP, obtaining a slightly better outcome for the latter in the long term, with significant differences at T5 and T6 for PPD. However, because of the absence of standardized protocols for PBM considering both therapeutic and research purposes, no definitive conclusions can be reached, and further studies are required.
1. Introduction
Periodontal disease is an inflammatory condition of soft and hard tooth-supporting tissues, representing the first cause of tooth loss [1] and the sixth most prevalent condition worldwide [2]. It derives from an untreated gingival inflammation caused by bacterial plaque accumulation. Clinically, it occurs as a bleeding of the marginal gum, with an irreversible periodontal attachment loss, rise of pockets and recessions, bone loss, tooth mobility, and exfoliation [3].
Several risk factors have been recognized: smoking [4], alteration of leukocytes [5], immunosuppression [6], diabetes [3], and genetic polymorphisms [7]. Among these, bacterial plaque accumulation remains the most significant as specific periodontopathogens cause an inflammation that, if prolonged, might lead to periodontal damage [8].
The therapy is based on the removal of the bacterial biofilm, thus stopping the causal mechanism of this process. Scaling and Root Planing (SRP) is the gold standard non-surgical therapy, which aims to remove dental plaque and calculus (scaling) as well as to smooth the root surfaces (root planing) [9]. However, recolonization from periodontal bacteria is a frequent event following SRP [10]. Accordingly, adjunctive therapeutic approaches have been proposed, such as the use of antibiotics [11], probiotics [12], ozone application [13], and the photodynamic therapy [14].
As regards ozone, its application in medicine and dentistry is increasing in recent years because of the broad-spectrum antimicrobial activity against bacteria, viruses, protozoa, and fungi [15,16,17,18,19]. Furthermore, ozone exerts an immunomodulant, anti-hypoxic, anti-inflammatory, and regenerative action [20,21]. Therefore, many clinical conditions have been contrasted by using ozone therapy, among which are the management of wound-healing, dental caries, oral lichen planus, gingivitis and periodontitis, halitosis, osteonecrosis of the jaw, post-surgical pain, plaque and biofilms, root canal treatment, dentin hypersensitivity, and temporomandibular joint disorders [21,22,23]. In particular, the use of ozone is indicated in all stages of gingival and periodontal diseases since it is able to exert anti-microbial activity, oxidize the microbial toxins implicated in periodontal diseases, and promote healing and tissue regeneration; moreover, ozone gaseous disinfection overcomes the limitations generally related to liquid chlorhexidine rinses [24].
Another adjuvant therapy in dentistry is represented using lasers to obtain photobiomodulation (PBM) [25]. This treatment, also known as Low-Level Laser Therapy (LLLT), has been particularly applied due to its analgesic action, which derives from the stimulation of nerve cells, the stabilization of membrane potentials, and the release of neurotransmitters in the inflammatory tissue [26]; the therapeutic window generally ranges from 1 to 500 mW, with wavelengths from 600 to 1000 nm [27]. The use of lasers has been recently raised as an adjunct therapy, even for nonsurgical periodontal treatment [28]. The beneficial effect of PBM on periodontal tissues has been evaluated both in vitro and in vivo; in particular, PBM administration has a positive effect on cell proliferation in gingival fibroblasts and results in increased FGF-b and type-1 collagen expression [29]. PBM is able to exert a beneficial action on periodontal health since it increases and accelerates the healing process in damaged tissue through bio-stimulation, and it normalizes the permeability of blood vessels and boosts microcirculation by causing vasodilation [30].
Based on these considerations, the aim of the present randomized clinical trial was to analyze the efficacy of PBM therapy with respect to ozone application, in addition to SRP for the improvement of periodontal clinical indexes. In particular, the Probing Pocket Depth (PPD), Bleeding Score (BS), and Plaque Index (PI) were evaluated since they are clinically relevant outcomes, which, respectively, indicate attachment loss, the presence of bleeding as sign of inflammation, and t plaque accumulation as etiological factors [31]. The first statistical null hypothesis of the study is that there are no significant intragroup differences in clinical indexes considering the different timepoints. The second null hypothesis of the study is that nor do intergroup differences occur between the two treatments at the corresponding timepoints.
2. Materials and Methods
2.1. Trial Design
This was a split-mouth, randomized, active controlled, and single-center trial with a 1:1 allocation ratio, approved by the Unit Internal Review Board (registration number: 2020-0610).
2.2. Participants
Patients attending the Unit of Dental Hygiene, Section of Dentistry, Department of Clinical, Surgical, Diagnostic and Pediatric Sciences of the University of Pavia (Pavia, Italy) for periodontal care were recruited in July 2020. The study lasted until July 2021. Informed consent of the patients was collected. Both interventions and outcome assessments were conducted at the same unit.
