In-Situ EC-AFM Study of Electrochemical P-Doping of Polymeric Nickel(II) Complexes with Schiff base Ligands
Abstract
1. Introduction
2. Results and Discussion
2.1. Electrochemical Synthesis of Polymer Films
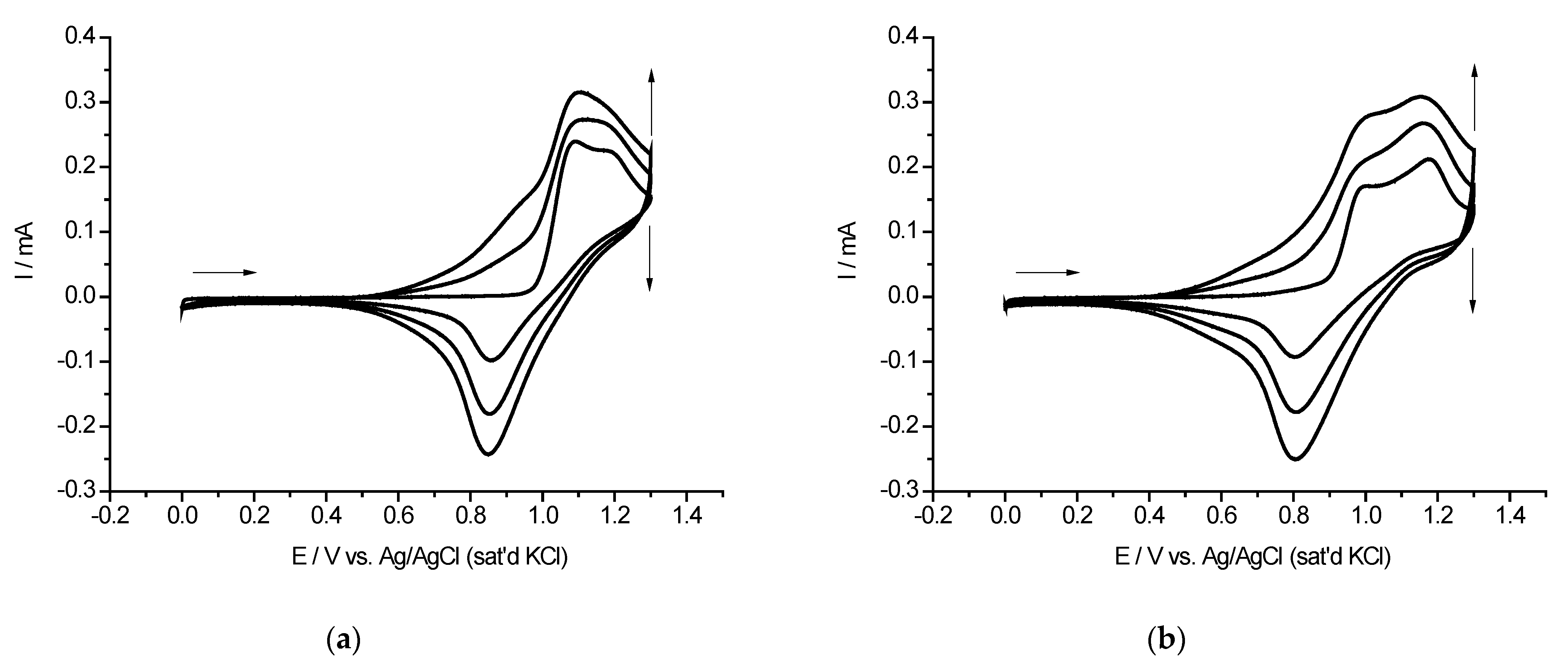
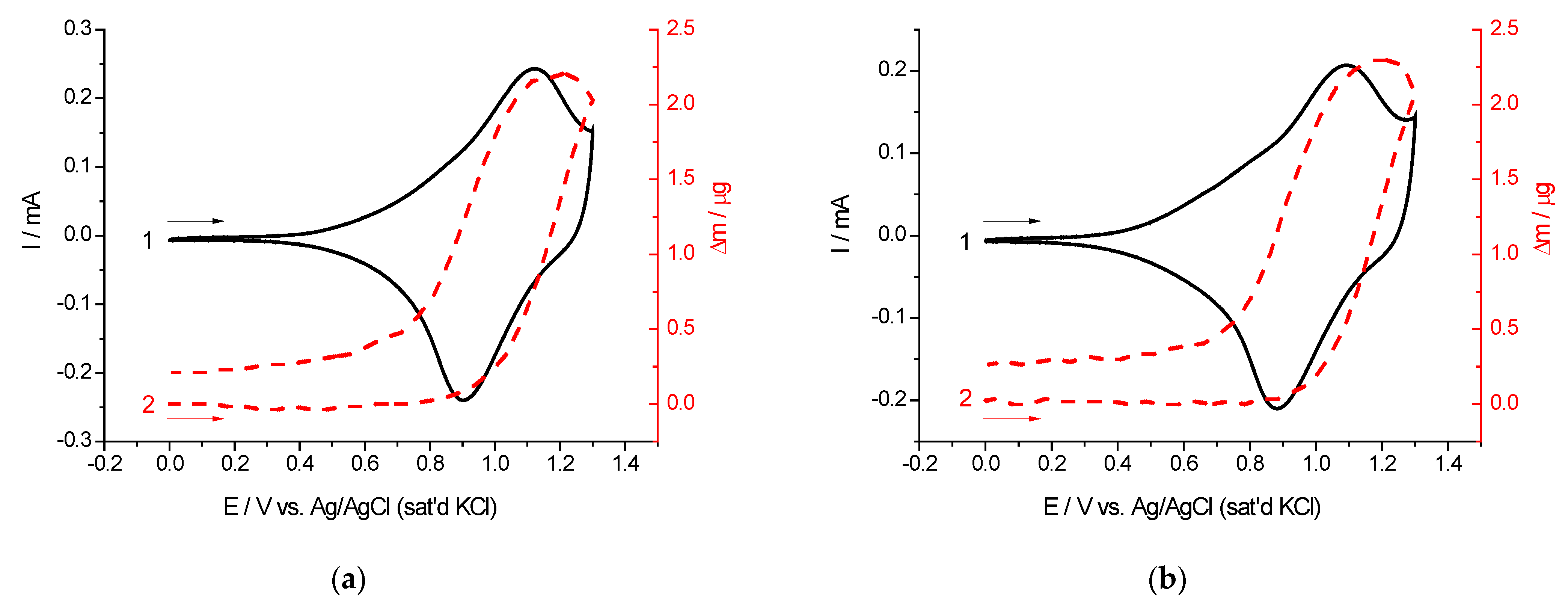
2.2. Cyclic Voltammetry and EQCM Measurements
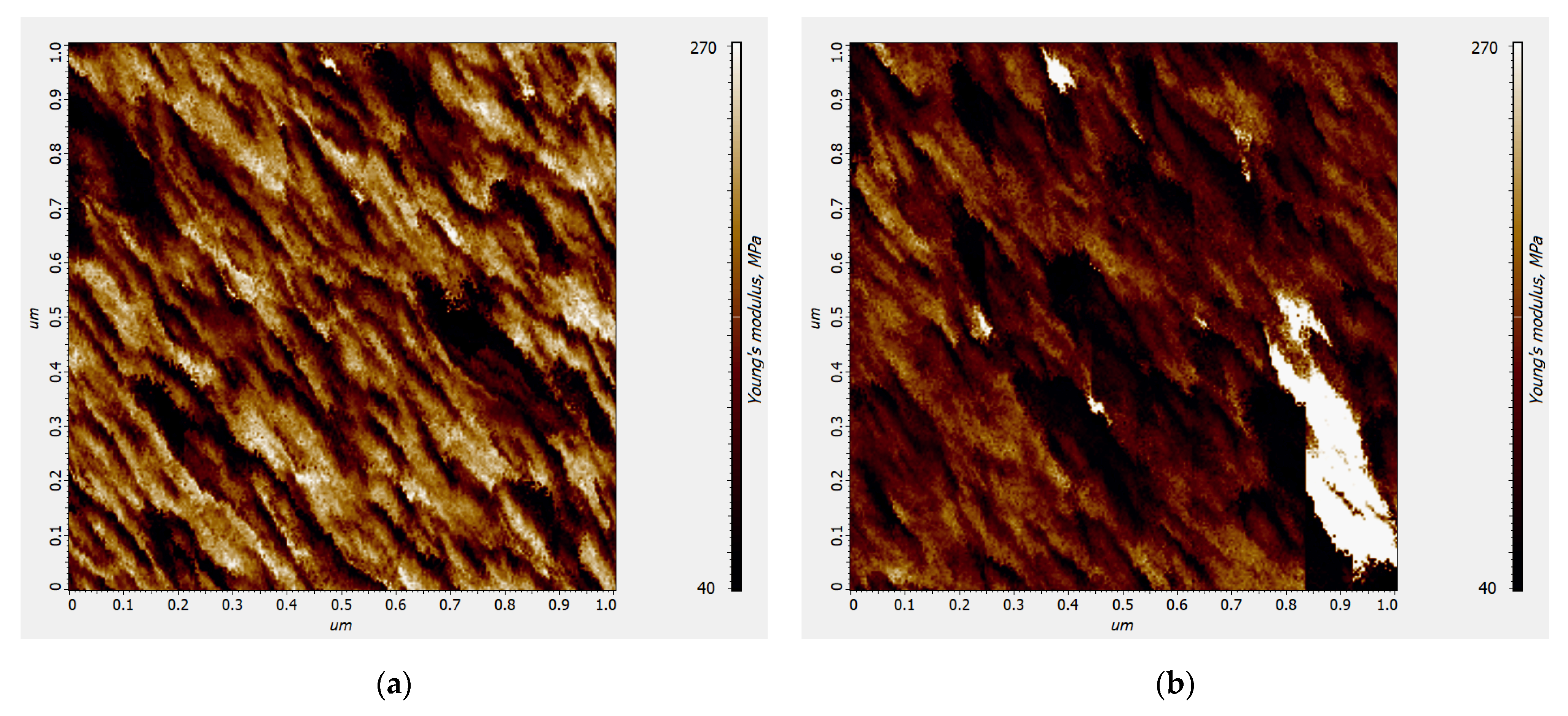
2.3. In-Situ Electrochemical Atomic Force Microscopy Measurements
3. Materials and Methods
3.1. Chemicals
3.2. Electrochemical Synthesis of Poly[NiSalphen] and poly[NiSalphen(CH3O)2]
3.3. Combination of Cyclic Voltammetry and EQCM Measurements
3.4. In-Situ EC-HD QNM AFM
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chepurnaya, I.A.; Karushev, M.P.; Alekseeva, E.V.; Lukyanov, D.A.; Levin, O.V. Redox-conducting polymers based on metal-salen complexes for energy storage applications. Pure Appl. Chem. 2020, 92, 1239–1258. [Google Scholar] [CrossRef]
- Freire, C.; Nunes, M.; Pereira, C.; Fernandes, D.M.; Peixoto, A.F.; Rocha, M. Metallo(salen) complexes as versatile building blocks for the fabrication of molecular materials and devices with tuned properties. Co-ord. Chem. Rev. 2019, 394, 104–134. [Google Scholar] [CrossRef]
- Beletskii, E.; Fedorova, A.; Lukyanov, D.; Kalnin, A.; Ershov, V.; Danilov, S.; Spiridonova, D.; Alekseeva, E.; Levin, O. Switchable resistance conducting-polymer layer for Li-ion battery overcharge protection. J. Power Sources 2021, 490, 229548. [Google Scholar] [CrossRef]
- Besedina, M.A.; Smirnova, E.A.; Poturai, D.O.; Karushev, M.P. The activity of monomeric and polymeric nickel complexes with Salen-type ligands as photosensitive materials for electrochemical solar cells. Russ. Chem. Bull. 2021, 70, 107–112. [Google Scholar] [CrossRef]
- Smirnova, E.A.; Timonov, A.M. A novel functional material for the electrochemical reduction of chlorinated organic compounds. Russ. Chem. Bull. 2021, 70, 1618–1621. [Google Scholar] [CrossRef]
