Nanostructured γ-Al2O3 Synthesis Using an Arc Discharge Method and its Application as an Antibacterial Agent against XDR Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticle Synthesis
2.2. Filtration and Characterization
2.3. In Vitro Studies
2.3.1. Antibacterial Activity
Disc Diffusion Method
Minimum Inhibitory Concentration (MIC)
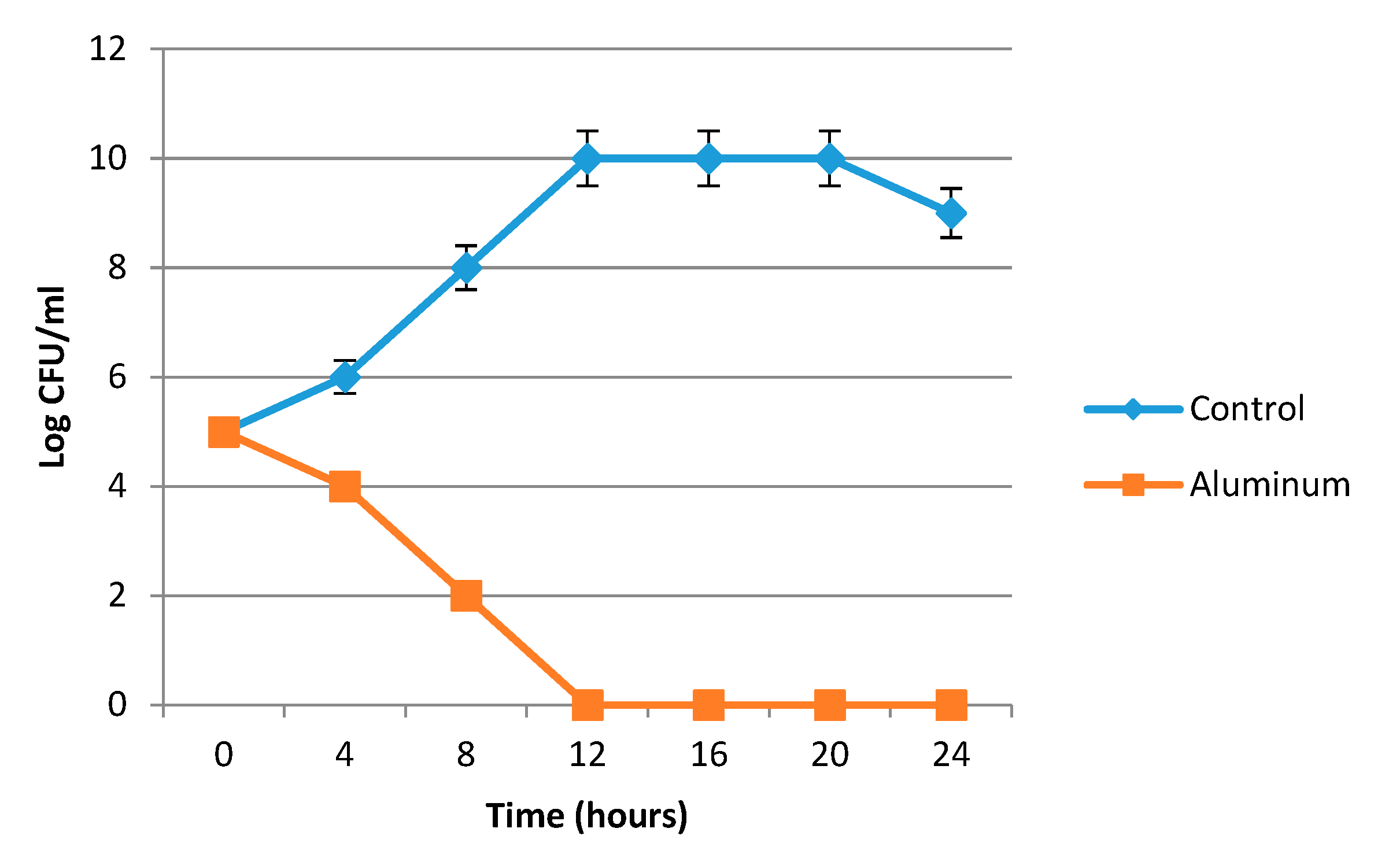
Bacterial Lethality Curve
2.3.2. Al2O3 Nanoparticle Mechanistic Action
Transmission Electron Microscope (TEM) Study
Reactive Oxygen Species (ROS) and Double-Strand Break Measurements
2.3.3. Evaluation of the Cytotoxic Effects of the Prepared γ-Al2O3
3. Results and Discussion
3.1. Preparation and Characterization of the Nanoyields
3.2. In Vitro Studies
3.2.1. Antibacterial Activity
3.2.2. γ-Al2O3 Nanoparticle Mechanistic Action
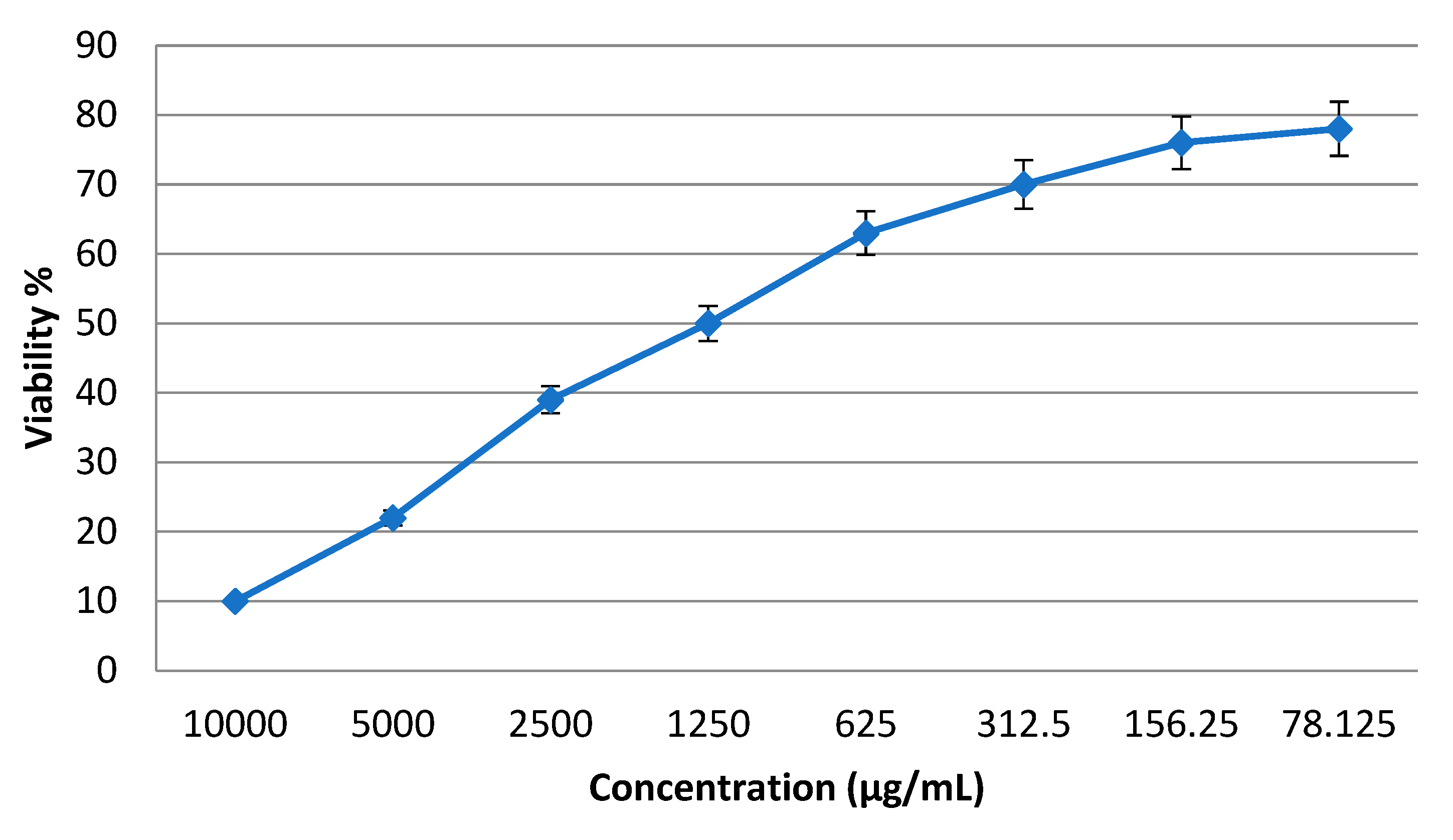
3.3. Cytotoxic Effects of the Prepared Nanoparticles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luis, A.I.S.; Campos, E.V.R.; de Oliveira, J.L.; Fraceto, L.F. Trends in aquaculture sciences: From now to use of nanotechnology for disease control. Rev. Aquac. 2019, 11, 119–132. [Google Scholar] [CrossRef]
- Adlhart, C.; Verran, J.; Azevedo, N.F.; Olmez, H.; Keinänen-Toivola, M.M.; Gouveia, I.; Melo, L.F.; Crijns, F. Surface modifications for antimicrobial effects in the healthcare setting: A critical overview. J. Hosp. Infect. 2018, 99, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Al-Humaidi, J.Y.; Hagar, M.; Bakr, B.A.; Elwakil, B.H.; Moneer, E.A.; El-Khatib, M. Decorative Multi-Walled Carbon Nanotubes by ZnO: Synthesis, Characterization, and Potent Anti-Toxoplasmosis Activity. Metals 2022, 12, 1246. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Elsafi, M.; Ghoneim, N.I.; Toderaş, M.; Sayyed, M.I.; Mohafez, H.; Islam, M.A.; Khandaker, M.U.; El-Khatib, M. Water Treatment from MB Using Zn-Ag MWCNT Synthesized by Double Arc Discharge. Materials 2021, 14, 7205. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Arora, N.; Sharma, N. Arc discharge synthesis of carbon nanotubes: Comprehensive review. Diam. Relat. Mater. 2014, 50, 135–150. [Google Scholar] [CrossRef]