The inclusion criteria were the following: age 20–70 years; presence of periodontal disease at stage II and III, grade A according to the latest Classification of Periodontal and Peri-Implant Diseases and Conditions (2017) [32]; patients with no surgical intervention in the last 12 months. The following exclusion criteria were also considered: the presence of systemic diseases; the presence of a cardiac pacemaker; pregnant and breastfeeding women; epilepsy; neuro-psychological disorders; smoking patients; patients taking antibiotics/anti-inflammatory drugs.
2.3. Interventions and Outcomes
At the baseline (T0), patients were asked to sign informed consent forms to participate in the study. Subsequently, an instructed operator collected the following periodontal clinical indices on each peri-implant site by means of a probe (UNC probe 15; Hu-Friedy, Chicago, IL, USA): Probing Pocket Depth (PPD) (measured on six sites per element), Bleeding on Probing (BOP), Bleeding Score (BS), and Plaque Index (PI) [12]. Then, a professional supragingival and subgingival oral hygiene was conducted using a piezoelectric instrument (Multipiezo, Mectron S.p.a, Carasco, Italy) and Gracey curettes (Hu-Friedy, Chicago, IL, USA), followed by supragingival and subgingival application of a decontaminating glycine powder (Glycine Powder, Mectron S.p.a., Carasco, Italy). At this stage, the two pathological sites with the highest PPD values per each quadrant were assigned to treatment: using a split-mouth design [33], the patients were randomly assigned to group A, in which topical ozone was administered (Ozone DTA generator, Sweden & Martina, Due Carrare, PD, Italy) to teeth belonging to the maxillary right and mandibular left quadrants (respectively, Q1 and Q3), while the remaining quadrants (Q2–Q4) were treated with PBM administration with a 980 nm AlGaInAs diode laser (Giotto model, Dental Medical Technologies, Lissone, MB, Italy) using a 200 nm optical fiber. In group B, the quadrants were inverted (Figure 1). Ozone was administered by inserting the probe type 3 at power 6 inside the periodontal pockets with a duration of about 1 min per cm2 [34]. Laser irradiation was conducted using an optic fiber of 200 nm, 980 nm wavelength, and 1.5 W power, with a pulsed irradiation of 1 min per site; the application was performed using punctual contact to reduce reflection, with the tip perpendicular to the gingival tissue [35]. The parameters of the devices are shown in Table 1.
Figure 1.
Treatments administered to patients from group A (left figure) and from group B (right figure).

Table 1.
Working parameters of the two treatments.
After 1 (T1), 2 (T2), 3 (T3), 4 (T4), 5 (T5), and 6 (T6) months, the periodontal indices were re-evaluated, and another treatment with ozone and PBM was performed for the same sites. The protocol of the study is shown in Table 2.

Table 2.
Protocol adopted for the study.
2.4. Sample Size
The sample size calculation (alpha = 0.05; power = 80%) for two independent study groups and a continuous primary endpoint was calculated.
Concerning the variable Probing Pocket Depth (the primary outcome), the expected difference between the means was supposed to be 0.1305 [36]; therefore, 30 patients could be enrolled using a split-mouth study. The calculation was performed with a Sample Size Calculator (Clin Calc LLC).
2.5. Randomization and Blinding
By means of a permuted block randomization table provided by the data analyst, 30 patients were randomized into group A or B according to a split-mouth design. An operator enrolled the participants and executed the professional oral procedures. Based on previously prepared sequentially numbered, opaque, sealed envelopes (SNOSE), an assistant assigned patients to the respective treatment. The order was randomized. The patients and operator could not be blinded as two different devices were used. The data analyst was blinded.
2.6. Statistical Methods
The data were submitted to statistical analysis with R Software (R version 3.1.3, R Development Core Team, R Foundation for Statistical Computing, Wien, Austria). For each group and variable, descriptive statistics (mean, standard deviation, minimum, median, and maximum) were calculated. PPD was calculated in millimeters; BOP and PI were calculated in percentages. Data normality was assessed with the Kolmogorov–Smirnov test. For the PPD variable, inferential comparisons among groups were performed using the ANOVA test for repeated measures with the post hoc Tukey test. For BOP and PI, we used the Kruskal–Wallis test followed by the Mann–Whitney U test. Significance was predetermined for p < 0.05 for all statistical tests.