- Polozhentseva, Y.A.; Novozhilova, M.V.; Chepurnaya, I.A.; Karushev, M.P. Polymeric Complexes of Nickel with Salen-Type Ligands as Multifunctional Components of Lithium Ion Battery Cathodes. Tech. Phys. Lett. 2021, 47, 83–87. [Google Scholar] [CrossRef]
- Hamnett, A.; Abel, J.; Eameaim, J.; Christensen, P.; Timonov, A.; Vasilyeva, S. A study of the polymerisation and electrochemical cycling of Pd methoxy-Salen derivatives using fast ellipsometry and FT-infrared spectroscopy. Phys. Chem. Chem. Phys. 1999, 1, 5147–5156. [Google Scholar] [CrossRef]
- Dmitrieva, E.; Rosenkranz, M.; Danilova, J.S.; Smirnova, E.A.; Karushev, M.P.; Chepurnaya, I.A.; Timonov, A.M. Radical formation in polymeric nickel complexes with N2O2 Schiff base ligands: An in situ ESR and UV–vis–NIR spectroelectrochemical study. Electrochim. Acta 2018, 283, 1742–1752. [Google Scholar] [CrossRef]
- Vilas-Boas, M.; Henderson, M.; Freire, C.; Hillman, A.; Vieil, E. A Combined Electrochemical Quartz-Crystal Microbalance Probe Beam Deflection (EQCM-PBD) Study of Solvent and Ion Transfers at a Poly[Ni(saltMe)]-Modified Electrode During Redox Switching. Chem. A Eur. J. 2000, 6, 1160–1167. [Google Scholar] [CrossRef]
- Sizov, V.; Novozhilova, M.V.; Alekseeva, E.V.; Karushev, M.; Timonov, A.; Eliseeva, S.; Vanin, A.A.; Malev, V.V.; Levin, O.V. Redox transformations in electroactive polymer films derived from complexes of nickel with SalEn-type ligands: Computational, EQCM, and spectroelectrochemical study. J. Solid State Electrochem. 2014, 19, 453–468. [Google Scholar] [CrossRef]
- Volkov, A.I.; Apraksin, R.V.; Falaleev, E.A.; Novoselova, J.V.; Volosatova, Y.A.; Lukyanov, D.A.; Alekseeva, E.V.; Levin, O.V. Tuning cationic transport in Nisalen polymers via pseudo-crown functionality. Electrochim. Acta 2022, 425, 140750. [Google Scholar] [CrossRef]
