A Novel Zero-Thermal-Quenching Red Phosphor with High Quantum Efficiency and Color Purity
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Targets
2.2. Characterization of Materials
3. Result and Discussion
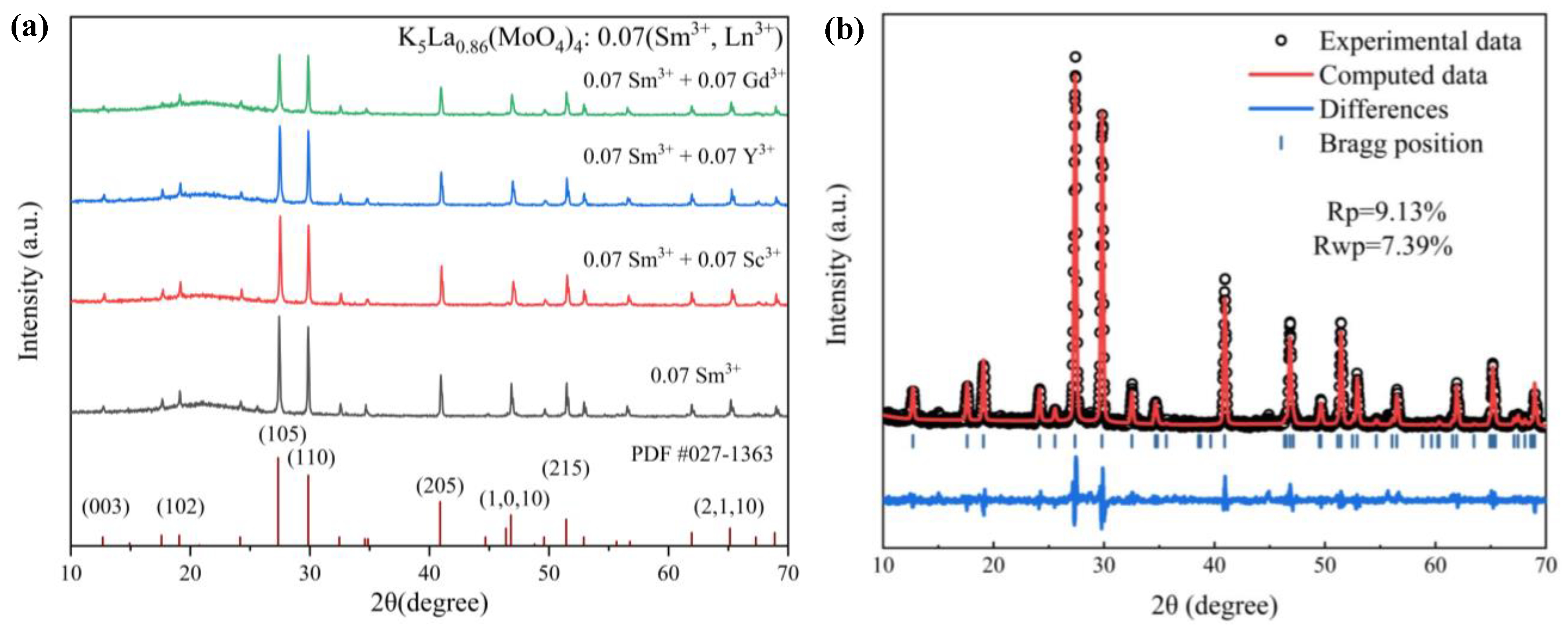
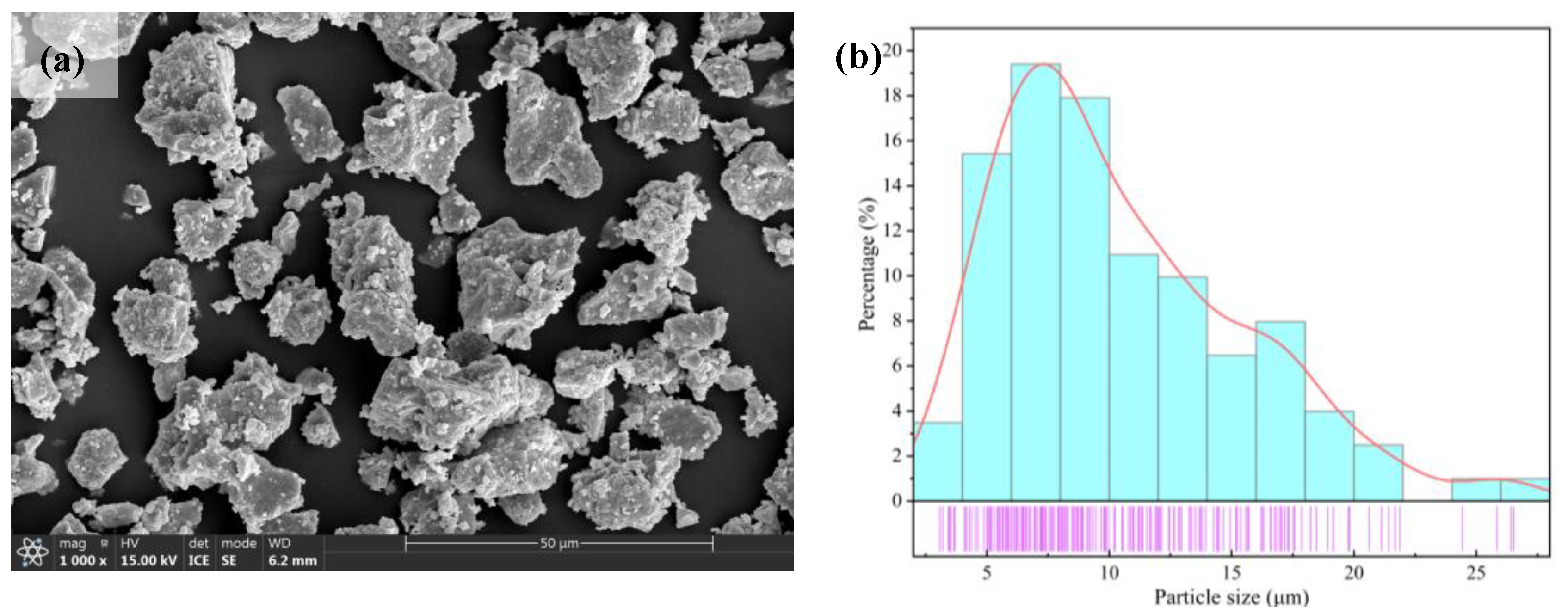
3.1. Phase Composition and Morphology
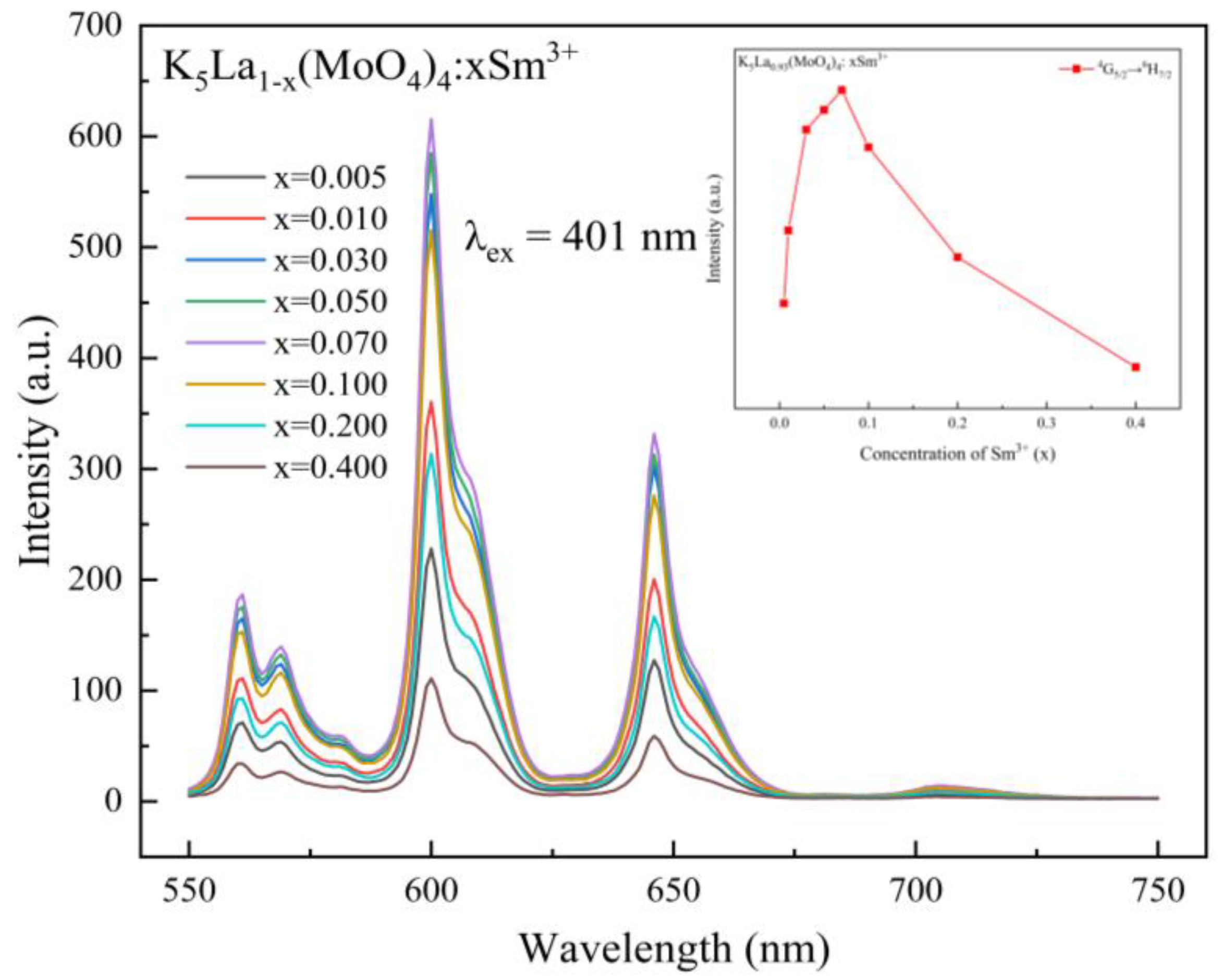
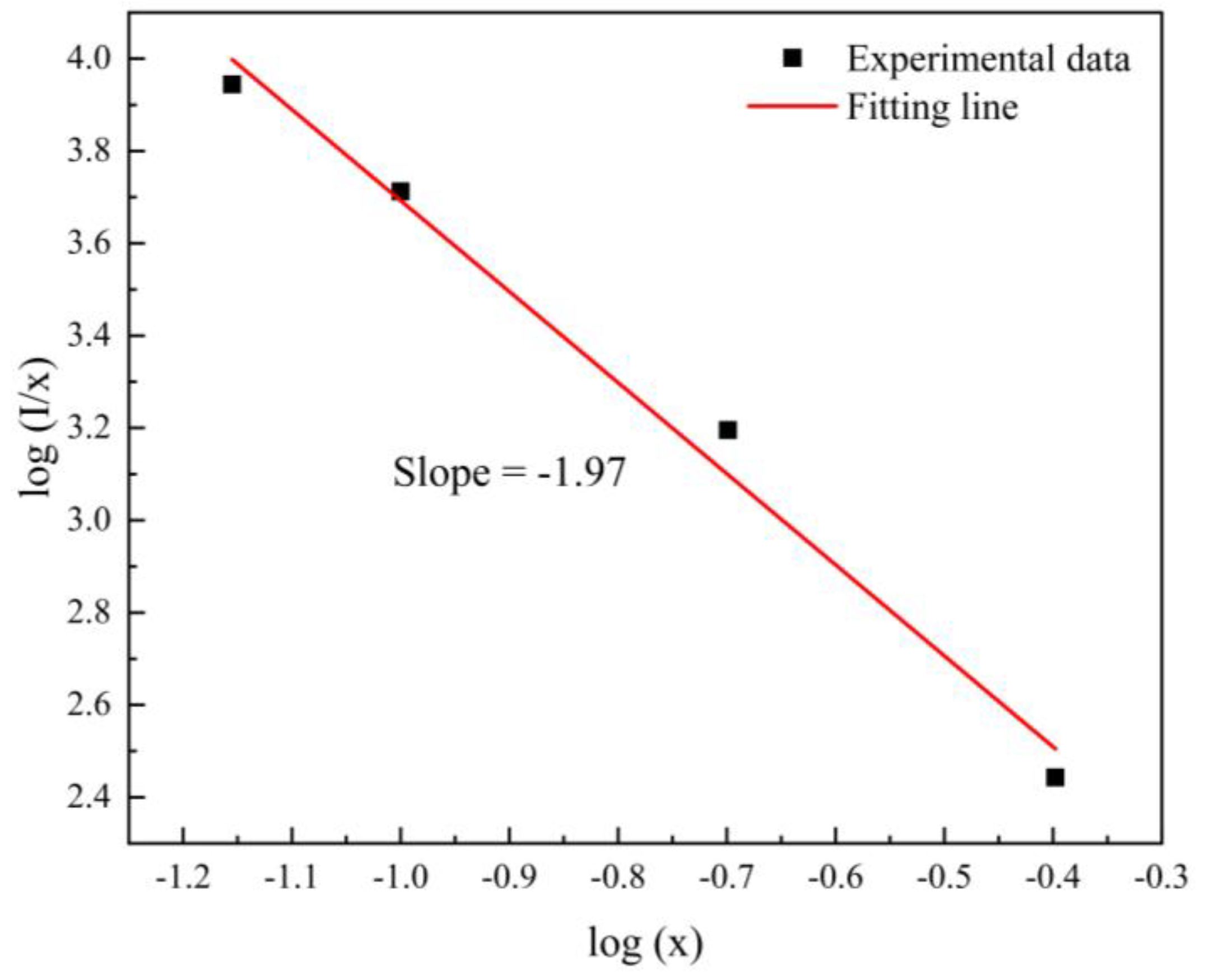
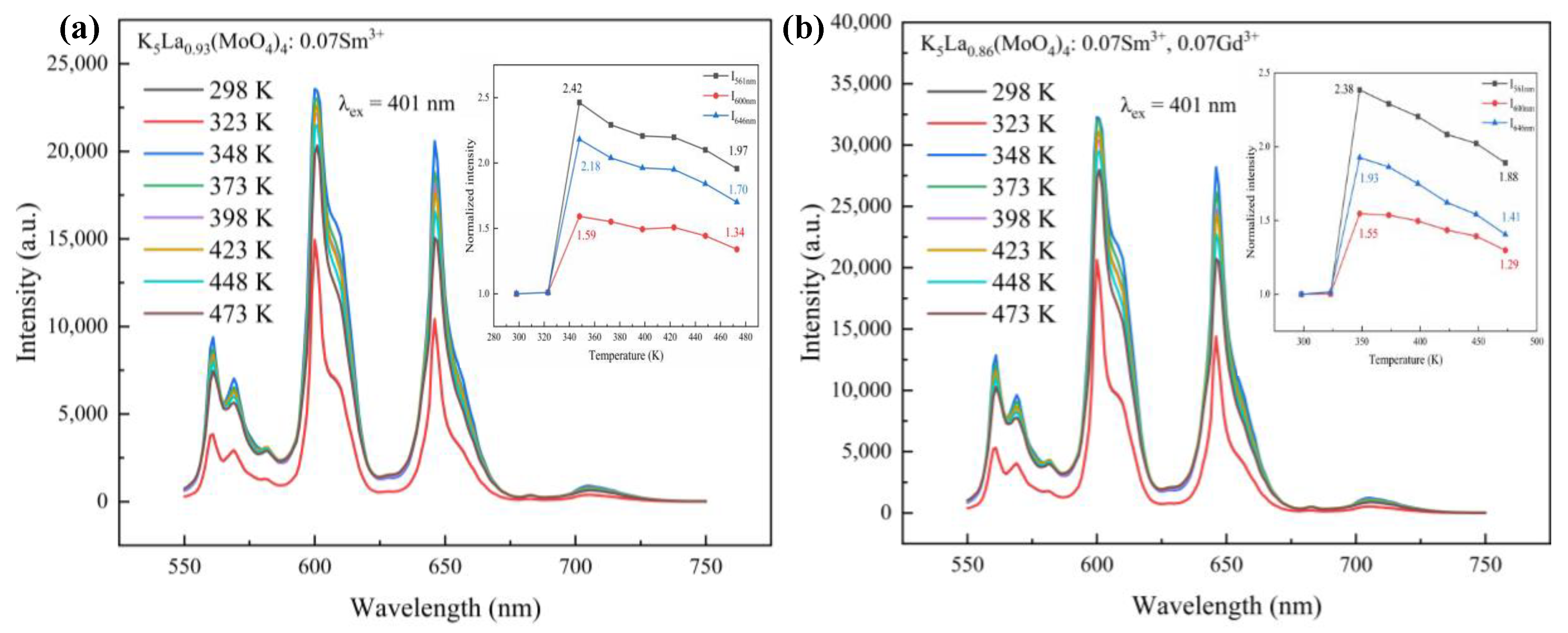
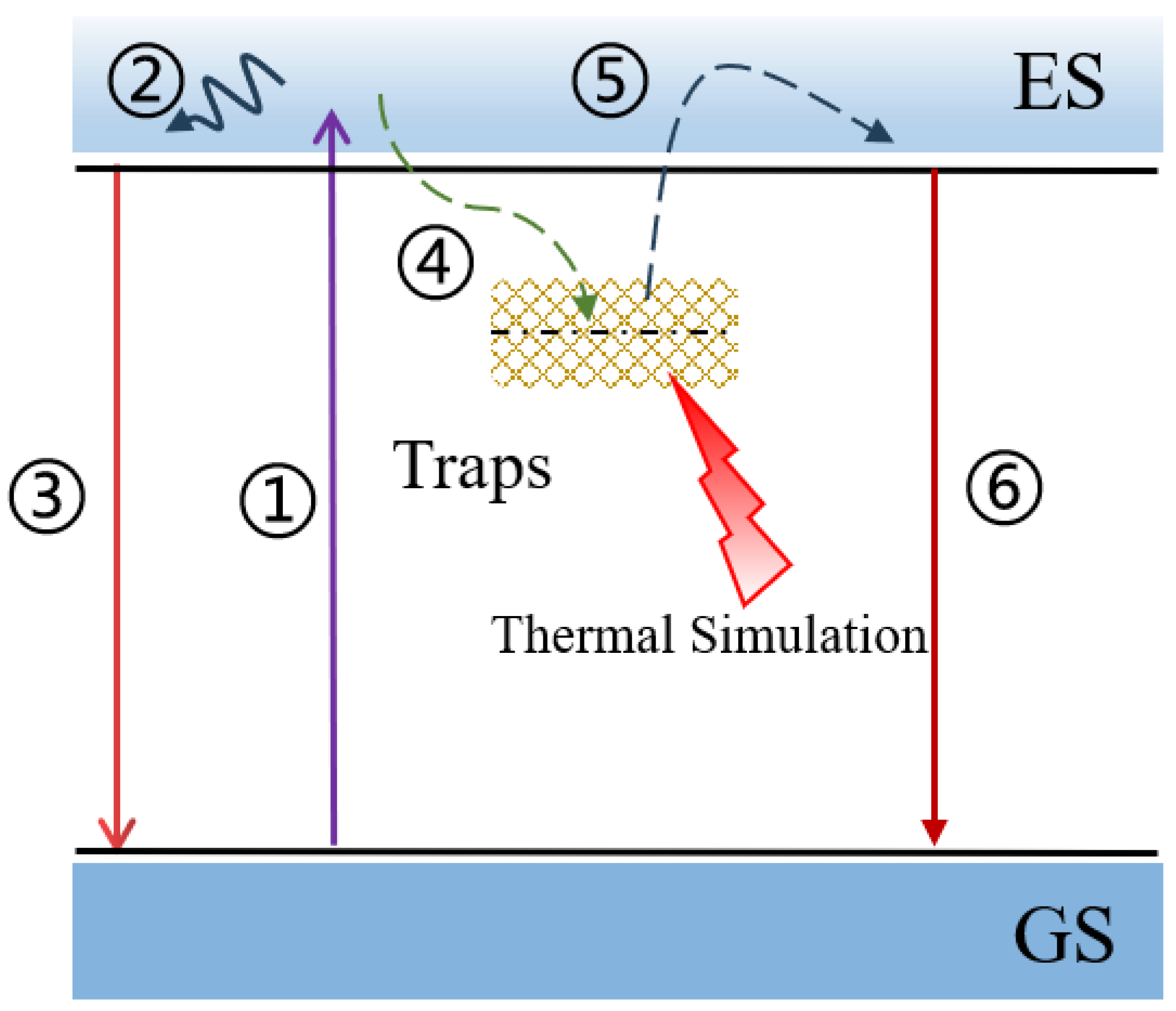
3.2. Fluorescence Property
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fang, M.-H.; Leaño, J.L., Jr.; Liu, R.-S. Control of Narrow-Band Emission in Phosphor Materials for Application in Light-Emitting Diodes. ACS Energy Lett. 2018, 3, 2573–2586. [Google Scholar] [CrossRef]
- Wang, L.; Xie, R.J.; Suehiro, T.; Takeda, T.; Hirosaki, N. Down-Conversion Nitride Materials for Solid State Lighting: Recent Advances and Perspectives. Chem. Rev. 2018, 118, 1951–2009. [Google Scholar] [CrossRef]
- Ye, S.; Xiao, F.; Pan, Y.X.; Ma, Y.Y.; Zhang, Q.Y. Phosphors in phosphor-converted white light-emitting diodes Recent advances in materials, techniques and properties. Mater. Sci. Eng. R-Rep. 2010, 71, 1–34. [Google Scholar] [CrossRef]
- Qiao, J.W.; Zhao, J.; Liu, Q.L.; Xia, Z.G. Recent advances in solid-state LED phosphors with thermally stable luminescence. J. Rare Earths 2019, 37, 565–572. [Google Scholar] [CrossRef]
- Schubert, E.F.; Kim, J.K. Solid-state light sources getting smart. Science 2005, 308, 1274–1278. [Google Scholar] [CrossRef]
- Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Prospects for LED lighting. Nat. Photonics 2009, 3, 179–181. [Google Scholar] [CrossRef]
- Lin, C.C.; Liu, R.S. Advances in Phosphors for Light-emitting Diodes. J. Phys. Chem. Lett. 2011, 2, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Nathan, M.I. The blue laser diode. GaN based light emitters and lasers. Science 1997, 277, 46–47. [Google Scholar]
- Bachmann, V.; Ronda, C.; Meijerink, A. Temperature Quenching of Yellow Ce3+ Luminescence in YAG:Ce. Chem. Mater. 2009, 21, 2077–2084. [Google Scholar] [CrossRef]
