Synthesis of Novel Li2O-CuO-Bi2O3-B2O3 Glasses for Radiation Protection: An Experimental and Theoretical Study
Abstract
:1. Introduction
2. Experimental Work
Synthesis of Glass Samples
3. X-ray Diffraction, Transmittance, and Density Measurements
3.1. Gamma Ray Shielding Parameter Studies
3.2. Optimization of the Sample Thickness
3.3. Computational Work
4. Results and Discussion
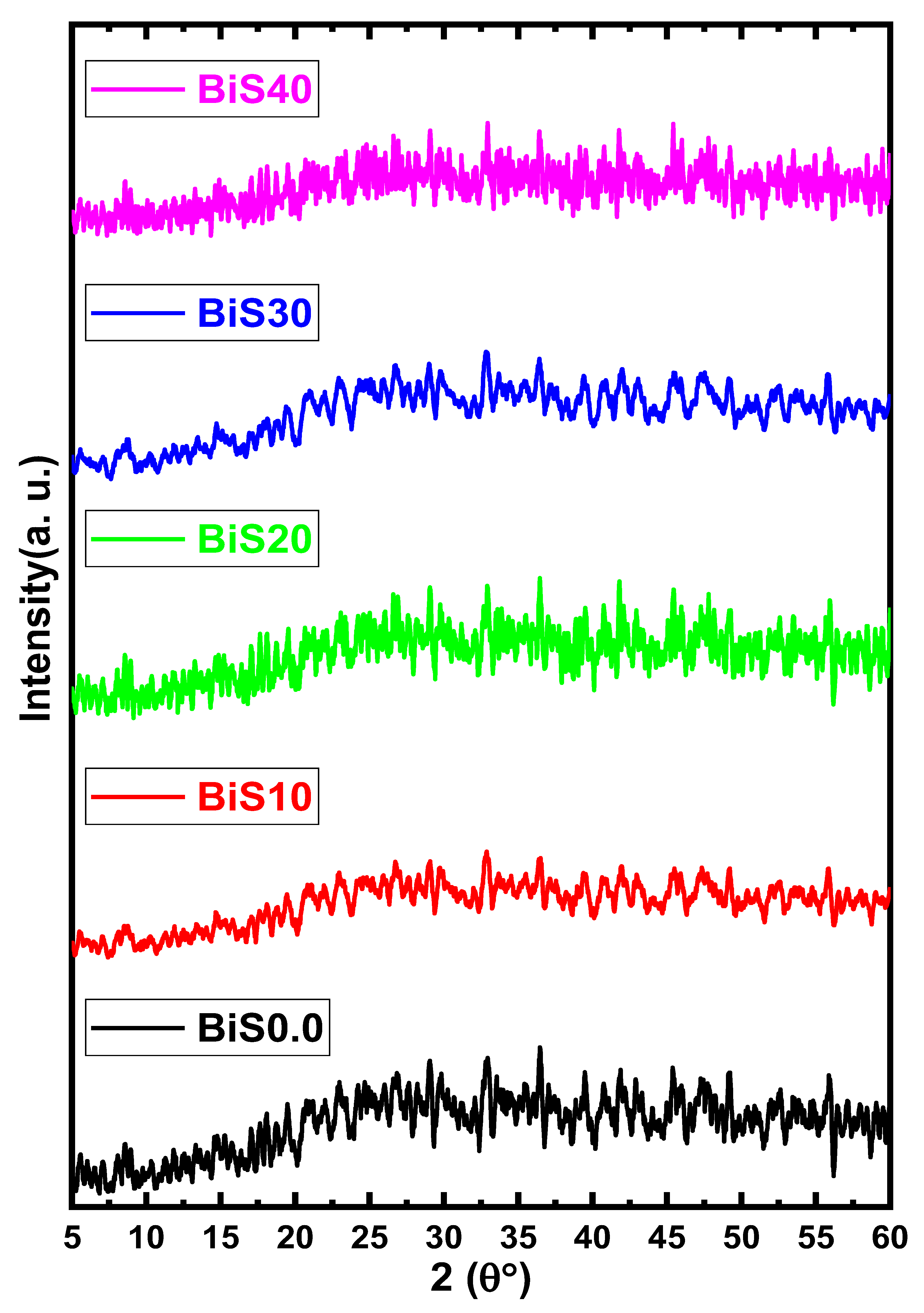
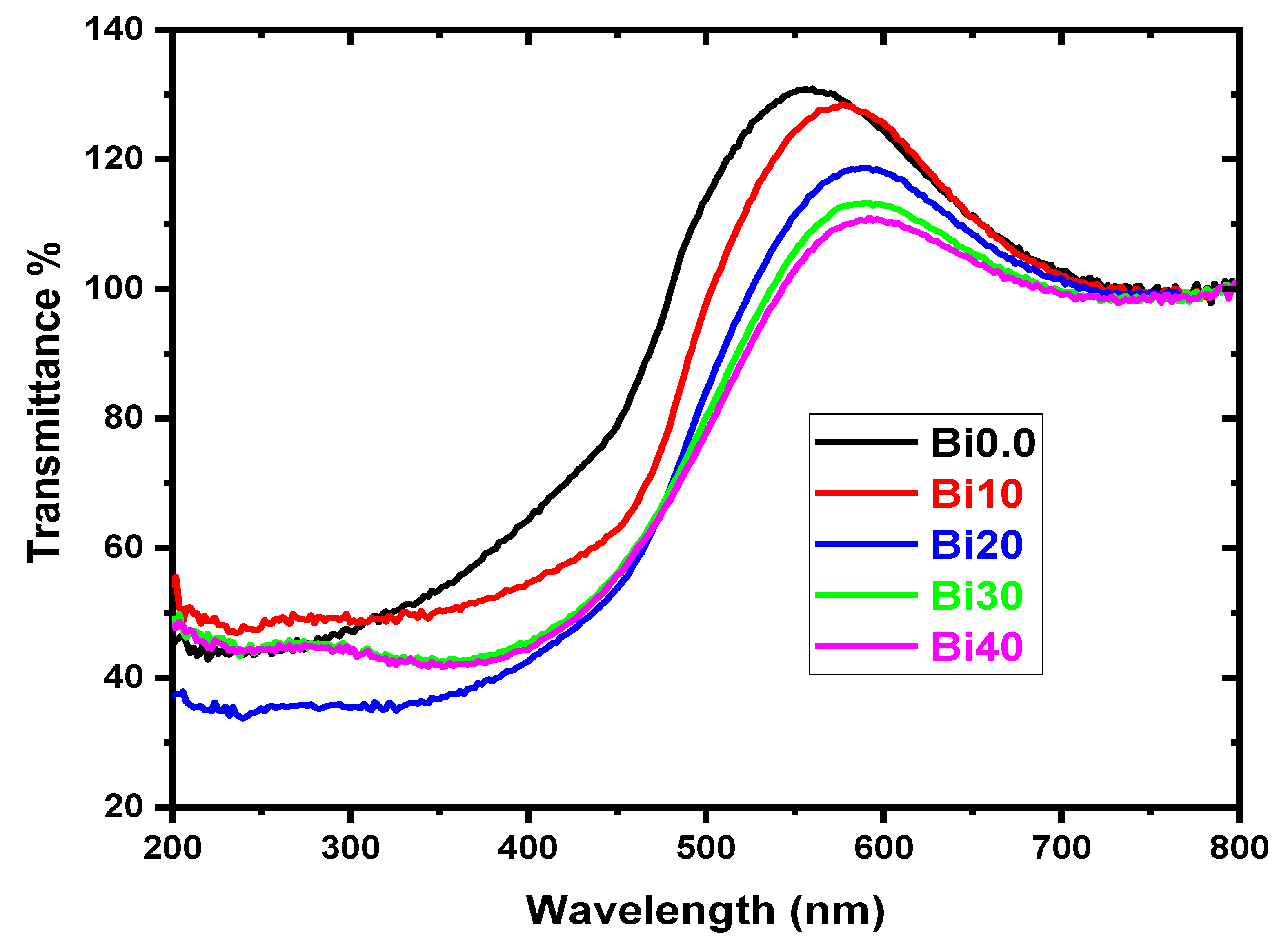
4.1. XRD, Transmittance, Density, and Molar Volume
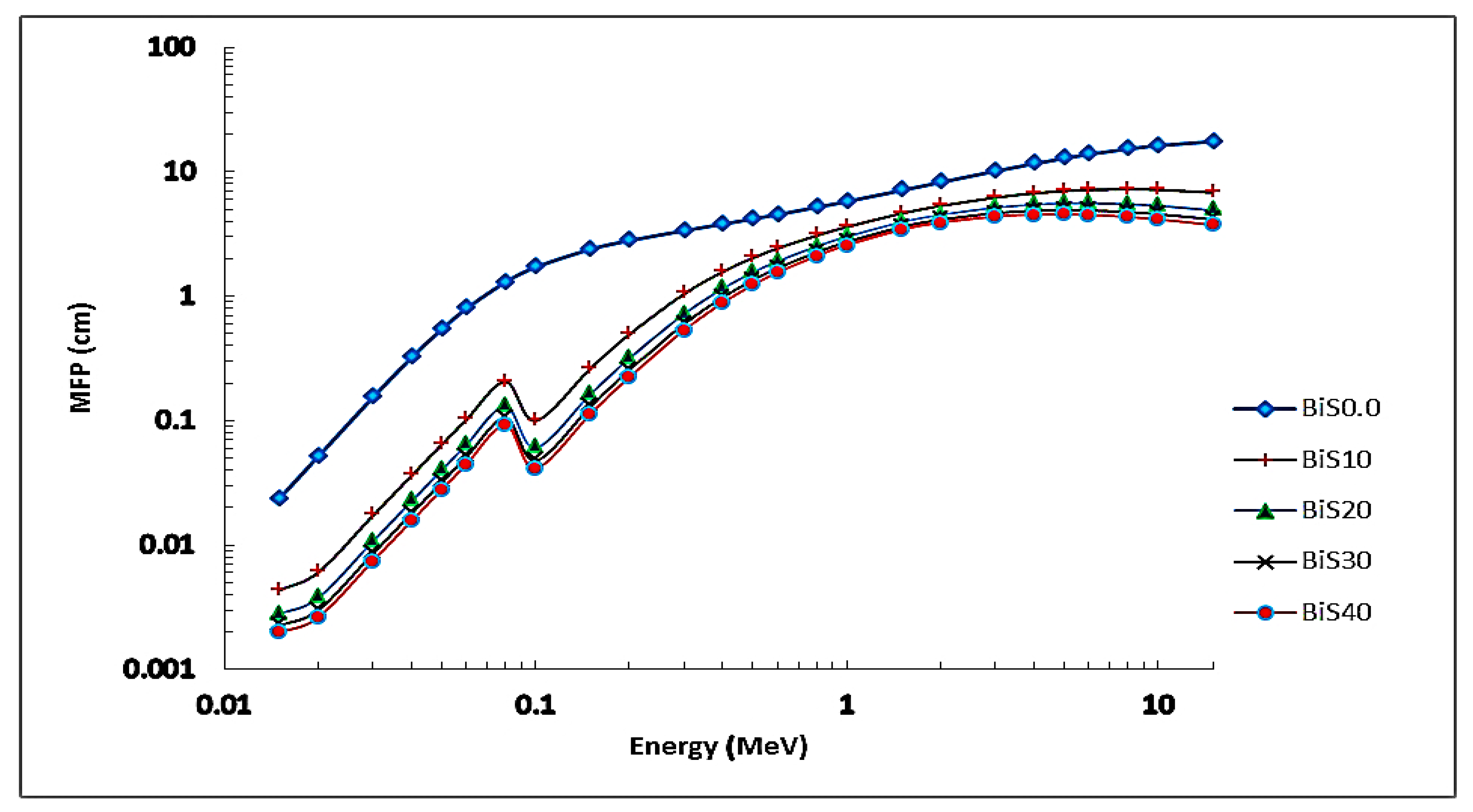
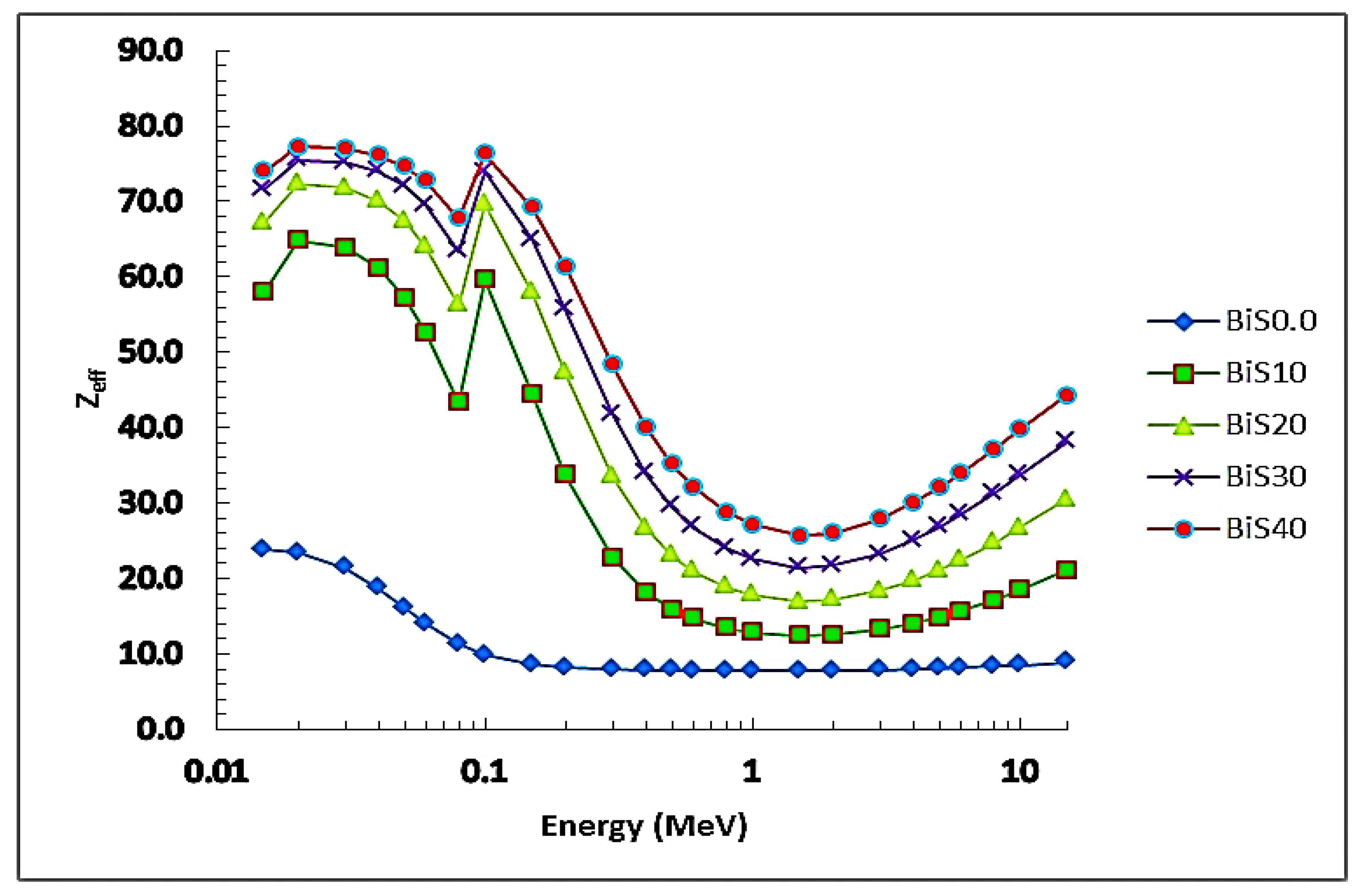
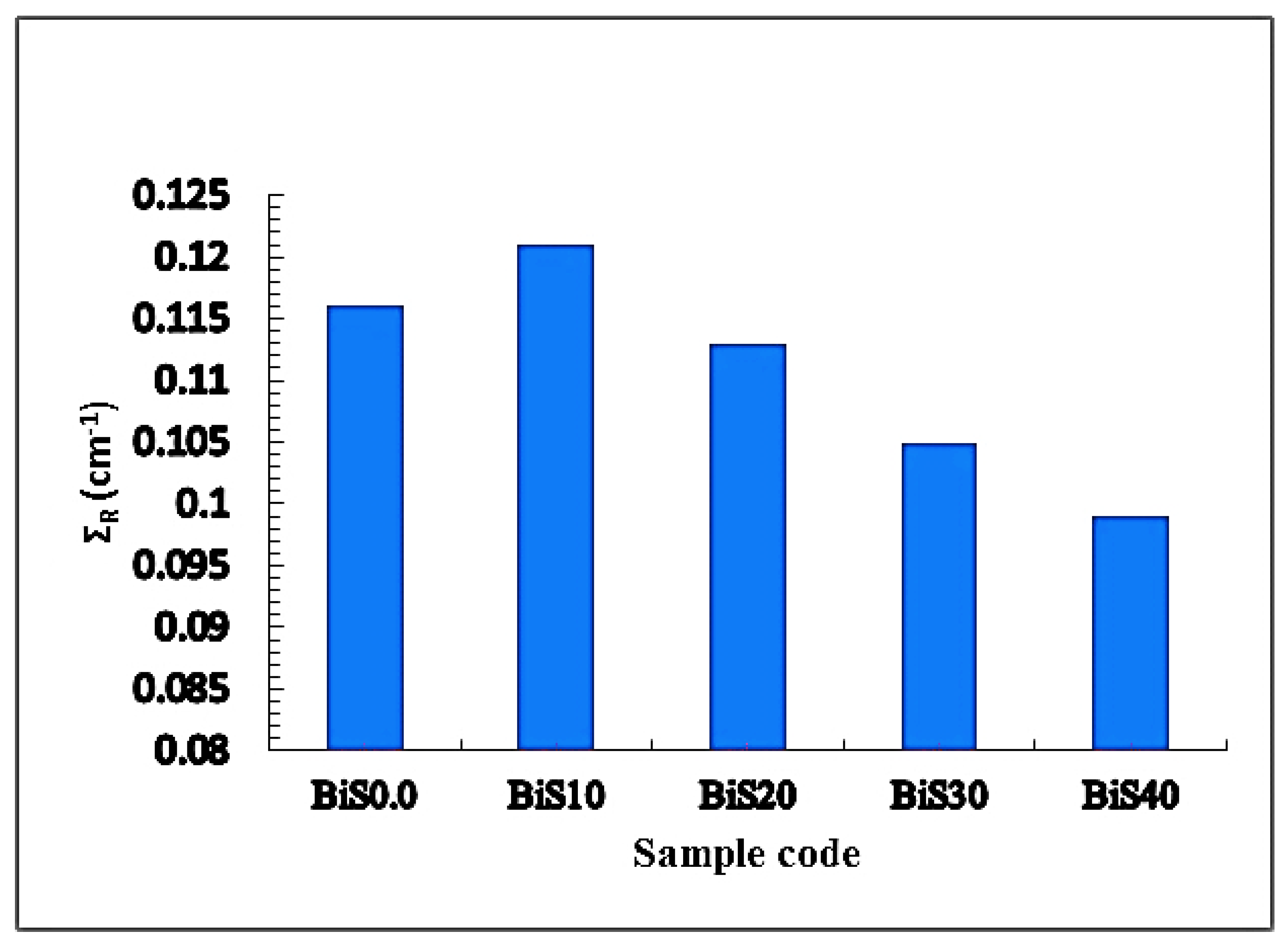
4.2. Nuclear Radiation Shielding
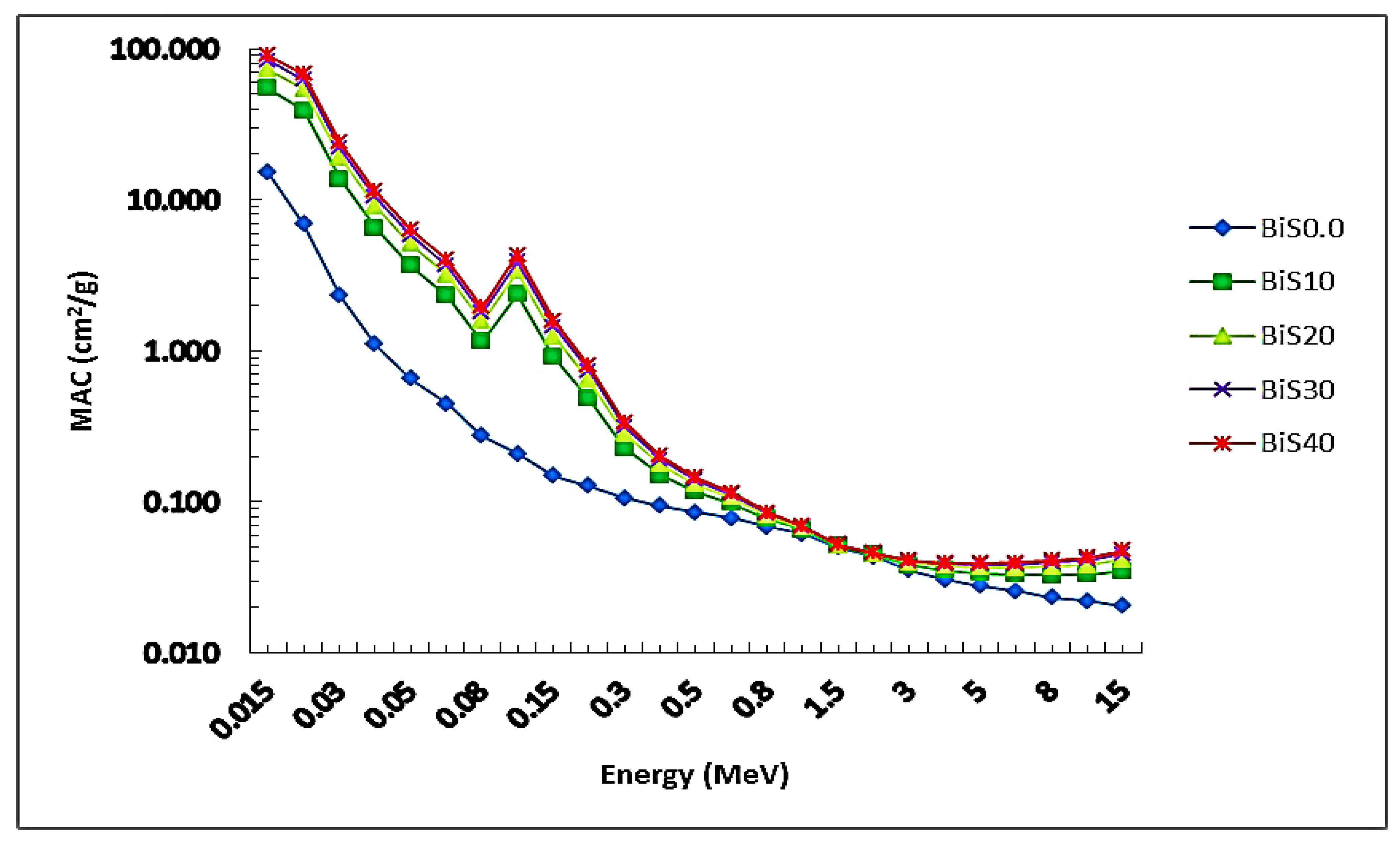
4.2.1. Experimental Results
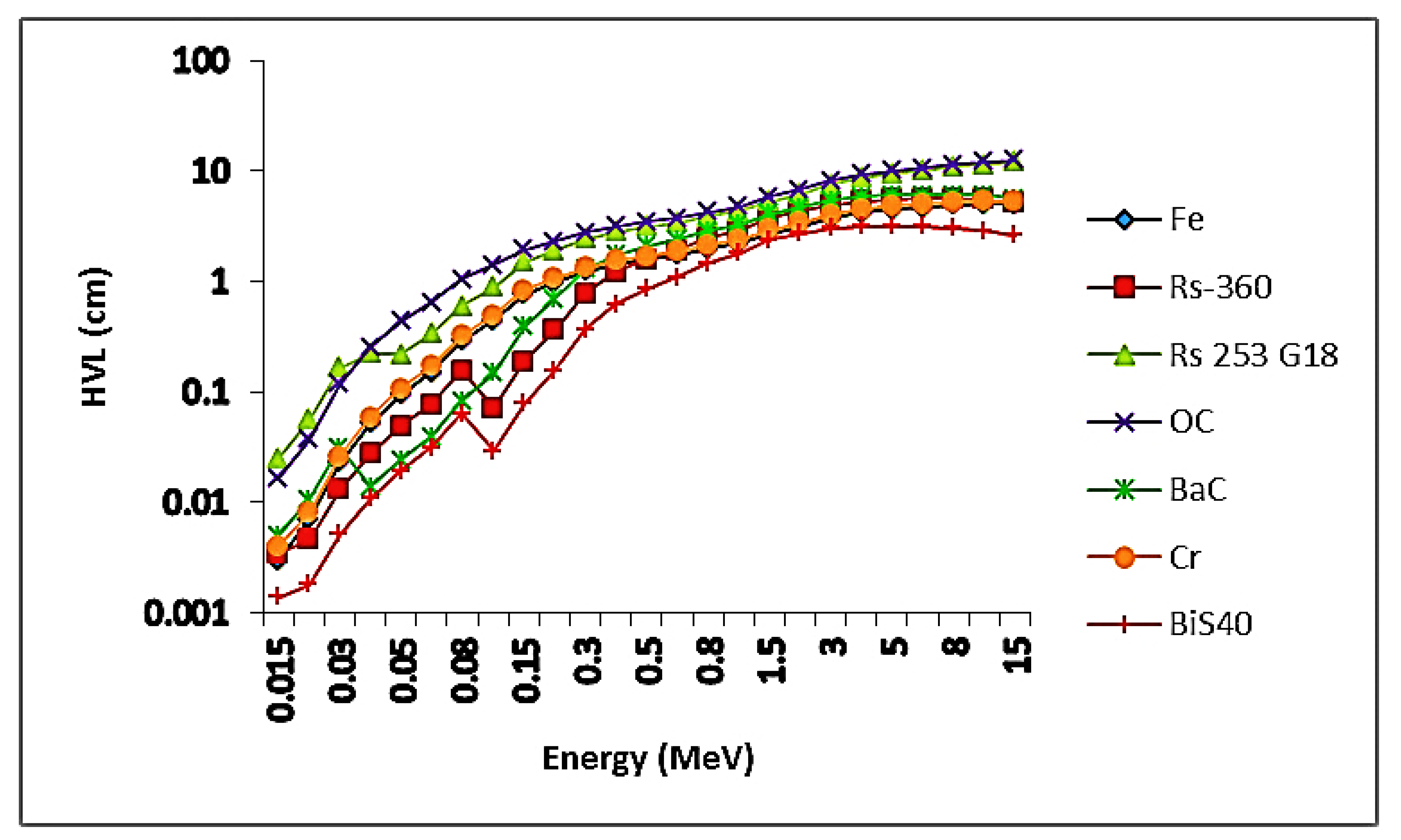
4.2.2. Theoretical Results
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saleh, E.E.; Algradee, M.A.; Al-Fakeh, M.S. Nuclear radiation shielding behavior for prepared LNZP glasses with (Cd+Te). Radiat. Phys. Chem. 2021, 189, 109743. [Google Scholar] [CrossRef]