3. Results
3.1. Participant Flow and Baseline Data
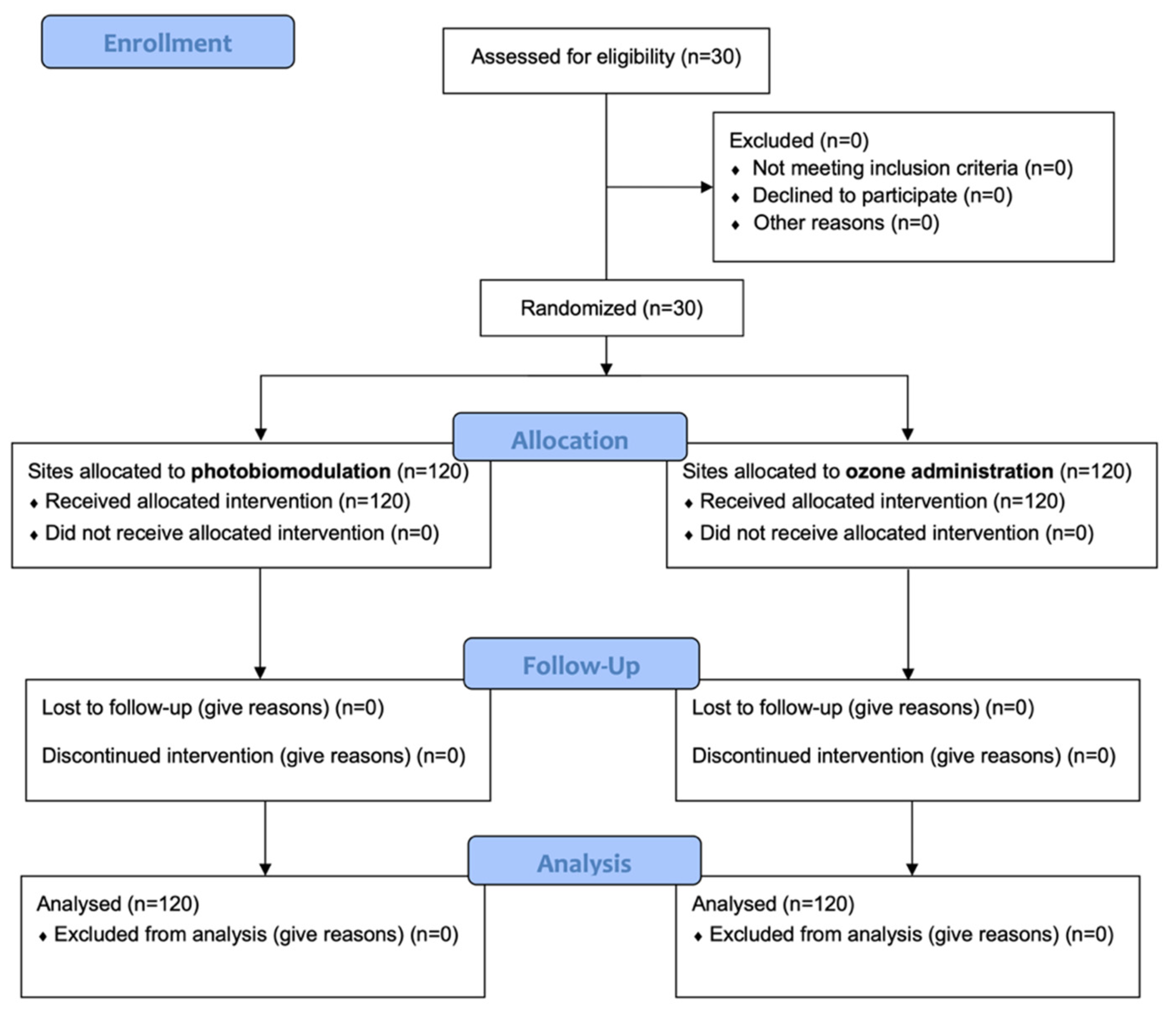
A total of 30 patients responding to the inclusion criteria were asked to participate in the study. They all agreed to participate and received the allocated interventions. No patient was excluded from analysis. The flow chart of the study is shown in Figure 2. At baseline, the sample showed a mean age of 47.1 ± 13.5 years (13 females, mean age 45.6 ± 12.1; 17 males, mean age 48.2 ± 14.7).
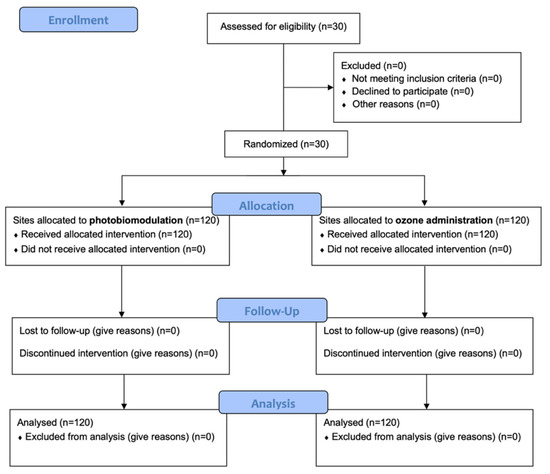
Figure 2.