- McKittrick, J.; Shea-Rohwer, L.E. Review: Down Conversion Materials for Solid-State Lighting. J. Am. Ceram. Soc. 2014, 97, 1327–1352. [Google Scholar] [CrossRef]
- Fan, N.S.; Du, Q.; Guo, R.; Luo, L.; Wang, L. Sol-Gel Synthesis and Photoluminescence Properties of a Far-Red Emitting Phosphor BaLaMgTaO6:Mn4+ for Plant Growth LEDs. Materials 2023, 16, 4029. [Google Scholar] [CrossRef]
- Li, Z.; Cheng, J.L.; Wang, Y.N.; Wu, K.Y.; Cao, J. Rapid synthesis of BaMoO4:Eu3+ red phosphors using microwave irradiation. Luminescence 2023, 38, 1414–1421. [Google Scholar] [CrossRef]
- Wang, D.; Guan, X.F.; Li, G.F. A novel scheelite-type LiCaGd(WO4)3:Eu3+ red phosphors with prominent thermal stability and high quantum efficiency. Int. J. Appl. Ceram. Technol. 2023, 20, 3171–3182. [Google Scholar] [CrossRef]
- Sun, G.H.; Chen, Q.L. Novel red-emitting Ca2LiScB4O10: Sm3+phosphor for WLED: Photoluminescence, thermal stability, crystal structure, Judd-Ofelt parameters and energy band gap studies. J. Alloys Compd. 2023, 936, 168263. [Google Scholar] [CrossRef]
- Feng, N.N.; Zhang, G.Q.; Bai, S.W.; Lu, D.Z.; Cao, D.Z.; Wu, G.; Han, X.Z.; Wang, F. Sm3+doped Ca3Y(AlO)3(BO3)4: A near-UV pumped orange-red phosphor with high thermal stability. Optik 2023, 275, 170554. [Google Scholar] [CrossRef]
- Pradhan, M.K.; Dash, S. Insights into structural and spectroscopic characterization of Sm3+ doped orange rich red emitting CsMgPO4 phosphors. J. Rare Earths 2022, 40, 1837–1848. [Google Scholar] [CrossRef]
- Qin, Z.Y.; Dong, L.P.; Zhang, G.H.; Liu, Y.F.; Zhao, G.Y.; Fang, Y.Z.; Hou, J.S. Hetero-valent substitution design of high thermal stability reddish-orange Sr3Ga2Sn1.5Si2.5O14: Sm3+phosphor for healthy lighting white-light-emitting-diodes applications. Opt. Mater. 2022, 131, 112640. [Google Scholar] [CrossRef]
- Tian, X.Y.; Wang, C.C.; Wen, J.; Lian, S.X.; Ji, C.Y.; Huang, Z.; Chen, Z.J.; Peng, H.X.; Wang, S.M.; Li, J.; et al. High temperature sensitivity phosphor based on an old material: Red emitting H3BO3 flux assisted CaTiO3: Pr3+. J. Lumin. 2019, 214, 116528. [Google Scholar] [CrossRef]