- Koyama, K.; Iijima, K.; Yoo, D.; Mori, T. Transistor properties of salen-type metal complexes. RSC Adv. 2020, 10, 29603–29609. [Google Scholar] [CrossRef] [PubMed]
- Memarzadeh, R.; Panahi, F.; Javadpour, S.; Ali, K.-N.; Noh, H.-B.; Shim, Y.-B. The Interaction of CO to the Co(salen) Complex in to PEDOT:PSS Film and Sensor Application. Bull. Korean Chem. Soc. 2012, 33, 1297–1302. [Google Scholar] [CrossRef]
- Nadeau, V.; Hildgen, P. AFM study of a New Carrier Based on PLA and Salen Copolymers for Gene Therapy. Molecules 2005, 10, 105–113. [Google Scholar] [CrossRef]
- Doroodmand, M.M.; Owji, S. Alternate layer by layered self assembly of conjugated and unconjugated Salen based nanowires as capacitive pseudo supercapacitor. Sci. Rep. 2021, 11, 18768. [Google Scholar] [CrossRef]
- Räisänen, M.T.; de Almeida, P.; Meinander, K.; Kemell, M.; Mutikainen, I.; Leskelä, M.; Repo, T. Cobalt salen functionalised polycrystalline gold surfaces. Thin Solid Films 2008, 516, 2948–2956. [Google Scholar] [CrossRef]
- Young, T.J.; Monclus, M.A.; Burnett, T.L.; Broughton, W.R.; Ogin, S.L.; Smith, P.A. The use of the PeakForceTMquantitative nanomechanical mapping AFM-based method for high-resolution Young′s modulus measurement of polymers. Meas. Sci. Technol. 2011, 22, 125703. [Google Scholar] [CrossRef]
- Belikov, S.; Magonov, S.; Erina, N.; Huang, L.; Su, C.; Rice, A.; Meyer, C.; Prater, C.; Ginzburg, V.; Meyers, G.; et al. Theoretical modelling and implementation of elastic modulus measurement at the nanoscale using atomic force microscope. J. Physics Conf. Ser. 2007, 61, 1303–1307. [Google Scholar] [CrossRef]