CONSORT flow-chart of the study.
Descriptive and inferential statistics are reported in the three following sections. Inter- and intra-group comparisons are shown with letter-based comparisons, which provide the assignment of the same letter/letters to groups with non-significantly different means. Accordingly, for pairwise comparisons, the presence of the same letter/letters for the means compared shows that no significant differences are present between them [37].
3.2. Probing Pocket Depth (PPD)
In Table 3, the PPD values are shown. PPD was significantly reduced from baseline to T6 in both the groups (p < 0.05). A significant difference between the two groups can be noted starting from T5, where the PPD values were significantly lower in the PBM group if compared to the ozone group (p < 0.05), and the trend was confirmed at T6 with a further reduction (p < 0.05), while in the ozone group there was no significant difference between T5 and T6 (p > 0.05).

Table 3.
Descriptive and inferential statistics of Probing Pocket Depth measurements (PPD).
3.3. Plaque Index (PI%)
As shown in Table 4, PI significantly decreased from baseline to T6 among the two groups (p < 0.05). A significant difference was found between T0 and T1 among the groups (p < 0.05), with a progressively higher reduction in the PBM group among the time frames of the study; however, the intergroup comparisons are not significant (p > 0.05).

Table 4.
Descriptive and inferential statistics of Plaque Index measurements (PI%).
3.4. Bleeding on Probing (BoP)
For BoP also, a significant decrease from baseline to T6 among the two groups was found (p < 0.05), as shown in Table 5. A significant difference was found between T0 and T1 among the groups (p < 0.05), with a progressively higher reduction in the PBM group among the time frames of the study; for this index also, the intergroup comparisons are not significant (p > 0.05).

Table 5.
Descriptive and inferential statistics of Bleeding on Probing measurements (BOP%).
4. Discussion
Oral infections are a serious concern in dentistry, and many efforts should be made to contrast bacterial colonization [38]. In addition to dental decay, even tooth-supporting tissues can be threatened by pathogenic microorganisms causing a dysbiosis leading to periodontitis [39]. Despite SRP being the gold standard treatment for periodontitis, many shortcomings, such as bacterial recolonization, are associated with it. On the basis of this consideration, the aim of the present randomized clinical trial was to evaluate the adjunctive efficacy of ozone and PBM, in addition to SRP, in improving periodontal clinical indexes. This study consisted of a split-mouth design, a kind of study introduced in dentistry to reduce patient-related biases.
The statistical null hypothesis of the study was partially rejected. Considering intra-group differences, both ozone and PBM had significantly reduced values of Probing Pocket Depth (PPD), Plaque Index (PI), and Bleeding on Probing (BoP), generally with a significant progressive reduction from baseline to the last evaluation timepoint. As regards intergroup comparisons, no significant differences were found, either for PI or for BoP; conversely, the PPD values were significantly different between the groups starting from T5 (5 months), with a significantly higher reduction for the sites treated with PBM. Additionally, considering the timepoint between T5 (5 months) and T6 (six months), no further significant reduction was assessed for PPD in the sites treated with ozone, differently from those treated with PBM. According to the results obtained in this study, both ozone and PBM appear as valuable tools as adjunctive treatment to SRP. No significant differences were noticed between the two treatment modalities, except for a higher reduction for BoP exerted by PBM in the long term.
Previously studies in the literature have evaluated the antimicrobial effect of ozone application. In particular, in vitro tests were conducted by Huth et al. [40] to compare the antimicrobial effectiveness of gaseous/aqueous ozone with respect to the gold standard chlorhexidine digluconate (CHX) against periodontal microorganisms. Specific periodontopathogens, i.e., Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Tannerella forsythia, and Parvimonas micra, were exposed for 1 min to gaseous ozone, aqueous ozone, chlorhexidine, or to a control substance (phosphate-buffered saline). Despite none of the agents managing to significantly reduce the A. actinomycetemcomitans count in biofilm cultures, 2% CHX and ozone gas at 53 g/m−3 eliminated P. gingivalis, T. forsythia, and P. micra, with a significantly greater antimicrobial effect against planktonic cultures with respect to biofilm-associated bacteria. No significant differences in the effectiveness of aqueous ozone or gaseous ozone were assessed compared with 2% CHX; however, the ozone treatment was more effective than the one based on the use of 0.2% CHX. Accordingly, the authors recognized that high-concentrated gaseous and aqueous ozone are valid antiseptics in periodontitis therapy. In fact, ozone therapy seems to be a reliable alternative to the conventional chlorhexidine-based products due to the absence of the shortcomings associated with these products, such as tooth staining, dysgeusia, and mucosal irritation [41].