- Xu, D.; Yu, P.L.; Tian, L.H. Luminescence properties of Pr3+ and Bi3+ co-doped NaCaTiNbO6 phosphor for red-LEDs. J. Rare Earths 2018, 36, 243–247. [Google Scholar] [CrossRef]
- Ma, Q.C.; Liu, Q.Y.; Wu, M.H.; Liu, Y.F.; Wang, R.; Zhou, R.X.; Xu, Y.H.; Wei, H.C.; Mi, R.Y.; Min, X.; et al. Eu3+-doped La2(MoO4)3 phosphor for achieving accurate temperature measurement and non-contact optical thermometers. Ceram. Int. 2023, 49, 8204–8211. [Google Scholar] [CrossRef]
- Krishnan, R.; Kroon, R.E.; Swart, H.C. Charge transfer characteristics and luminescence properties of Eu3+ activated Ba2YMoO6 and BaY2(MoO4)4 phosphors. Mater. Res. Bull. 2022, 145, 111554. [Google Scholar] [CrossRef]
- Lin, Y.; He, D.; Jiang, K.; Qin, R.; Wang, J.; Shao, Y.; Yan, J.; Yu, R.; Zhang, D.; Xie, S.; et al. A novel red-emitting K5La(MoO4)4:Eu3+ phosphor with a high quantum efficiency for w-LEDs and visualization of latent fingerprints. J. Alloys Compd. 2023, 960, 170563. [Google Scholar] [CrossRef]
- Huang, D.Y.; Ouyang, Q.Y.; Liu, B.; Chen, B.K.; Wang, Y.T.; Yuan, C.G.; Xiao, H.; Lian, H.Z.; Lin, J. Mn2+/Mn4+ co-doped LaM1-xAl11-yO19 (M = Mg, Zn) luminescent materials: Electronic structure, energy transfer and optical thermometric properties. Dalton Trans. 2021, 50, 4651–4662. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, D.C.; Gao, W.; Han, T.; Peng, L.L.; Tu, M.J. Effect of doping Gd3+ on crystal structure and luminescent properties of Sr2SiO4:Eu2+ phosphor. J. Rare Earths 2015, 33, 693–699. [Google Scholar] [CrossRef]
- Zhao, C.; Zhu, D.C.; Pu, Y.; Peng, L.L.; Han, T.; Tu, M.J. The effect of doping La3+ on structure and luminescent properties of Sr2SiO4:Eu2+ phosphors. Ceram. Int. 2015, 41, 13341–13347. [Google Scholar] [CrossRef]
- Jose, T.A.; Krishnapriya, T.; Jose, A.; C S, R.; Joseph, C.; Biju, P.R. A study on structural and luminescence properties of novel orange-red emitting Sm3+ doped Sr3LiSbO6 nanorod phosphor with high color purity for W-LEDs. J Alloy Compd 2023, 963, 171104. [Google Scholar] [CrossRef]
- Lei, B.F.; Li, B.; Zhang, H.R.; Li, W.L. Preparation and luminescence properties of CaSnO3: Sm3+ phosphor emitting in the reddish orange region. Opt. Mater. 2007, 29, 1491–1494. [Google Scholar] [CrossRef]