- Schoetz, T.; Kurniawan, M.; Stich, M.; Peipmann, R.; Efimov, I.; Ispas, A.; Bund, A.; de Leon, C.P.; Ueda, M. Understanding the charge storage mechanism of conductive polymers as hybrid battery-capacitor materials in ionic liquids by in situ atomic force microscopy and electrochemical quartz crystal microbalance studies. J. Mater. Chem. A 2018, 6, 17787–17799. [Google Scholar] [CrossRef]
- Łępicka, K.; Majewska, M.; Nowakowski, R.; Kutner, W.; Pieta, P. High electrochemical stability of meso-Ni-salen based conducting polymer manifested by potential-driven reversible changes in viscoelastic and nanomechanical properties. Electrochim. Acta 2018, 297, 94–100. [Google Scholar] [CrossRef]
- Novoa-Ramírez, C.S.; Silva-Becerril, A.; Olivera-Venturo, F.L.; García-Ramos, J.C.; Flores-Alamo, M.; Ruiz-Azuara, L. N/N Bridge Type and Substituent Effects on Chemical and Crystallographic Properties of Schiff-Base (Salen/Salphen) Niii Complexes. Crystals 2020, 10, 616. [Google Scholar] [CrossRef]
- Mendes, R.A.; Germino, J.C.; Fazolo, B.R.; Thaines, E.H.; Ferraro, F.; Santana, A.M.; Ramos, R.J.; de Souza, G.L.; Freitas, R.G.; Vazquez, P.A.; et al. Electronic and magnetic properties of the [Ni(salophen)]: An experimental and DFT study. J. Adv. Res. 2017, 9, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bei, F.-L.; Xu, X.-Y.; Yang, X.-J.; Wang, X. Crystal structure and characterization of 1,2-N,N-disallicydene-phenylamineato nickel(II) complex. J. Chem. Crystallogr. 2003, 33, 845–849. [Google Scholar] [CrossRef]
- Danilova, J.S.; Avdoshenko, S.M.; Karushev, M.P.; Timonov, A.M.; Dmitrieva, E. Infrared spectroscopic study of nickel complexes with salen-type ligands and their polymers. J. Mol. Struct. 2021, 1241, 130668. [Google Scholar] [CrossRef]
- Smirnova, E.; Baichurin, R.; Viktorov, N.; Spiridonova, D.; Timonov, A.; Karushev, M. N,N′-4,5-Dimethoxy-1,2-phenylenebis(salicylideneiminato)nickel(II). Molbank 2022, 2022, M1512. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Deng, F.; Li, J. Electropolymerization and electrochemical behavior of nickel Schiff base complexes with different groups between imine linkages. RSC Adv. 2016, 6, 79894–79899. [Google Scholar] [CrossRef]