In addition to laboratory studies, clinical trials have also assessed the efficacy of ozone therapy for the treatment of gingivitis, periodontitis, and peri-implantitis. In particular, in a recent work by our group, patients suffering from peri-implantitis were recruited to evaluate the efficacy of a clinical protocol based on the irrigation of pathological sites by means of ozonized water, compared to pure water [13]. Evaluations were conducted at baseline, as well as after one month and two months. The results of the study highlighted a significant reduction in PPD, PI, BoP, and Bleeding Score (BS). This outcome did not occur in the control group, thus confirming the role of ozone as an adjuvant agent within an oral hygiene protocol. This result is in accordance with previous evidence in the literature [24,42,43]. Moreover, in the research by Rapone and colleagues [44], the adjunctive use of the gaseous ozone therapy was evaluated for its effect on similar clinical indexes evaluated in the present report, such as BoP and PPD; both of these indexes were significantly decreased at six months as highlighted in this study.
Beside ozone therapy, other adjunctive approaches have been proposed in oral hygiene, such as the use of antibiotics [11], probiotics [12], and lasers [14,25]. As regards these approaches, the administration of PBM using coherent, collimated, and monochromatic light energy is exploited to perform therapeutic procedures; these devices are included in the category of so-called “non-surgical lasers”, which are opposed to “surgical lasers” that are able to incise, excise, and ablate tissues [43]. The term “photobiomodulation” (also defined as Low-Level Laser Therapy, LLLT) describes how PBM lasers work, considering that they use photons (light energy) to modulate biological processes. Various transcription factors are switched on by PBM, such as the antimicrobial peptide h-BD-2 (human β-defensin-2). PBM partially shares its mechanism of action with photodynamic therapy (PDT); the difference between the two is that while PBM is used to improve wound healing or as pain relief, PDT is a combination of light- and photo-sensitive drugs targeted at chromophores to destroy microbes or oncogenic cells [45].
PBM lasers have been generally used in dentistry for pain management, especially in orthodontics in the case of band application [26]. In addition to this, PBM has been more recently proposed as an adjunctive approach to oral hygiene treatments in the case of periodontal pathologies. A previous study has investigated the local effect of PBM on the treatment of periodontal pockets in patients with periodontitis and type 2 diabetes [34]. In particular, periodontal patients with pockets reporting PPD and CAL (Clinical Attachment Level) ≥ 5 mm were selected. Pockets were randomly assigned to receive only mechanical debridement (SRP) (control group) or SRP with PBM (experimental group). PPD, CAL, BoP, and PI were compared at baseline, 3, 6, and 12 months. After 12 months, no significant difference was assessed for PPD and CAL between the control and experimental group. The frequency of pockets with PPD 5–6 mm was significantly lower for the PBM group at 6 months with respect to the control group. Pockets with PPD ≥ 7 mm changed significantly between baseline and 3, 6, and 12 months for the PBM group, whereas, for the control group, a statistical significance was only observed between baseline and 6 months. The authors concluded that, despite PBM protocol not providing significant changes for PPD and CAL in periodontal pockets when compared to mechanical therapy only, the former treatment was more effective in reducing the percentage of moderate and severe periodontal pockets at 3, 6, and 12 months in patients with type 2 diabetes mellitus. This agrees with our study where a similar reduction in PPD was found from baseline to the subsequent 6 months, despite no evaluations have been conducted at 12 months. However, the aforementioned study did not find significant changes when comparing PPD and CAL in periodontal pockets treated with PBM or mechanical therapy only. Accordingly, no direct comparisons can be made considering that, in the present research, no control group exposed to mechanical therapy alone was considered.
An analogue study conducted among betel chewers confirmed the additional effect of PBM for the management of periodontitis, compared to SRP alone [46].
Despite the positive results assessed for PBM in the present study, as well as in previous research, no conclusive evidence has been demonstrated yet. According to recent and high-quality systematic reviews, the current evidence lacks sufficient information regarding PBM dosimetry, which is fundamental in determining standardized, and thus replicable, protocols for both therapeutic and research purposes; additionally, the substantial differences in the methodologies and the high risk of bias assessed for the studies included have caused their classification to be of low quality [47,48].
The present report presents some limitations. Firstly, as regards PBM, no validated protocols have been published in the literature until now [46]; conversely, different non-standardized parameters have been used in studies for both laser wavelength [46,47,48] and exposure time [34,47,48]. Moreover, according to some findings, PBM could exert systemic effects even in the case of a local irradiation, thus limiting the necessity of split-mouth studies [49]. Finally, regarding ozone, further approaches are available in addition to gaseous administration, such as ozonated water and oil/gel [13,24]. The efficacy of these other methods deserves to be evaluated and compared to PBM.