- Algradee, M.A.; Saleh, E.E.; Samir, O.M.; Alwany, A.B.; Sherbini, T.M.E. Evaluation of structural, elastic properties and nuclear radiation shielding competence of Nd3+ doped lithium-zinc-phosphate glasses. J. Non-Cryst. Solids 2022, 576, 121304. [Google Scholar] [CrossRef]
- Alwany, A.B.; Youssef, G.M.; Saleh, E.E.; Samir, O.M.; Algradee, M.A.; Alnehia, A. Structural, optical and radiation shielding properties of ZnS nanoparticles QDs. Optik 2022, 260, 169124. [Google Scholar] [CrossRef]
- Al-Buriahi, M.; Bakhsh, E.M.; Tonguc, B.; Khan, S.B. Mechanical and radiation shielding properties of tellurite glasses doped with ZnO and NiO. Ceram. Int. 2020, 46, 19078–19083. [Google Scholar] [CrossRef]
- Almisned, G.; Tekin, H.; Kavaz, E.; Bilal, G.; Issa, S.; Zakaly, H.; Ene, A. Gamma, Fast Neutron, Proton, and Alpha Shielding Properties of Borate Glasses: A Closer Look on Lead (II) Oxide and Bismuth (III) Oxide Reinforcement. Appl. Sci. 2021, 11, 6837. [Google Scholar] [CrossRef]
- Sathiyapriya, G.; Naseer, K.A.; Marimuthu, K.; Kavaz, E.; Alalawi, A.; Al-Buriahi, M.S. Structural, optical and nuclear radiation shielding properties of strontium barium borate glasses doped with dysprosium and niobium. J. Mater. Sci. Mater. Electron. 2021, 32, 8570–8592. [Google Scholar] [CrossRef]
- Sayyed, M.; Lakshminarayana, G.; Dong, M.; Ersundu, M.; Ersundu, A.; Kityk, I. Investigation on gamma and neutron radiation shielding parameters for BaO/SrO–Bi2O3–B2O3 glasses. Radiat. Phys. Chem. 2018, 145, 26–33. [Google Scholar] [CrossRef]
- Lakshminarayana, G.; Kumar, A.; Tekin, H.; Issa, S.A.; Al-Buriahi, M.; Lee, D.-E.; Yoon, J.; Park, T. Binary B2O3-Bi2O3 glasses, scrutinization of directly and indirectly ionizing radiations shielding abilities. J. Mater. Res. Technol. 2020, 9, 14549–14567. [Google Scholar] [CrossRef]