- Niu, P.; Liu, X.; Xie, Z.; Zhao, W. Photoluminescence properties of a novel red-emitting nanowire phosphor Ba6Gd2W3O18:Eu3+. J. Lumin. 2018, 198, 34–39. [Google Scholar] [CrossRef]
- Hooda, A.; Khatkar, S.P.; Khatkar, A.; Malik, R.K.; Kumar, M.; Devi, S.; Taxak, V.B. Reddish-orange light emission via combustion synthesized Ba3Y4O9: Sm3+ nanocrystalline phosphor upon near ultraviolet excitation. J. Lumin. 2020, 217, 116806. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in oxidic phosphors. Phys. Lett. A 1968, 28, 444–445. [Google Scholar] [CrossRef]
- Blasse, G. Energy transfer in oxidic phosphors. Philips Res. Rep. 1969, 24, 131. [Google Scholar] [CrossRef]
- Li, K.; Chen, D.; Xu, J.; Zhang, R.; Yu, Y.; Wang, Y. Phase transition and multicolor luminescence of Eu2+/Mn2+-activated Ca3(PO4)2 phosphors. Mater. Res. Bull. 2014, 49, 677–681. [Google Scholar] [CrossRef]
- Dexter, D.L.; Schulman, J.H. Theory of concentration quenching in inorganic phosphors. J. Chem. Phys. 1954, 22, 1063–1070. [Google Scholar] [CrossRef]
- Dexter, D.L. A theory of sensitized luminescence in solids. J. Chem. Phys. 1953, 21, 836–850. [Google Scholar] [CrossRef]
- Chen, R. On the calculation of activation energies and frequency factors from glow curves. J. Appl. Phys. 1969, 40, 570–584. [Google Scholar] [CrossRef]
- Yang, C.G.; Liu, Y.G.; Yu, H.J.; Liu, Y.K.; Yang, J.Y.; Xie, C.A.; Wang, L.L.; Mi, R.Y. Using energy transfer to obtain warm white-emitting and thermally stable Ca3Y(AlO)3(BO3)4:Dy3+, Eu3+ phosphors. J. Lumin. 2023, 260, 119875. [Google Scholar] [CrossRef]
- Monika; Yadav, R.S.; Bahadur, A.; Rai, S.B. 37. Monika; Yadav, R.S.; Bahadur, A.; Rai, S.B. Multicolor tunable bright photoluminescence in Ca2+/Mg2+ modified Eu3+ doped ZnGa2O4 phosphors under UV excitation for solid state lighting applications. RSC Adv. 2023, 13, 20164–20178. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, J.; Liu, C.; Kuang, X.; Su, Q. A host sensitized reddish-orange Gd2MoO6:Sm3+ phosphor for light emitting diodes. Appl. Phys. Lett. 2011, 98, 081917. [Google Scholar] [CrossRef]