- El-Khatib, A.M.; El-khatib, M. Synthesis of hexagonal nanozinc by arc discharge for antibacterial water treatment. Surf. Innov. 2020, 8, 165–171. [Google Scholar] [CrossRef]
- El-Khatib, A.; Khalil, A.; Ismail, M.; Elkhatib, M. The combined effects of multisized silver nanoparticles and pulsed magnetic field on K. pneumonia. Bioinspired Biomim. Nanobiomater. 2019, 8, 154–160. [Google Scholar] [CrossRef]
- Baghdadi, A.M.; Saddiq, A.A.; Aissa, A.; Algamal, Y.; Khalil, N.M. Structural refinement and antimicrobial activity of aluminum oxide nanoparticles. J. Ceram. Soc. Jpn. 2022, 130, 257–263. [Google Scholar] [CrossRef]
- Jiao, Z.; Liu, Z.; Zhao, X. Cathode-arc-anode behavior in cooling-induced cathode-focusing GTA system: A unified numerical model. Int. J. Heat Mass Transf. 2022, 199, 123484. [Google Scholar] [CrossRef]
- Li, W.F.; Ma, X.L.; Zhang, W.S.; Zhang, W.; Li, Y.; Zhang, Z.D. Synthesis and characterization of γ-Al2O3 nanorods. Phys. Status Solidi 2006, 203, 294–299. [Google Scholar] [CrossRef]
- Delaportas, D.; Svarnas, P.; Alexandrou, I.; Siokou, A.; Black, K.; Bradley, J.W. γ-Al2O3 nanoparticle production by arc-discharge in water: In situ discharge characterization and nanoparticle investigation. J. Phys. D Appl. Phys. 2009, 42, 245204. [Google Scholar] [CrossRef]
- Humphries, R.M.; Kircher, S.; Ferrell, A.; Krause, K.M.; Malherbe, R.; Hsiung, A.; Burnham, C.-A.D. The Continued Value of Disk Diffusion for Assessing Antimicrobial Susceptibility in Clinical Laboratories: Report from the Clinical and Laboratory Standards Institute Methods Development and Standardization Working Group. J. Clin. Microbiol. 2018, 56, e00437-18. [Google Scholar] [CrossRef] [PubMed]