- Aboalatta, A.; Asad, J.; Humaid, M.; Musleh, H.; Shaat, S.K.K.; Ramadan, K.; Sayyed, M.I.; Alajerami, Y.; Aldahoudi, N. Experimental investigation of zinc sodium borate glass systems containing barium oxide for gamma radiation shielding applications. Nucl. Eng. Technol. 2021, 53, 3058–3067. [Google Scholar] [CrossRef]
- Al-Buriahi, M.S.; Eke, C.; Alomairy, S.; Mutuwong, C.; Sfina, N. Micro-hardness and gamma-ray attenuation properties of lead iron phosphate glasses. J. Mater. Sci. Mater. Electron. 2021, 32, 13906–13916. [Google Scholar] [CrossRef]
- Al-Buriahi, M.; Singh, V.; Alalawi, A.; Sriwunkum, C.; Tonguc, B.T. Mechanical features and radiation shielding properties of TeO2-Ag2O-WO3 glasses. Ceram. Int. 2020, 46, 15464–15472. [Google Scholar] [CrossRef]
- Algradee, M.A.; Saleh, E.E.; Sherbini, T.M.E.; El-Mallawany, R. Optical and gamma-ray shielding features of Nd3+ doped lithium-zinc-borophosphorate glasses. Optik 2021, 242, 167059. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K.; Thakur, S.; Singh, P.; Bajwa, B. Investigation of bismuth borate glass system modified with barium for structural and gamma-ray shielding properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 206, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Saleh, E.E.; Algradee, M.A.; Alresheedi, F.; Al-Fakeh, M.S.; El-Fiki, S.A.; Youssef, G.M.; El Sharbini, T.M. Synthesis and nuclear radiation shielding ability of Li2O-ZnO-P2O5 glasses: The role of Yb2O3. J. Electron. Mater. 2022, 51, 7283–7296. [Google Scholar] [CrossRef]
- Saleh, E.E.; Algradee, M.A.; El-Fiki, S.; Youssef, G. Fabrication of novel lithium lead borate bismuth glasses for nuclear radiation shielding. Radiat. Phys. Chem. 2021, 193, 109939. [Google Scholar] [CrossRef]
- Nordfors, B. The statistical error in X-ray absorption measurements. Ark. Fys. 1960, 18, 37. [Google Scholar]