- Martin, J.L.B.; Reid, M.F.; Wells, J.-P.R. Dipole–Quadrupole interactions between Sm3+ ions in KY3F10 nanocrystals. J. Alloys Compd. 2023, 937, 168394. [Google Scholar] [CrossRef]
- Pang, R.; Li, C.Y.; Shi, L.L.; Su, Q. A novel blue-emitting long-lasting proyphosphate phosphor Sr2P2O7:Eu2+, Y3+. J. Phys. Chem. Solids 2009, 70, 303–306. [Google Scholar] [CrossRef]
- Chen, H.T.; Huang, X.F.; Huang, W.G. Effect of Mn doping on the electronic structure and absorption spectrum of Ba2SiO4:Eu2+ phosphor. Chin. J. Phys. 2016, 54, 931–939. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, W.T.; Wang, X.M.; Huang, X.; Du, H.Y.; Lin, J.H. Enhancing the photoluminescence performance of Ca5(PO4)2SiO4:Re3+ (Re = Eu, Sm) phosphors with A(3+) (A = La, Bi) codoping and white light-emitting diode application. Ceram. Int. 2022, 48, 13080–13089. [Google Scholar] [CrossRef]
- Yang, L.X.; Wang, J.S.; Zhu, D.C.; Pu, Y.; Zhao, C.; Han, T. The effect of doping Mg2+ on structure and properties of Sr1.992-xMgxSiO4: 0.008Eu2+ blue phosphor synthesized by co-precipitation method. Opt. Mater. 2018, 75, 887–892. [Google Scholar] [CrossRef]
- Ullah, I.; Sarumaha, C.S.; Angnanon, A.; Khan, I.; Shoaib, M.; Khattak, S.A.; Kothan, S.; Sangwaranatee, N.; Rooh, G.; Kaewkhao, J. Spectral features of Gd2O3 modified Sm3+doped lithium strontium borate glasses for solid-state laser applications. Ceram. Int. 2023, 49, 13774–13782. [Google Scholar] [CrossRef]
- Xu, C.L.; Strzep, A.; Zhang, Z.H.; Kou, H.M.; Ma, F.K.; Su, L.B. Growth and spectroscopic properties of CaxSr1-xF2: Sm: Gd single crystals. J. Lumin. 2022, 249, 119008. [Google Scholar] [CrossRef]
- Luo, L.; Liu, R.H.; Liu, Y.H.; Zhuang, W.D.; Li, Y.F.; Qin, S.W. Enhanced luminescence performances of Mn4+-activated Sr4Al14O25 red phosphors by doping with Sc3+ ions. J. Rare Earths 2021, 39, 380–385. [Google Scholar] [CrossRef]
- Hao, J.R.; Cao, L.; Wei, Y.; Yan, C.J.; Li, G.G. Improving photoluminescence and thermal stability of CaSi2O2N2:Eu2+ phosphors by codoping lanthanide ions (Ln = Sc, La, Gd, Yb, Lu). Mater. Lett. 2018, 211, 122–125. [Google Scholar] [CrossRef]
