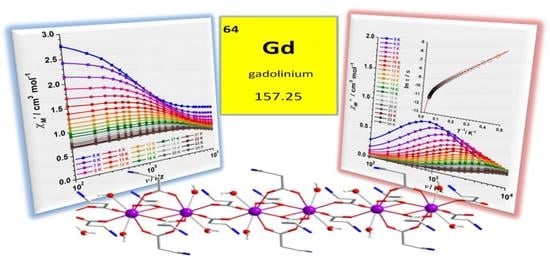
One-Dimensional Gadolinium (III) Complexes Based on Alpha- and Beta-Amino Acids Exhibiting Field-Induced Slow Relaxation of Magnetization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthetic Procedure
2.2. Description of the Crystal Structures
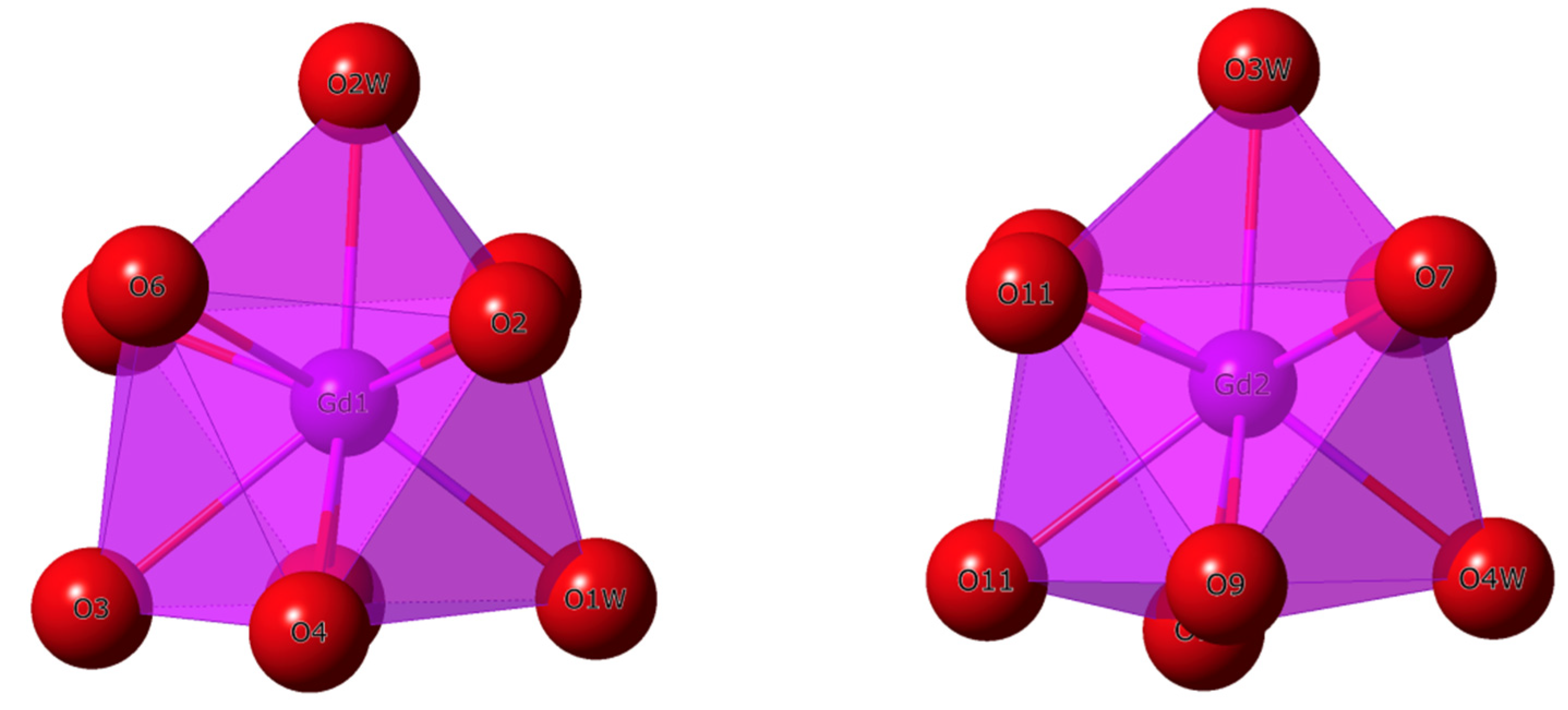
2.3. Analysis of the Polyhedral Structures
2.4. Magnetic Properties
3. Experimental Section
3.1. Preparation of the Complexes
3.1.1. Synthesis of {[Gd2(gly)6(H2O)4](ClO4)6·5H2O}n (1)
3.1.2. Synthesis of {[Gd2(β-ala)6(H2O)4](ClO4)6·H2O)}n (2)
3.2. X-ray Data Collection and Structure Refinement
3.3. Physical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.Y.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.Y.; Kaizu, Y. Mononuclear Lanthanide Complexes with a Long Magnetization Relaxation Time at High Temperatures: A New Category of Magnets at the Single-Molecular Level. J. Phys. Chem. B 2004, 108, 11265–11271. [Google Scholar] [CrossRef]
- Habib, F.; Murugesu, M. Lessons learned from dinuclear lanthanide nano-magnets. Chem. Soc. Rev. 2013, 42, 3278–3288. [Google Scholar] [CrossRef] [Green Version]
- Ferrando-Soria, J.; Vallejo, J.; Castellano, M.; Martínez-Lillo, J.; Pardo, E.; Cano, J.; Castro, I.; Lloret, F.; Ruiz-García, R.; Julve, M. Molecular magnetism, quo vadis? A historical perspective from a coordination chemist viewpoint. Coord. Chem. Rev. 2017, 339, 17–103. [Google Scholar] [CrossRef]
- Liu, J.-L.; Chen, Y.-C.; Tong, M.-L. Symmetry strategies for high performance lanthanide-based single-molecule magnets. Chem. Soc. Rev. 2018, 47, 2431–2453. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Kalita, P.; Chandrasekhar, V. Lanthanide(III)-Based Single-Ion Magnets. ACS Omega 2018, 3, 9462–9475. [Google Scholar] [CrossRef] [PubMed]
- Zabala-Lekuona, A.; Seco, J.M.; Colacio, E. Single-Molecule Magnets: From Mn12-ac to dysprosium metallocenes, a travel in time. Coord. Chem. Rev. 2021, 441, 213984. [Google Scholar] [CrossRef]
- Gebrezgiabher, M.; Bayeh, Y.; Gebretsadik, T.; Gebreslassie, G.; Elemo, F.; Thomas, M.; Linert, W. Lanthanide-Based Single-Molecule Magnets Derived from Schiff Base Ligands of Salicylaldehyde Derivatives. Inorganics 2020, 8, 66. [Google Scholar] [CrossRef]
- McAdams, S.G.; Ariciu, A.-M.; Kostopoulos, A.K.; Walsh, J.P.S.; Tuna, F. Molecular single-ion magnets based on lanthanides and actinides: Design considerations and new advances in the context of quantum technologies. Coord. Chem. Rev. 2017, 346, 216–239. [Google Scholar] [CrossRef] [Green Version]