- De Bellefeuille, D.; Askari, M.S.; Lassalle-Kaiser, B.; Journaux, Y.; Aukauloo, A.; Orio, M.; Thomas, F.; Ottenwaelder, X. Reversible Double Oxidation and Protonation of the Non-Innocent Bridge in a Nickel(II) Salophen Complex. Inorg. Chem. 2012, 51, 12796–12804. [Google Scholar] [CrossRef]
- Ding, B.; Solomon, M.B.; Leong, C.F.; D′Alessandro, D.M. Redox-active ligands: Recent advances towards their incorporation into coordination polymers and metal-organic frameworks. Co-ord. Chem. Rev. 2021, 439, 213891. [Google Scholar] [CrossRef]
- Calbo, J.; Golomb, M.J.; Walsh, A. Redox-active metal–organic frameworks for energy conversion and storage. J. Mater. Chem. A 2019, 7, 16571–16597. [Google Scholar] [CrossRef]
- Shagisultanova, G.A.; Ardasheva, L.P.; Orlova, I. Electro- and Photoelectroactivity of Thin-Layer Polymers Based on [NiSalen] and [NiSalphen] Complexes. Russ. J. Appl. Chem. 2003, 76, 1631–1636. [Google Scholar] [CrossRef]
- Novozhilova, M.; Anischenko, D.; Chepurnaya, I.; Dmitrieva, E.; Malev, V.; Timonov, A.; Karushev, M. Metal-centered redox activity in a polymeric Cobalt(II) complex of a sterically hindered salen type ligand. Electrochim. Acta 2020, 353, 136496. [Google Scholar] [CrossRef]
- Deng, F.; Li, X.; Ding, F.; Niu, B.; Li, J. Pseudocapacitive Energy Storage in Schiff Base Polymer with Salphen-Type Ligands. J. Phys. Chem. C 2018, 122, 5325–5333. [Google Scholar] [CrossRef]
- Pfeiffer, P.; Breith, E.; Lübbe, E.; Tsumaki, T. Tricyclische orthokondensierte Nebenvalenzringe. Eur. J. Org. Chem. 1933, 503, 84–130. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung danner Schichten und zur Mikrowägung. Z. Phys. 2005, 155, 206–222. [Google Scholar] [CrossRef]