Based on these considerations, there is the need to conduct further well-designed RCTs with the goal of determining the efficacy of PBM, if any, and comparing it with different ozone treatments available nowadays. Clinicians should be aware of the possibility of adopting both PBM and ozone as adjunctive therapies to SRP for their positive effects when combined with professional protocols of dental hygiene.
5. Conclusions
PBM therapy could be a reliable adjunctive agent to mechanical debridement in periodontal disease, with a slightly better effect than ozone in the long term. However, no definitive conclusions can be reached for the former therapeutic approach due to the absence of standardized protocols regarding its administration.
Author Contributions
Conceptualization, A.B. and A.S.; methodology, A.S. and A.B.; software, A.S.; validation, A.S., A.B., A.V. and A.M.; formal analysis, A.S.; investigation, R.S. and F.D.F.; resources, A.B.; data curation, R.S., F.D.F., L.P. and A.S.; writing—original draft preparation, S.G. and M.P.; writing—review and editing, A.S., S.G. and M.P.; visualization, A.S. and A.B.; supervision, A.S. and A.B.; project administration, A.S.; funding acquisition, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Internal Review Board (registration number: 2020-0610).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreño, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [Green Version]
- Kinane, D.F.; Chestnutt, I.G. Smoking and periodontal disease. Crit. Rev. Oral Biol. Med. 2000, 11, 356–365. [Google Scholar]
- Taba, M., Jr.; Souza, S.L.; Mariguela, V.C. Periodontal disease: A genetic perspective. Braz. Oral Res. 2012, 26, 32–38. [Google Scholar] [CrossRef]
- Pólvora, T.L.S.; Nobre, Á.V.V.; Tirapelli, C.; Taba, M., Jr.; Macedo, L.D.; Santana, R.C.; Pozzetto, B.; Lourenço, A.G.; Motta, A.C.F. Relationship between human immunodeficiency virus (HIV-1) infection and chronic periodontitis. Expert Rev. Clin. Immunol. 2018, 14, 315–327. [Google Scholar] [CrossRef]
- Shapira, L.; Wilensky, A.; Kinane, D.F. Effect of genetic variability on the inflammatory response to periodontal infection. J. Clin. Periodontol. 2005, 32, 72–86. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32, 7–15. [Google Scholar] [CrossRef]
- Berezow, A.B.; Darveau, R.P. Microbial shift and periodontitis. Periodontology 2000 2011, 55, 36–47. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000 2018, 76, 85–96. [Google Scholar] [CrossRef]
- Feres, M. Antibiotics in the treatment of periodontal diseases: Microbiological basis and clinical applications. Ann. R. Australas. Coll. Dent. Surg. 2008, 19, 37–44. [Google Scholar]
- Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Butera, A.; Gallo, S.; Pascadopoli, M.; Luraghi, G.; Scribante, A. Ozonized Water Administration in Peri-Implant Mucositis Sites: A Randomized Clinical Trial. Appl. Sci. 2021, 11, 7812. [Google Scholar] [CrossRef]
- Meimandi, M.; Talebi Ardakani, M.R.; Esmaeil Nejad, A.; Yousefnejad, P.; Saebi, K.; Tayeed, M.H. The Effect of Photodynamic Therapy in the Treatment of Chronic Periodontitis: A Review of Literature. J. Lasers Med. Sci. 2017, 8, S7–S11. [Google Scholar] [CrossRef]
- Sechi, L.A.; Lezcano, I.; Nunez, N.; Espim, M.; Duprè, I.; Pinna, A.; Molicotti, P.; Fadda, G.; Zanetti, S. Antibacterial activity of ozonized sunflower oil (Oleozon). J. Appl. Microbiol. 2001, 90, 279–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lezcano, I.; Nuñez, N.; Espino, M.; Gómez, M. Antibacterial activity of ozonized sunflower oil, oleozon, against Staphylococcus aureus and Staphylococcus epidermidis. Ozone Sci. Eng. 2000, 22, 207–214. [Google Scholar] [CrossRef]
- Mascarenhas, L.A.B.; Oliveira, F.O.; da Silva, E.S.; dos Santos, L.M.C.; de Alencar Pereira Rodrigues, L.; Neves, P.R.F.; Santos, A.Á.B.; Moreira, G.A.F.; Lobato, G.M.; Nascimento, C.; et al. Technological Advances in Ozone and Ozonized Water Spray Disinfection Devices. Appl. Sci. 2021, 11, 3081. [Google Scholar] [CrossRef]