- Karotsis, G.; Evangelisti, M.; Dalgarno, S.J.; Brechin, E.K. A Calix[4 ]arene 3d/4f Magnetic Cooler. Angew. Chem. Int. Ed. 2009, 48, 9928–9931. [Google Scholar] [CrossRef]
- Ghosh, T.K.; Maity, S.; Mayans, J.; Ghosh, A. Family of Isomeric CuII–LnIII (Ln = Gd, Tb, and Dy) Complexes Presenting Field-Induced Slow Relaxation of Magnetization Only for the Members Containing GdIII. Inorg. Chem. 2020, 60, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.; Murugavel, R. Magnetic relaxation in single-ion magnets formed by less-studied lanthanide ions Ce(III), Nd(III), Gd(III), Ho(III), Tm(II/III) and Yb(III). Coord. Chem. Rev. 2022, 453, 214288. [Google Scholar] [CrossRef]
- Maciel, J.W.D.O.; Lemes, M.A.; Valdo, A.K.; Rabelo, R.; Martins, F.T.; Maia, L.J.Q.; Costa de Santana, R.; Lloret, F.; Julve, M.; Cangussu, D. Europium(III), Terbium(III), and Gadolinium(III) Oxamato-Based Coordination Polymers: Visible Luminescence and Slow Magnetic Relaxation. Inorg. Chem. 2021, 60, 6176–6190. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Barea, B.; Mayans, J.; Rabelo, R.; Sanchis-Perucho, A.; Moliner, N.; Martínez-Lillo, J.; Julve, M.; Lloret, F.; Ruiz-García, R.; Cano, J. Holmium(III) Single-Ion Magnet for Cryomagnetic Refrigeration Based on an MRI Contrast Agent Derivative. Inorg. Chem. 2021, 60, 12719–12723. [Google Scholar] [CrossRef]
- Orts-Arroyo, M.; Rabelo, R.; Carrasco-Berlanga, A.; Moliner, N.; Cano, J.; Julve, M.; Lloret, F.; De Munno, G.; Ruiz-García, R.; Mayans, J.; et al. Field-induced slow magnetic relaxation and magnetocaloric effects in an oxalato-bridged gadolinium(III)-based 2D MOF. Dalton Trans. 2021, 50, 3801–3805. [Google Scholar] [CrossRef]
- Arauzo, A.; Lazarescu, A.; Shova, S.; Bartolomé, E.; Cases, R.; Luzón, J.; Bartolomé, J.; Turta, C. Structural and magnetic properties of some lanthanide (Ln = Eu(III), Gd(III) and Nd(III)) cyanoacetate polymers: Field-induced slow magnetic relaxation in the Gd and Nd substitutions. Dalton Trans. 2014, 43, 12342–12356. [Google Scholar] [CrossRef]
- Izuogu, D.C.; Yoshida, T.; Zhang, H.; Cosquer, G.; Katoh, K.; Ogata, S.; Hasegawa, M.; Nojiri, H.; Damjanović, M.; Wernsdorfer, W.; et al. Slow Magnetic Relaxation in a Palladium–Gadolinium Complex Induced by Electron Density Donation from the Palladium Ion. Chem. Eur. J. 2018, 24, 9285–9294. [Google Scholar] [CrossRef]