- Halbus, A.F.; Horozov, T.S.; Paunov, V.N. Colloid particle formulations for antimicrobial applications. Adv. Colloid Interface Sci. 2017, 249, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.A.; Murray, B.E. Antibiotic-Resistant Bugs in the 21st Century—A Clinical Super-Challenge. N. Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Basak, S.; Singh, P.; Rajurkar, M. Multidrug Resistant and Extensively Drug Resistant Bacteria: A Study. J. Pathog. 2016, 2016, 4065603. [Google Scholar] [CrossRef] [PubMed]
- El-Khatib, A.M.; Elsafi, M.; Sayyed, M.; Abbas, M.; El-Khatib, M. Impact of micro and nano aluminium on the efficiency of photon detectors. Results Phys. 2021, 30, 104908. [Google Scholar] [CrossRef]
- Sulaiman, S.; Mahmud, A.A.A.; Bakar, M.H.A.; Noor, A.Z.M. Aluminum Nanoparticles Preparation via Plasma Arc Discharge. In Progress in Engineering Technology III; Advanced Structured, Materials; Abu Bakar, M.H., Nurhidayat Zahelem, M., Öchsner, A., Eds.; Springer: Cham, Switzerland, 2021; Volume 148. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Elwakil, B.H.; Elshewemi, S.S.; El-Naggar, M.Y.; A Bekhit, A.; A Olama, Z. Novel Siwa propolis and colistin-integrated chitosan nanoparticles: Elaboration; in vitro and in vivo appraisal. Nanomedicine 2020, 15, 1269–1284. [Google Scholar] [CrossRef]
- Aljohani, F.S.; Hamed, M.T.; Bakr, B.A.; Shahin, Y.H.; Abu-Serie, M.M.; Awaad, A.K.; El-Kady, H.; Elwakil, B.H. In vivo bio-distribution and acute toxicity evaluation of greenly synthesized ultra-small gold nanoparticles with different biological activities. Sci. Rep. 2022, 12, 6269. [Google Scholar] [CrossRef] [PubMed]
- Dorgham, R.A.; Abd Al Moaty, M.N.; Chong, K.P.; Elwakil, B.H. Molasses-Silver Nanoparticles: Synthesis, Optimization, Characterization, and Antibiofilm Activity. Int. J. Mol. Sci. 2022, 23, 10243. [Google Scholar] [CrossRef] [PubMed]