- Giuroiu, C.L.; Andrian, S.; Stoleriu, S.; Scurtu, M.; Țănculescu, O.; Poroch, V.; Sălceanu, M. The Combination of Diode Laser and Ozonated Water in the Treatment of Complicated Pulp Gangrene. Appl. Sci. 2020, 10, 4203. [Google Scholar] [CrossRef]
- Silva, V.; Peirone, C.; Capita, R.; Alonso-Calleja, C.; Marques-Magallanes, J.A.; Pires, I.; Maltez, L.; Pereira, J.E.; Igrejas, G.; Poeta, P. Topical Application of Ozonated Oils for the Treatment of MRSA Skin Infection in an Animal Model of Infected Ulcer. Biology. 2021, 10, 372. [Google Scholar] [CrossRef]
- Di Mauro, R.; Cantarella, G.; Bernardini, R.; Di Rosa, M.; Barbagallo, I.; Distefano, A.; Longhitano, L.; Vicario, N.; Nicolosi, D.; Lazzarino, G.; et al. The Biochemical and Pharmacological Properties of Ozone: The Smell of Protection in Acute and Chronic Diseases. Int. J. Mol. Sci. 2019, 20, 634. [Google Scholar] [CrossRef] [Green Version]
- Monzillo, V.; Lallitto, F.; Russo, A.; Poggio, C.; Scribante, A.; Arciola, C.R.; Bertuccio, F.R.; Colombo, M. Ozonized Gel Against Four Candida Species: A Pilot Study and Clinical Perspectives. Materials 2020, 13, 1731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallo, S.; Scribante, A. Ozone therapy in dentistry: From traditional applications towards innovative ones. A review of the literature. IOP Conf. Ser. Earth Environ. Sci. 2021, 707, 012001. [Google Scholar] [CrossRef]
- Colombo, M.; Gallo, S.; Garofoli, A.; Poggio, C.; Arciola, C.R.; Scribante, A. Ozone Gel in Chronic Periodontal Disease: A Randomized Clinical Trial on the Anti-Inflammatory Effects of Ozone Application. Biology 2021, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Mansi, B. Ozone therapy in periodontics. J. Med. Life 2012, 5, 59–67. [Google Scholar]
- Merigo, E.; Rocca, J.P.; Pinheiro, A.L.B.; Fornaini, C. Photobiomodulation Therapy in Oral Medicine: A Guide for the Practitioner with Focus on New Possible Protocols. Photobiomodul. Photomed. Laser Surg. 2019, 37, 669–680. [Google Scholar] [CrossRef]
- Sfondrini, M.F.; Vitale, M.; Pinheiro, A.L.B.; Gandini, P.; Sorrentino, L.; Iarussi, U.M.; Scribante, A. Photobiomodulation and Pain Reduction in Patients Requiring Orthodontic Band Application: Randomized Clinical Trial. Biomed. Res. Int. 2020, 2020, 7460938. [Google Scholar] [CrossRef]
- Sun, G.; Tunér, J. Low-level laser therapy in dentistry. Dent. Clin. N. Am. 2004, 48, 1061–1076. [Google Scholar] [CrossRef]
- Caruso, U.; Nastri, L.; Piccolomini, R.; d’Ercole, S.; Mazza, C.; Guida, L. Use of diode laser 980 nm as adjunctive therapy in the treatment of chronic periodontitis. A randomized controlled clinical trial. New Microbiol. 2008, 31, 513–518. [Google Scholar] [PubMed]
- Saygun, I.; Nizam, N.; Ural, A.U.; Serdar, M.A.; Avcu, F.; Tözüm, T.F. Low-level laser irradiation affects the release of basic fibroblast growth factor (bFGF), insulin-like growth factor-I (IGF-I), and receptor of IGF-I (IGFBP3) from osteoblasts. Photomed. Laser Surg. 2012, 30, 149–154. [Google Scholar] [CrossRef]
- Özberk, S.S.; Gündoğar, H.; Özkaya, M.; Taner, İ.L.; Erciyas, K. The effect of photobiomodulation therapy on nonsurgical periodontal treatment in patients with type 2 diabetes mellitus: A randomized controlled, single-blind, split-mouth clinical trial. Lasers Med. Sci. 2020, 35, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Karkhanechi, M.; Chow, D.; Sipkin, J.; Sherman, D.; Boylan, R.J.; Norman, R.G.; Craig, R.G.; Cisneros, G.J. Periodontal status of adult patients treated with fixed buccal appliances and removable aligners over one year of active orthodontic therapy. Angle Orthod. 2013, 83, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef] [Green Version]