- Guo, F.-S.; Day, B.M.; Chen, Y.-C.; Tong, M.-L.; Mansikkamäki, A.; Layfield, R.A. Magnetic hysteresis up to 80 kelvin in a dysprosium metallocene single-molecule magnet. Science 2018, 362, 1400–1403. [Google Scholar] [CrossRef] [Green Version]
- Ullah, A.; Cerdá, J.; Baldoví, J.J.; Varganov, S.A.; Aragó, J.; Gaita-Ariño, A. In Silico Molecular Engineering of Dysprosocenium-Based Complexes to Decouple Spin Energy Levels from Molecular Vibrations. J. Phys. Chem. Lett. 2019, 10, 7678–7683. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Peng, Y.-Y.; Liu, J.-L.; Tong, M.-L. Field-induced slow magnetic relaxation in a mononuclear Gd(III) complex. Inorg. Chem. Commun. 2019, 107, 107449. [Google Scholar] [CrossRef]
- Mayans, J.; Escuer, A. Correlating the axial Zero Field Splitting with the slow magnetic relaxation in GdIII SIMs. Chem. Commun. 2021, 57, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-L.; Liu, B.-P.; Tan, Z.-C.; Zhang, D.-S.; Li, L.; Chen, Y.-D.; Shi, Q. Synthesis, Crystal Structure and Themochemical Study of [Gd2(Gly)6(H2O)4](ClO4)6(H2O)5. Acta Chim. Sin. Chin. Ed. 2007, 65, 2349–2355. [Google Scholar]
- Liang, F.-P.; Ma, L.-F.; Huang, S.-W.; Huang, Y.-Q. Synthesis, Crystal Structure and Antibacterial Activity of β-Alanine Complexes with Nd, Gd, Er. Chin. J. Appl. Chem. 2004, 21, 58–62. [Google Scholar]
- Orts-Arroyo, M.; Ten-Esteve, A.; Ginés-Cárdenas, S.; Castro, I.; Martí-Bonmatí, L.; Martínez-Lillo, J. A Gadolinium(III) Complex Based on the Thymine Nucleobase with Properties Suitable for Magnetic Resonance Imaging. Int. J. Mol. Sci. 2021, 22, 4586. [Google Scholar] [CrossRef]
- Legendziewicz, J.; Huskowska, E.; Waśkowska, A.; Argay, G. Spectroscopy and crystal structure of neodymium coordination compound with glycine: Nd2(Gly)6·(ClO4)6·9H2O. Inorg. Chim. Acta 1984, 92, 151–157. [Google Scholar] [CrossRef]
- Orts-Arroyo, M.; Castro, I.; Lloret, F.; Martínez-Lillo, J. Field-induced slow relaxation of magnetisation in two one-dimensional homometallic dysprosium(III) complexes based on alpha- and beta-amino acids. Dalton Trans. 2020, 49, 9155–9163. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Dolan, N.; Brechin, E.K. A cationic and ferromagnetic hexametallic Mn(III) single-molecule magnet based on the salicylamidoxime ligand. Dalton Trans. 2013, 42, 12824–12827. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Lillo, J.; Dolan, N.; Brechin, E.K. A family of cationic oxime-based hexametallic manganese(III) single-molecule magnets. Dalton Trans. 2014, 43, 4408–4414. [Google Scholar] [CrossRef]