- Bhuvaneshwari, M.; Bairoliya, S.; Parashar, A.; Chandrasekaran, N.; Mukherjee, A. Differential toxicity of Al2O3 particles on Gram-positive and Gram-negative sediment bacterial isolates from freshwater. Environ. Sci. Pollut. Res. 2016, 23, 12095–12106. [Google Scholar] [CrossRef] [PubMed]
- Elwakil, B.H.; Toderas, M.; El-Khatib, M. Arc discharge rapid synthesis of engineered copper oxides nano shapes with potent antibacterial activity against multi-drug resistant bacteria. Sci. Rep. 2022, 12, 20209. [Google Scholar] [CrossRef] [PubMed]
- El-Shaer, H.; Elwakil, B.H.; Bakr, B.A.; Eldrieny, A.M.; El-Khatib, M.; Chong, K.P.; Gazia, A.A.A. Physiotherapeutic Protocol and ZnO Nanoparticles: A Combined Novel Treatment Program against Bacterial Pyomyositis. Biology 2022, 11, 1393. [Google Scholar] [CrossRef]
- Bell, T.E.; González-Carballo, J.M.; Tooze, R.P.; Torrente-Murciano, L. Single-step synthesis of nanostructured γ-alumina with solvent reusability to maximise yield and morphological purity. J. Mater. Chem. A 2015, 3, 6196–6201. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, D.; Zhou, G.; Zhang, C.; Zhang, P.; Hou, X. Synthesis of nano-sized γ-Al2O3 with controllable size by simple homogeneous precipitation method. Mater. Lett. 2020, 279, 128476. [Google Scholar] [CrossRef]
- Al-Rubayee, W.T.; Abdul-Rasheed, O.F.; Ali, N.M. Preparation of a Modified Nanoalumina Sorbent for the Removal of Alizarin Yellow R and Methylene Blue Dyes from Aqueous Solutions. J. Chem. 2016, 2016, 4683859. [Google Scholar] [CrossRef]
- Zemek, P.; Van Gompel, J.; Plowman, S. PPM-Level HCl Measurements from Cement Kilns and Waste Incinerators by FTIR Spectroscopy. MIDAC Corporation. Available online: http://www.midac.com/files/AP-212.pdf (accessed on 22 November 2022).