- Gu, Y.X.; Zhang, Q.H.; Li, Y.G.; Wang, H.Z.; Xie, R.J. Enhanced emission from CaSi2O2N2:Eu2+ phosphors by doping with Y3+ ions. Mater. Lett. 2009, 63, 1448–1450. [Google Scholar] [CrossRef]
- Nanda, S.S.; Nayak, P.; Gupta, S.K.; Rawat, N.S.; Goutam, U.K.; Dash, S. Structural, optical spectroscopy and energy transfer features of Tb3+-activated (Y, Gd)F3 nanophosphors for UV-based LEDs. New J. Chem. 2022, 46, 15617–15627. [Google Scholar] [CrossRef]
- Ullah, I.; Rooh, G.; Khattak, S.A.; Khan, I.; Shoaib, M.; Kothan, S.; Srisittipokakun, N.; Kaewkhao, J. Spectral characteristics and energy transfer in Gd3+and Nd3+ doped borate glasses for NIR laser applications. Infrared Phys. Technol. 2022, 125, 104272. [Google Scholar] [CrossRef]
- Lv, P.; Fang, L.Z.; Xia, H.P.; Song, H.W.; Chen, B.J. The effect of GdF3 on red emission of Eu3+: LiYF4 single crystals grown by the Bridgman method. J. Mater. Sci.-Mater. Electron. 2022, 33, 21628–21637. [Google Scholar] [CrossRef]
- Kumar, K.N.; Vijayalakshmi, L.; Kim, J.S. Enhanced red luminescence quantum yield from Gd3+/Eu3+: CaLa2ZnO5 phosphor spheres for photonic applications. Mater. Res. Bull. 2018, 103, 234–241. [Google Scholar] [CrossRef]
- Zhou, L.; Tanner, P.A.; Ning, L.; Zhou, W.; Liang, H.; Zheng, L. Spectral Properties and Energy Transfer between Ce3+ and Yb3+ in the Ca3Sc2Si3O12 Host: Is It an Electron Transfer Mechanism? J. Phys. Chem. A 2016, 120, 5539–5548. [Google Scholar] [CrossRef]
- Qin, X.; Liu, X.W.; Huang, W.; Bettinelli, M.; Liu, X.G. Lanthanide-Activated Phosphors Based on 4f-5d Optical Transitions: Theoretical and Experimental Aspects. Chem. Rev. 2017, 117, 4488–4527. [Google Scholar] [CrossRef]
- Fu, A.J.; Pang, Q.; Yang, H.; Zhou, L.Y. Ba2YNbO6:Mn4+-based red phosphor for warm white light-emitting diodes (WLEDs): Photoluminescent and thermal characteristics. Opt. Mater. 2017, 70, 144–152. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, S.; Jiang, G.; Chen, Y.; Duan, C.; Yin, M. Temperature dependent luminescence of Dy3+ doped BaYF5 nanoparticles for optical thermometry. Curr. Appl. Phys. 2014, 14, 1067–1071. [Google Scholar] [CrossRef]