- Rojas-Dotti, C.; Moliner, N.; Lloret, F.; Martínez-Lillo, J. Ferromagnetic Oxime-Based Manganese(III) Single-Molecule Magnets with Dimethylformamide and Pyridine as Terminal Ligands. Crystals 2019, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Jianxue, L.; Ninghai, H.; Chunji, N.; Qingbo, M. The crystal structure of a samarium complex with β-alanine. J. Alloys Compd. 1992, 184, L1–L3. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE 2.1; Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Alvarez, S.; Alemany, P.; Casanova, D.; Cirera, J.; Llunell, M.; Avnir, D. Shape maps and polyhedral interconversion paths in transition metal chemistry. Coord. Chem. Rev. 2005, 249, 1693–1708. [Google Scholar] [CrossRef]
- Ruiz-Martínez, A.; Casanova, D.; Alvarez, S. Polyhedral Structures with an Odd Number of Vertices: Nine-Coordinate Metal Compounds. Chem. Eur. J. 2008, 14, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lillo, J.; Cañadillas-Delgado, L.; Cano, J.; Lloret, F.; Julve, M.; Faus, J. A heteropentanuclear oxalato-bridged [ReIV4GdIII] complex: Synthesis, crystal structure and magnetic properties. Chem. Commun. 2012, 48, 9242–9244. [Google Scholar] [CrossRef]
- Roy, L.E.; Hughbanks, T. Magnetic Coupling in Dinuclear Gd Complexes. J. Am. Chem. Soc. 2006, 128, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Cañadillas-Delgado, L.; Fabelo, O.; Cano, J.; Ruiz-Pérez, C. Magnetic Interactions in Oxo-Carboxylate Bridged Gadolinium(III) Complexes Synthesis, Crystal Structures and Magnetic Properties; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2010. [Google Scholar]
- Handzlik, G.; Magott, M.; Arczyński, M.; Sheveleva, A.M.; Tuna, F.; Baran, S.; Pinkowicz, D. Identical anomalous Raman relaxation exponent in a family of single ion magnets: Towards reliable Raman relaxation determination? Dalton Trans. 2020, 49, 11942–11949. [Google Scholar] [CrossRef]
- SHELXTL-2013/4, Bruker Analytical X-Ray Instruments; Sheldrick, G.M. (Ed.) Bruker Analytical X-ray Systems, Inc.: Madison, WI, USA, 2013. [Google Scholar]
- Diamond 4.5.0; Crystal Impact GbR; Crystal Impact: Bonn, Germany, 2018.
- CrystalMaker 8.7.5; CrystalMaker Software Ltd.: Oxford, UK, 2013.