- Lesaffre, E.; Philstrom, B.; Needleman, I.; Worthington, H. The design and analysis of split-mouth studies: What statisticians and clinicians should know. Stat. Med. 2009, 28, 3470–3482. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Ferrara, E.; Corsalini, M.; Converti, I.; Grassi, F.R.; Santacroce, L.; Topi, S.; Gnoni, A.; Scacco, S.; Scarano, A.; et al. The Effect of Gaseous Ozone Therapy in Conjunction with Periodontal Treatment on Glycated Hemoglobin Level in Subjects with Type 2 Diabetes Mellitus: An Unmasked Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 5467. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Dias, S.B.; de Marco, A.C.; Santamaria, M., Jr.; Kerbauy, W.D.; Jardini, M.A.; Santamaria, M.P. Connective tissue graft associated or not with low laser therapy to treat gingival recession: Randomized clinical trial. J. Clin. Periodontol. 2015, 42, 54–61. [Google Scholar] [CrossRef]
- Yılmaz, S.; Algan, S.; Gursoy, H.; Noyan, U.; Kuru, B.E.; Kadir, T. Evaluation of the clinical and antimicrobial effects of the Er:YAG laser or topical gaseous ozone as adjuncts to initial periodontal therapy. Photomed. Laser Surg. 2013, 31, 293–298. [Google Scholar] [CrossRef]
- Piepho, H.P. An Algorithm for a Letter-Based Representation of All-Pairwise Comparisons. J. Comput. Graph. Stat. 2004, 13, 456–466. [Google Scholar] [CrossRef]
- Scribante, A.; Poggio, C.; Gallo, S.; Riva, P.; Cuocci, A.; Carbone, M.; Arciola, C.R.; Colombo, M. In Vitro Re-Hardening of Bleached Enamel Using Mineralizing Pastes: Toward Preventing Bacterial Colonization. Materials 2020, 13, 818. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.L.; Szafrański, S.P.; Jarek, M.; Bhuju, S.; Wagner-Döbler, I. Dysbiosis in chronic periodontitis: Key microbial players and interactions with the human host. Sci. Rep. 2017, 7, 3703–3715. [Google Scholar] [CrossRef] [Green Version]
- Huth, K.C.; Quirling, M.; Lenzke, S.; Paschos, E.; Kamereck, K.; Brand, K.; Hickel, R.; Ilie, N. Effectiveness of ozone against periodontal pathogenic microorganisms. Eur. J. Oral Sci. 2011, 119, 204–210. [Google Scholar] [CrossRef]
- Gandhi, K.K.; Cappetta, E.G.; Pavaskar, R. Effectiveness of the adjunctive use of ozone and chlorhexidine in patients with chronic periodontitis. BDJ Open. 2019, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Talmaç, A.C.; Çalişir, M. Efficacy of gaseous ozone in smoking and non-smoking gingivitis patients. Ir. J. Med. Sci. 2021, 190, 325–333. [Google Scholar] [CrossRef]
- Convissar, R.; Ross, G. Photobiomodulation Lasers in Dentistry. Semin. Orthod. 2020, 26, 102–106. [Google Scholar] [CrossRef]
- Rapone, B.; Ferrara, E.; Santacroce, L.; Topi, S.; Gnoni, A.; Dipalma, G.; Mancini, A.; Di Domenico, M.; Tartaglia, G.M.; Scarano, A.; et al. The Gaseous Ozone Therapy as a Promising Antiseptic Adjuvant of Periodontal Treatment: A Randomized Controlled Clinical Trial. Int. J. Environ. Res. Public Health 2022, 19, 985. [Google Scholar] [CrossRef]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation—Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef] [PubMed]
- Al-Rabiah, M.; Al-Hamoudi, N.; Al-Aali, K.A.; Slapar, L.; AlHelal, A.; Al Deeb, M.; Mokeem, S.A.; Vohra, F.; Abduljabbar, T. Efficacy of Scaling and Root Planing with Photobiomodulation for Treating Periodontitis in Gutka Chewers: A Randomized Controlled Trial. Photobiomodul. Photomed. Laser Surg. 2020, 38, 545–551. [Google Scholar] [CrossRef]
- Ren, C.; McGrath, C.; Jin, L.; Zhang, C.; Yang, Y. The effectiveness of low-level laser therapy as an adjunct to non-surgical periodontal treatment: A meta-analysis. J. Periodontal. Res. 2017, 52, 8–20. [Google Scholar] [CrossRef] [Green Version]
- Dalvi, S.; Benedicenti, S.; Hanna, R. Effectiveness of Photobiomodulation as an Adjunct to Nonsurgical Periodontal Therapy in the Management of Periodontitis- A Systematic Review of in vivo Human Studies. Photochem. Photobiol. 2021, 97, 223–242. [Google Scholar] [CrossRef] [PubMed]
- Cronshaw, M.; Parker, S.; Anagnostaki, E.; Mylona, V.; Lynch, E.; Grootveld, M. Photobiomodulation and Oral Mucositis: A Systematic Review. Dent. J. 2020, 8, 87. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).