- Liu, S.F.; Ming, H.; Cui, J.; Liu, S.B.; You, W.X.; Ye, X.Y.; Yang, Y.M.; Nie, H.P.; Wang, R.X. Color-Tunable Upconversion Luminescence and Multiple Temperature Sensing and Optical Heating Properties of Ba3Y4O9:Er3+/Yb3+ Phosphors. J. Phys. Chem. C 2018, 122, 16289–16303. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, M.; Chen, Z.; Gao, P.X.; Hintzen, H.T.; Wong, W.Y.; Wang, J.; Zhou, Z. Pyrophosphate Phosphor Solid Solution with High Quantum Efficiency and Thermal Stability for Efficient LED Lighting. Iscience 2020, 23, 100892. [Google Scholar] [CrossRef]
- Qian, Y.; Zhu, D.; Pu, Y. A zero-thermal-quenching phosphor Sr3La(AlO)3(BO3)4: Dy3+ for near ultraviolet excitation white-LEDs. J. Lumin. 2022, 243, 118610. [Google Scholar] [CrossRef]
- Garlick, G.F.J.; Gibson, A.F. The Electron Trap Mechanism of Luminescence in Sulphide and Silicate Phosphors. Proc. Phys. Soc. Lond. 1948, 60, 574–590. [Google Scholar] [CrossRef]
- Geng, X.; Xie, Y.; Chen, S.N.; Luo, J.M.; Li, S.C.; Wang, T.; Zhao, S.C.; Wang, H.; Deng, B.; Yu, R.J.; et al. Enhanced local symmetry achieved zero-thermal-quenching luminescence characteristic in the Ca2InSbO6:Sm3+ phosphors for w-LEDs. Chem. Eng. J. 2021, 410, 128396. [Google Scholar] [CrossRef]
- Van den Eeckhout, K.; Smet, P.F.; Poelman, D. Persistent Luminescence in Eu2+-Doped Compounds: A Review. Materials 2010, 3, 2536–2566. [Google Scholar] [CrossRef]
- Miao, S.H.; Liang, Y.J.; Shi, R.Q.; Wang, W.L.; Li, Y.F.; Wang, X.J. Broadband Short-Wave Infrared-Emitting MgGa2O4:Cr3+, Ni2+ Phosphor with Near-Unity Internal Quantum Efficiency and High Thermal Stability for Light-Emitting Diode Applications. Acs Appl. Mater. Interfaces 2023, 15, 32580–32588. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Tian, J.H.; Zhuang, W.D. Near-Infrared LuCa2ScZrGa2GeO12:Cr3+ Garnet Phosphor with Ultra-broadband Emission for NIR LED Applications. Inorg. Chem. 2023, 62, 10772–10779. [Google Scholar] [CrossRef]


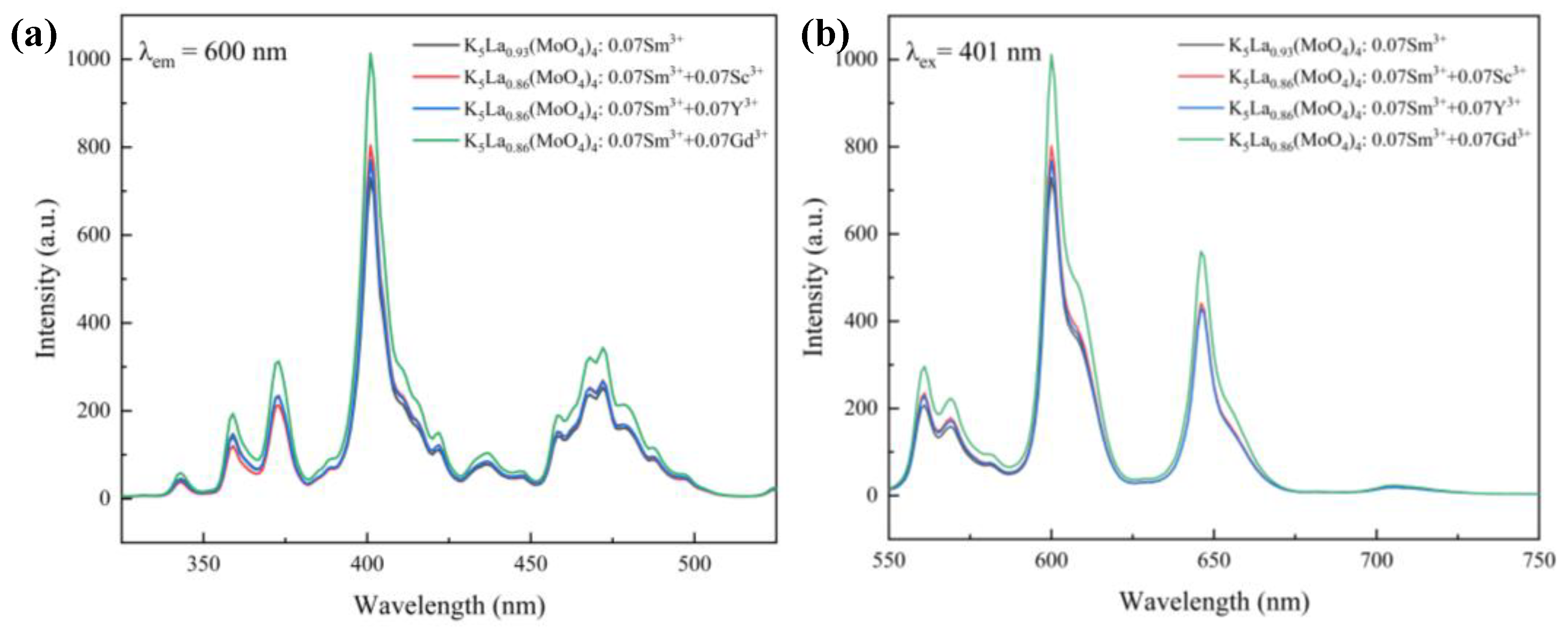
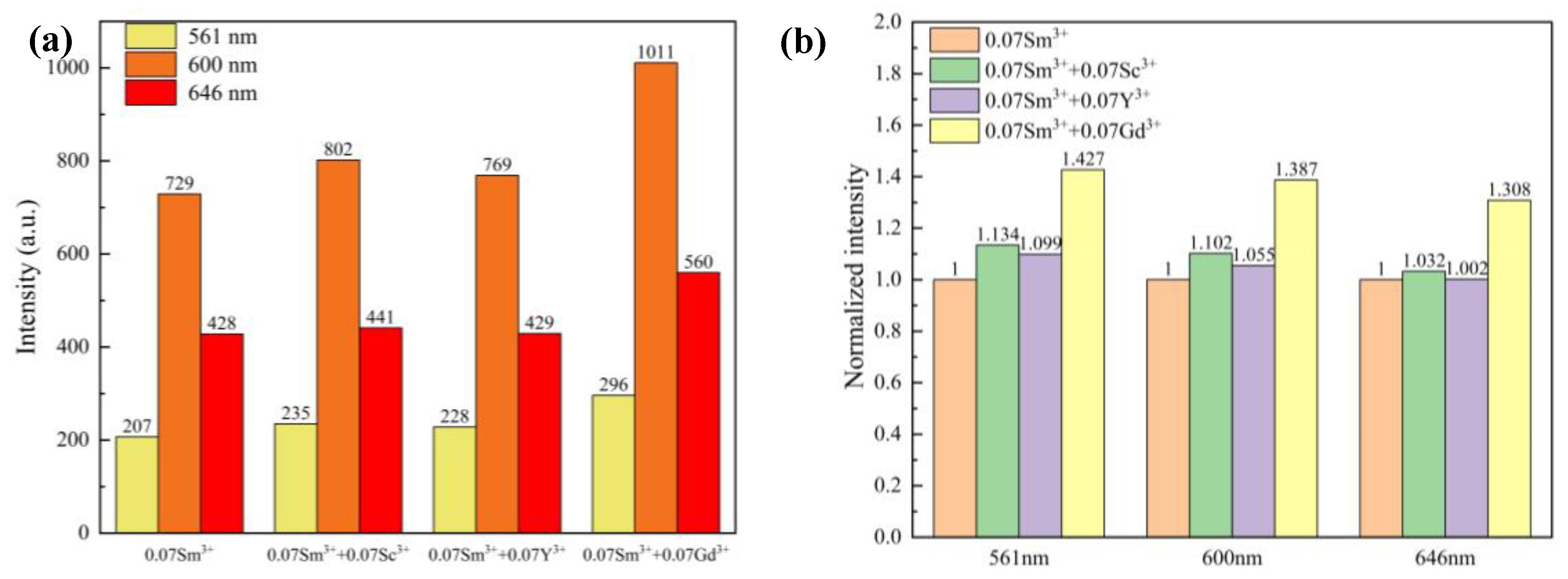
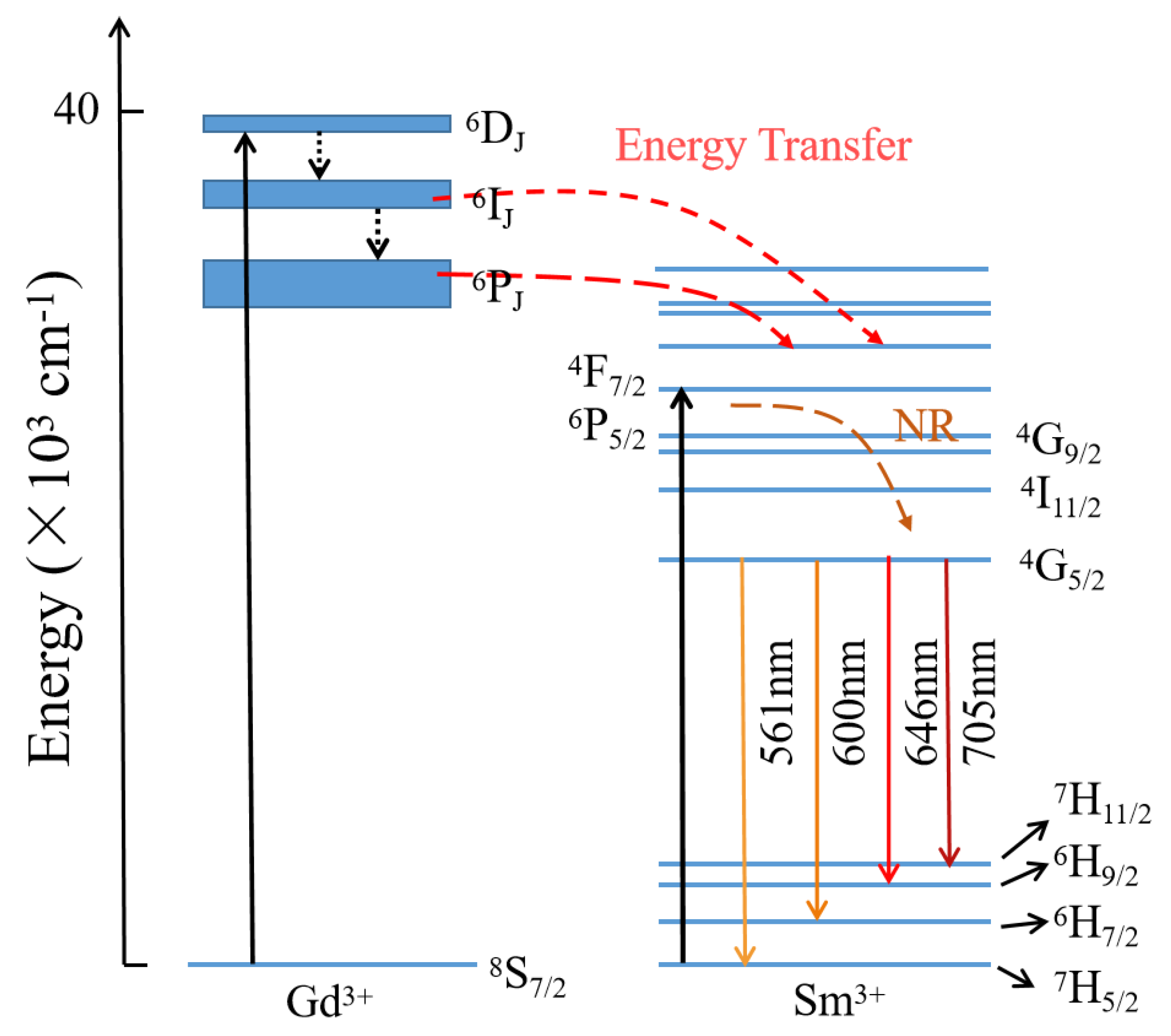
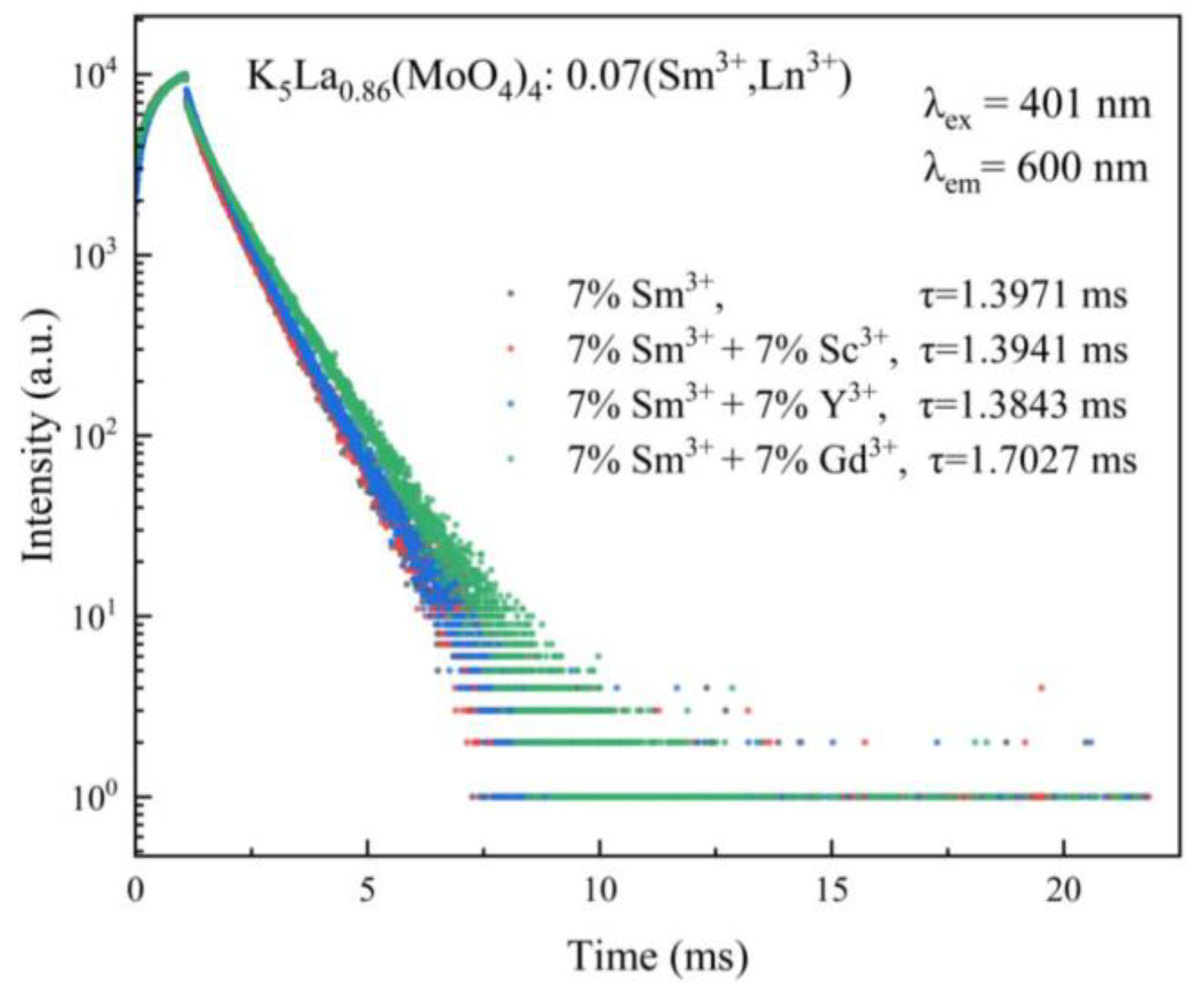




| Label | Elem | Mult | x | y | z | Frac |
|---|---|---|---|---|---|---|
| K1 | K + 1 | 3 | 0 | 0 | 0 | 0.418 |