- Bain, G.A.; Berry, J.F. Diamagnetic Corrections and Pascal’s Constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]








| Compound | 1 | 2 |
|---|---|---|
| CIF | 2149741 | 2149742 |
| Formula | C12H48Cl6N6O45Gd2 | C18H52Cl6N6O41Gd2 |
| Fw/g mol−1 | 1523.76 | 1535.85 |
| Temperature/K | 120 (2) | 120 (2) |
| Crystal system | Triclinic | Triclinic |
| Space group | Pī | Pī |
| a/Å | 11.401 (1) | 9.172 (1) |
| b/Å | 13.986 (1) | 12.733 (1) |
| c/Å | 15.506 (1) | 21.558 (1) |
| α/° | 96.47 (1) | 76.39 (1) |
| β/° | 102.59 (1) | 81.26 (1) |
| γ/° | 105.99 (1) | 82.47 (1) |
| V/Å3 | 2280.1 (2) | 2406.9 (2) |
| Z | 2 | 2 |
| Dc/g cm−3 | 2.219 | 2.119 |
| μ (Mo − Kα)/mm−1 | 3.370 | 3.187 |
| F (000) | 1504 | 1520 |
| Goodness-of-fit on F2 | 1.013 | 0.989 |
| R1 [I > 2σ(I)]/all data | 0.0160/0.0175 | 0.0274/0.0302 |
| wR2 [I > 2σ(I)]/all data | 0.0417/0.0426 | 0.0741/0.0760 |
| Metal Ion | HBPY | CU | SAPR | TDD | JGBF | JETBPY | BTPR | JSD | TT |
|---|---|---|---|---|---|---|---|---|---|
| Gd(1) | 17.116 | 11.317 | 1.254 | 1.763 | 13.137 | 27.406 | 0.732 | 3.440 | 11.813 |
| Gd(2) | 13.944 | 9.336 | 0.915 | 2.077 | 12.464 | 28.179 | 1.167 | 3.889 | 9.984 |
| Metal Ion | HPY | JTC | JCCU | CSAPR | JTCTPR | TCTPR | JTDIC | HH | MFF |
|---|---|---|---|---|---|---|---|---|---|
| Gd(1) | 19.213 | 15.400 | 10.340 | 1.117 | 2.255 | 1.543 | 12.866 | 10.100 | 1.368 |
| Gd(2) | 18.545 | 14.843 | 10.477 | 1.208 | 2.134 | 1.561 | 13.375 | 9.502 | 1.489 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orts-Arroyo, M.; Sanchis-Perucho, A.; Moliner, N.; Castro, I.; Lloret, F.; Martínez-Lillo, J. One-Dimensional Gadolinium (III) Complexes Based on Alpha- and Beta-Amino Acids Exhibiting Field-Induced Slow Relaxation of Magnetization. Inorganics 2022, 10, 32. https://doi.org/10.3390/inorganics10030032
Orts-Arroyo M, Sanchis-Perucho A, Moliner N, Castro I, Lloret F, Martínez-Lillo J. One-Dimensional Gadolinium (III) Complexes Based on Alpha- and Beta-Amino Acids Exhibiting Field-Induced Slow Relaxation of Magnetization. Inorganics. 2022; 10(3):32. https://doi.org/10.3390/inorganics10030032
Chicago/Turabian StyleOrts-Arroyo, Marta, Adrián Sanchis-Perucho, Nicolas Moliner, Isabel Castro, Francesc Lloret, and José Martínez-Lillo. 2022. "One-Dimensional Gadolinium (III) Complexes Based on Alpha- and Beta-Amino Acids Exhibiting Field-Induced Slow Relaxation of Magnetization" Inorganics 10, no. 3: 32. https://doi.org/10.3390/inorganics10030032
APA StyleOrts-Arroyo, M., Sanchis-Perucho, A., Moliner, N., Castro, I., Lloret, F., & Martínez-Lillo, J. (2022). One-Dimensional Gadolinium (III) Complexes Based on Alpha- and Beta-Amino Acids Exhibiting Field-Induced Slow Relaxation of Magnetization. Inorganics, 10(3), 32. https://doi.org/10.3390/inorganics10030032