- Geoprincy, G.; Gandhi, N.N.; Renganathan, S. Novel antibacterial effects of alumina nanoparticles on Bacillus cereus and Bacillus subtilis in comparison with antibiotics. Int. J. Pharm. Pharm. Sci. 2012, 4, 544–548. [Google Scholar]
- Jwad, K.H.; Saleh, T.H.; Abd-Alhamza, B. Preparation of Aluminum Oxide Nanoparticles by Laser Ablation and a Study of Their Applications as Antibacterial and Wounds Healing Agent. Nano Biomed. Eng. 2019, 11, 313–319. [Google Scholar] [CrossRef]
- Pakrashi, S.; Kumar, D.; Iswarya, V.; Bhuvaneshwari, M.; Chandrasekaran, N.; Mukherjee, A. A comparative ecotoxicity analysis of α- and γ-phase aluminium oxide nanoparticles towards a freshwater bacterial isolate Bacillus licheniformis. Bioprocess Biosyst. Eng. 2014, 37, 2415–2423. [Google Scholar] [CrossRef] [PubMed]
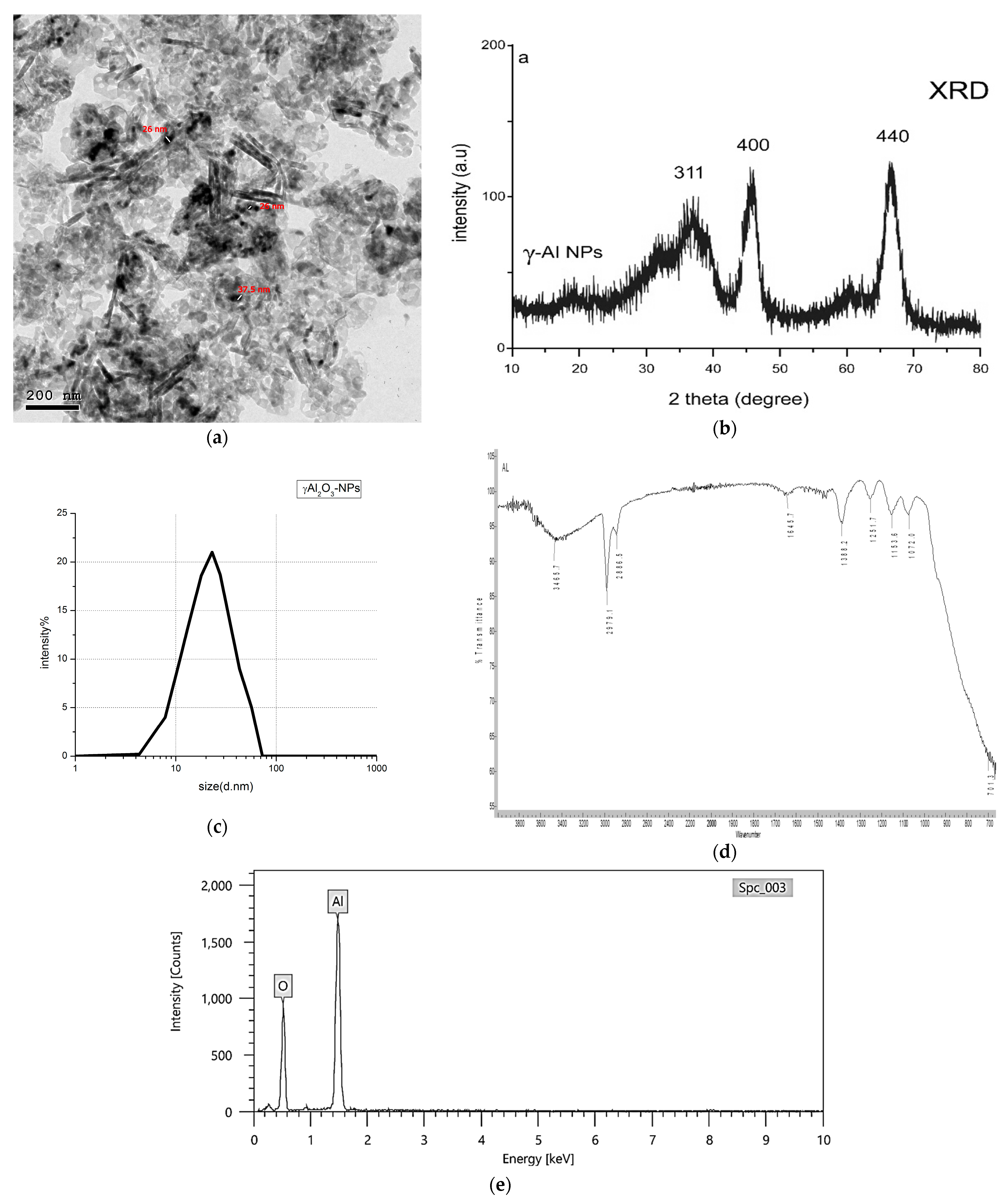

| (2Ө) Degree | Intensity (Counts) | (h, k, and l) MillerIndices | FWHM Degree | Grain Size (nm) |
|---|---|---|---|---|
| 35.91 | 78.21 | (311) | 8.73 | 1.00 |
| 45.73 | 102.34 | (400) | 1.93 | 4.67 |
| 66.79 | 111.69 | (440) | 2.13 | 4.67 |
| Tested Strains | γ-Al2O3 NPs | |
|---|---|---|
| IZ (mm) | MIC (mg/mL) | |
| A. baumannii 1 | 15 | 8 |
| A. baumannii 2 | 10 | 16 |
| A. baumannii 3 | 19 | 2 |
| A. baumannii 4 | 15 | 8 |
| A. baumannii 5 | 6 | 64 |
| A. baumannii 6 | 13 | 16 |
| A. baumannii 7 | 6 | 64 |