| La1 | La + 3 | 3 | 0 | 0 | 0 | 0.495 |
| Mo1 | Mo + 6 | 6 | 0 | 0 | 0.400674 | 0.983 |
| K2 | K + 1 | 6 | 0 | 0 | 0.195013 | 1 |
| O1 | O − 2 | 18 | −0.043738 | 0.043738 | 0.318691 | 0.262 |
| O2 | O − 2 | 36 | −0.1855 | 0.1509 | 0.4173 | 0.13 |
| O3 | O − 2 | 18 | −0.144455 | 0.144455 | 0.429581 | 0.569 |
| Cation | Radius (Å) (CN = 6) | Dr (%) |
|---|---|---|
| La3+ | 1.032 | \ |
| Sm3+ | 0.958 | 7.17 |
| Sc3+ | 0.745 | 27.8 |
| Y3+ | 0.900 | 12.8 |
| Gd3+ | 0.938 | 9.11 |
| Number | x | CIE (xc, yc) | CCT (K) | Color Purity (%) |
|---|---|---|---|---|
| 1 | 0.005 | (0.5880, 0.4113) | 1764 | 99.97 |
| 2 | 0.01 | (0.5888, 0.4105) | 1761 | 99.97 |
| 3 | 0.03 | (0.5895, 0.4098) | 1759 | 99.97 |
| 4 | 0.05 | (0.5896, 0.4097) | 1759 | 99.97 |
| 5 | 0.07 | (0.5898, 0.4095) | 1758 | 99.97 |
| 6 | 0.10 | (0.5899, 0.4094) | 1758 | 99.97 |
| 7 | 0.20 | (0.5894, 0.4099) | 1759 | 99.97 |
| 8 | 0.40 | (0.5870, 0.4123) | 1767 | 99.97 |
| Number | Dopant | CIE (xc, yc) | CCT (K) | Color Purity (%) |
|---|---|---|---|---|
| 1 | 0.07Sm3+ | (0.5898, 0.4095) | 1758 | 99.97 |
| 2 | 0.07Sm3+ + 0.07Sc3+ | (0.5922, 0.4071) | 1753 | 99.97 |
| 3 | 0.07Sm3+ + 0.07Y3+ | (0.5919, 0.4074) | 1754 | 99.97 |
| 4 | 0.07Sm3+ + 0.07Gd3+ | (0.5924, 0.4069) | 1753 | 99.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, T.; Zhang, S.; Zhu, D. A Novel Zero-Thermal-Quenching Red Phosphor with High Quantum Efficiency and Color Purity. Inorganics 2023, 11, 406. https://doi.org/10.3390/inorganics11100406
Zhao T, Zhang S, Zhu D. A Novel Zero-Thermal-Quenching Red Phosphor with High Quantum Efficiency and Color Purity. Inorganics. 2023; 11(10):406. https://doi.org/10.3390/inorganics11100406
Chicago/Turabian StyleZhao, Tianyang, Shiqi Zhang, and Dachuan Zhu. 2023. "A Novel Zero-Thermal-Quenching Red Phosphor with High Quantum Efficiency and Color Purity" Inorganics 11, no. 10: 406. https://doi.org/10.3390/inorganics11100406
APA StyleZhao, T., Zhang, S., & Zhu, D. (2023). A Novel Zero-Thermal-Quenching Red Phosphor with High Quantum Efficiency and Color Purity. Inorganics, 11(10), 406. https://doi.org/10.3390/inorganics11100406