- Sader, J.E.; Chon, J.; Mulvaney, P. Calibration of rectangular atomic force microscope cantilevers. Rev. Sci. Instruments 1999, 70, 3967–3969. [Google Scholar] [CrossRef]



| Polymer | Mass of Dry Polymer Film, m, µg (±0.1) | Number of Electrons Exchanged by Each Monomer Unit, n (±0.1) | Effective Molar Mass of the Electrolyte Species, M (±10), g mol−1 | Proposed Composition of the Electrolyte Species |
|---|---|---|---|---|
| poly[NiSalphen] | 5.1 | 1.2 | 190 | BF4− + PC |
| poly[NiSalphen(CH3O)2] | 4.9 | 1.5 | 245 | BF4− + 1.5 PC |
| Polymer | Average Young’s Modulus, MPa | |
|---|---|---|
| Uncharged State | Charged State | |
| poly[NiSalphen] | 175 ± 8 | 108 ± 4 |
| poly[NiSalphen(CH3O)2] | 145 ± 5 | 95 ± 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnova, E.; Ankudinov, A.; Chepurnaya, I.; Timonov, A.; Karushev, M. In-Situ EC-AFM Study of Electrochemical P-Doping of Polymeric Nickel(II) Complexes with Schiff base Ligands. Inorganics 2023, 11, 41. https://doi.org/10.3390/inorganics11010041
Smirnova E, Ankudinov A, Chepurnaya I, Timonov A, Karushev M. In-Situ EC-AFM Study of Electrochemical P-Doping of Polymeric Nickel(II) Complexes with Schiff base Ligands. Inorganics. 2023; 11(1):41. https://doi.org/10.3390/inorganics11010041
Chicago/Turabian StyleSmirnova, Evgenia, Alexander Ankudinov, Irina Chepurnaya, Alexander Timonov, and Mikhail Karushev. 2023. "In-Situ EC-AFM Study of Electrochemical P-Doping of Polymeric Nickel(II) Complexes with Schiff base Ligands" Inorganics 11, no. 1: 41. https://doi.org/10.3390/inorganics11010041
APA StyleSmirnova, E., Ankudinov, A., Chepurnaya, I., Timonov, A., & Karushev, M. (2023). In-Situ EC-AFM Study of Electrochemical P-Doping of Polymeric Nickel(II) Complexes with Schiff base Ligands. Inorganics, 11(1), 41. https://doi.org/10.3390/inorganics11010041