| A. baumannii 8 | 10 | 16 |
| A. baumannii 9 | 7 | 64 |
| A. baumannii 10 | 17 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almotairy, A.R.Z.; Amer, A.M.; El-Kady, H.; Elwakil, B.H.; El-Khatib, M.; Eldrieny, A.M. Nanostructured γ-Al2O3 Synthesis Using an Arc Discharge Method and its Application as an Antibacterial Agent against XDR Bacteria. Inorganics 2023, 11, 42. https://doi.org/10.3390/inorganics11010042
Almotairy ARZ, Amer AM, El-Kady H, Elwakil BH, El-Khatib M, Eldrieny AM. Nanostructured γ-Al2O3 Synthesis Using an Arc Discharge Method and its Application as an Antibacterial Agent against XDR Bacteria. Inorganics. 2023; 11(1):42. https://doi.org/10.3390/inorganics11010042
Chicago/Turabian StyleAlmotairy, Awatif Rashed Z., A. M. Amer, Hadir El-Kady, Bassma H. Elwakil, Mostafa El-Khatib, and Ahmed M. Eldrieny. 2023. "Nanostructured γ-Al2O3 Synthesis Using an Arc Discharge Method and its Application as an Antibacterial Agent against XDR Bacteria" Inorganics 11, no. 1: 42. https://doi.org/10.3390/inorganics11010042
APA StyleAlmotairy, A. R. Z., Amer, A. M., El-Kady, H., Elwakil, B. H., El-Khatib, M., & Eldrieny, A. M. (2023). Nanostructured γ-Al2O3 Synthesis Using an Arc Discharge Method and its Application as an Antibacterial Agent against XDR Bacteria. Inorganics, 11(1), 42. https://doi.org/10.3390/inorganics11010042