- Chantler, C.T.; Tran, C.Q.; Barnea, Z.; Paterson, D.; Cookson, D.J.; Balaic, D.X. Measurement of the X-ray mass attenuation coefficient of copper using 8.85–20 keV synchrotron radiation. Phys. Rev. A 2001, 64, 062506. [Google Scholar] [CrossRef] [Green Version]
- Şakar, E.; Özpolat, Ö.F.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X/PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2019, 166, 108496. [Google Scholar] [CrossRef]
- Mohsen, M.; Gomaa, E.; Al-Kotb, M.S.; Abdel-Baki, M.; Fathy, N. Positron annihilation Lifetime and Fourier transform infrared spectroscopic studies on Bi2O3–B2O3 glasses. J. Non-Cryst. Solids 2016, 436, 1–8. [Google Scholar] [CrossRef]
- Alajerami, Y.S.; Drabold, D.A.; Thapa, R.; Sayyed, M.I.; Mhareb, M.H.A. Physical, structural, optical and gamma-ray shielding properties of Na2O-CdO-Bi2O3-B2O3 glasses. Int. J. Appl. Glass Sci. 2021, 12, 259–273. [Google Scholar] [CrossRef]
- Schott, Co. Available online: https://www.schott.com/advanced_optics/english/products/optical-materials/specialmaterials/radiation-shielding-glasses/index.html (accessed on 10 June 2022).








| Sample Code | Mol% | ρ (g/cm3) | Vm (cm3/mole) | |||
|---|---|---|---|---|---|---|
| B2O3 | CuO | Li2O | BiO | |||
| BiS0.0 | 70 | 20 | 10 | 0.0 | 2.813 | 24.048 |
| BiS10 | 60 | 20 | 10 | 10 | 4.222 | 25.407 |
| BiS20 | 50 | 20 | 10 | 20 | 4.913 | 29.899 |
| BiS30 | 40 | 20 | 10 | 30 | 5.320 | 35.065 |
| BiS40 | 30 | 20 | 10 | 40 | 5.584 | 40.504 |
| Energy (MeV) | BiS0.0 | BiS10 | BiS20 | BiS30 | BiS40 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Exp. | Phy-X | Exp. | Phy-X | Exp. | Phy-X | Exp. | Phy-X | Exp. | Phy-X | |
| 0.662 | 0.075 ± 0.002 | 0.075 | 0.089 ± 0.003 | 0.090 | 0.095 ± 0.003 | 0.096 | 0.100 ± 0.003 | 0.100 | 0.113 ± 0.004 | 0.103 |
| 1.173 | 0.056 ± 0.002 | 0.057 | 0.058 ± 0.002 | 0.059 | 0.059 ± 0.002 | 0.060 | 0.061 ± 0.002 | 0.061 | 0.060 ± 0.002 | 0.061 |
| 1.333 | 0.053 ± 0.002 | 0.053 | 0.054 ± 0.002 | 0.055 | 0.054 ± 0.002 | 0.055 | 0.054 ± 0.002 | 0.056 | 0.055 ± 0.002 | 0.056 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Fakeh, M.S.; Saleh, E.E.; Alresheedi, F. Synthesis of Novel Li2O-CuO-Bi2O3-B2O3 Glasses for Radiation Protection: An Experimental and Theoretical Study. Inorganics 2023, 11, 27. https://doi.org/10.3390/inorganics11010027
Al-Fakeh MS, Saleh EE, Alresheedi F. Synthesis of Novel Li2O-CuO-Bi2O3-B2O3 Glasses for Radiation Protection: An Experimental and Theoretical Study. Inorganics. 2023; 11(1):27. https://doi.org/10.3390/inorganics11010027
Chicago/Turabian StyleAl-Fakeh, Maged S., Emran Eisa Saleh, and Faisal Alresheedi. 2023. "Synthesis of Novel Li2O-CuO-Bi2O3-B2O3 Glasses for Radiation Protection: An Experimental and Theoretical Study" Inorganics 11, no. 1: 27. https://doi.org/10.3390/inorganics11010027





