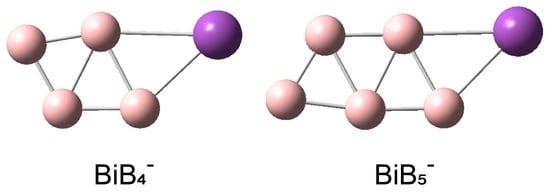
The Structures and Bonding of Bismuth-Doped Boron Clusters: BiB4− and BiB5−
Abstract
:1. Introduction
2. Experimental and Theoretical Methods
2.1. Photoelectron Spectroscopy
2.2. Theoretical Methods
3. Results
3.1. Experimental Results
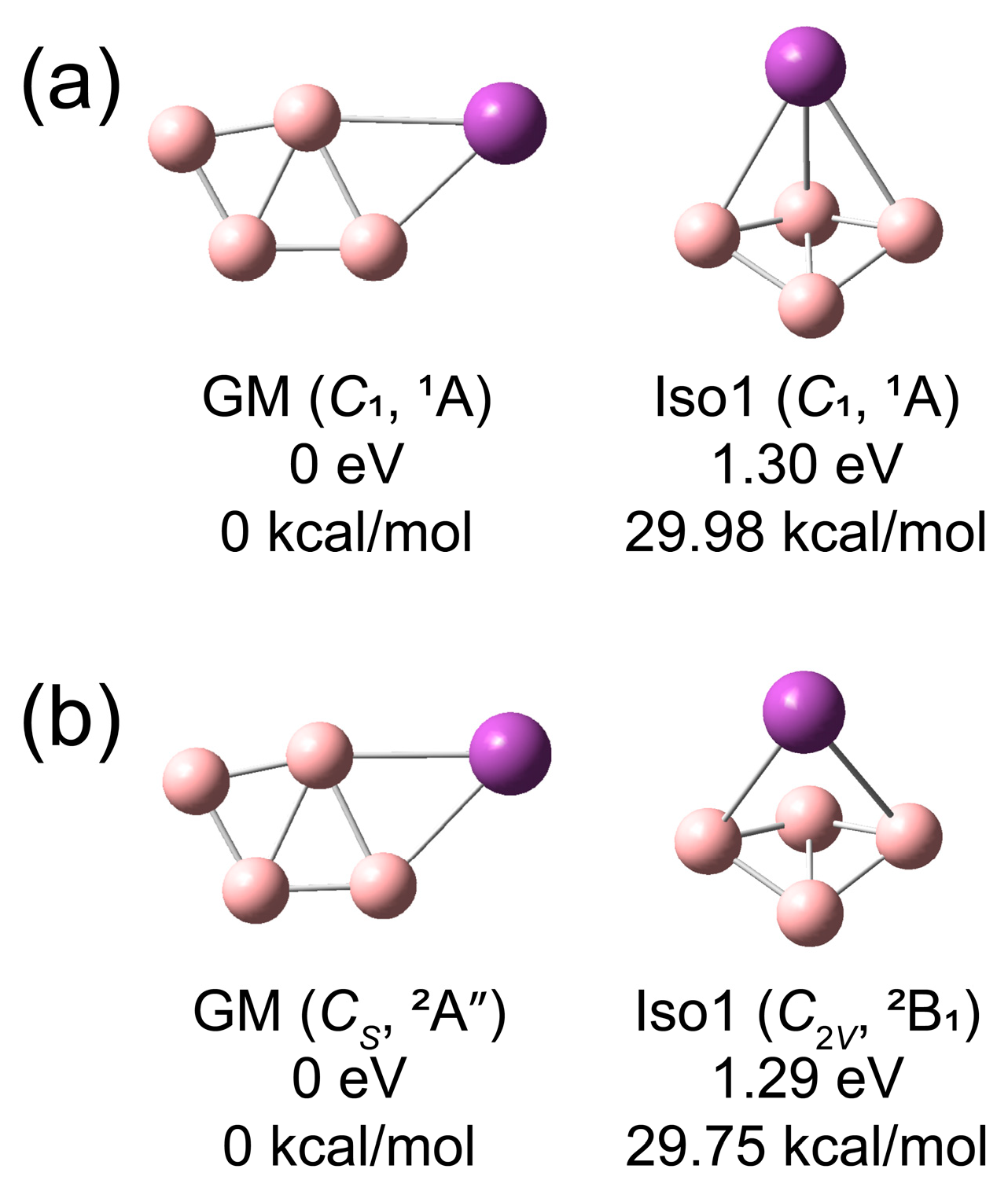
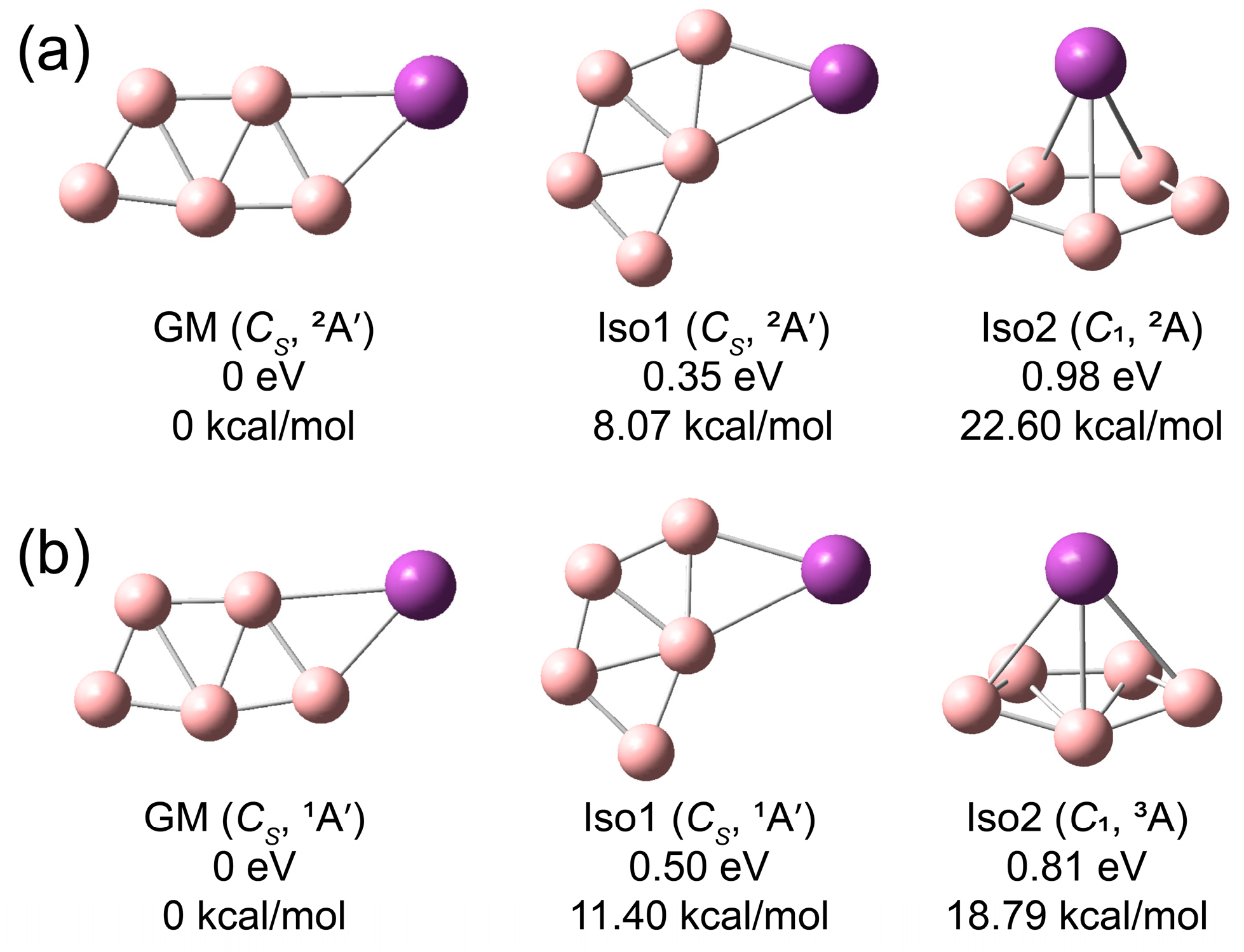
3.2. Theoretical Results
4. Discussion
4.1. Comparison between Experiment and Theory
4.1.1. BiB4−
4.1.2. BiB5−
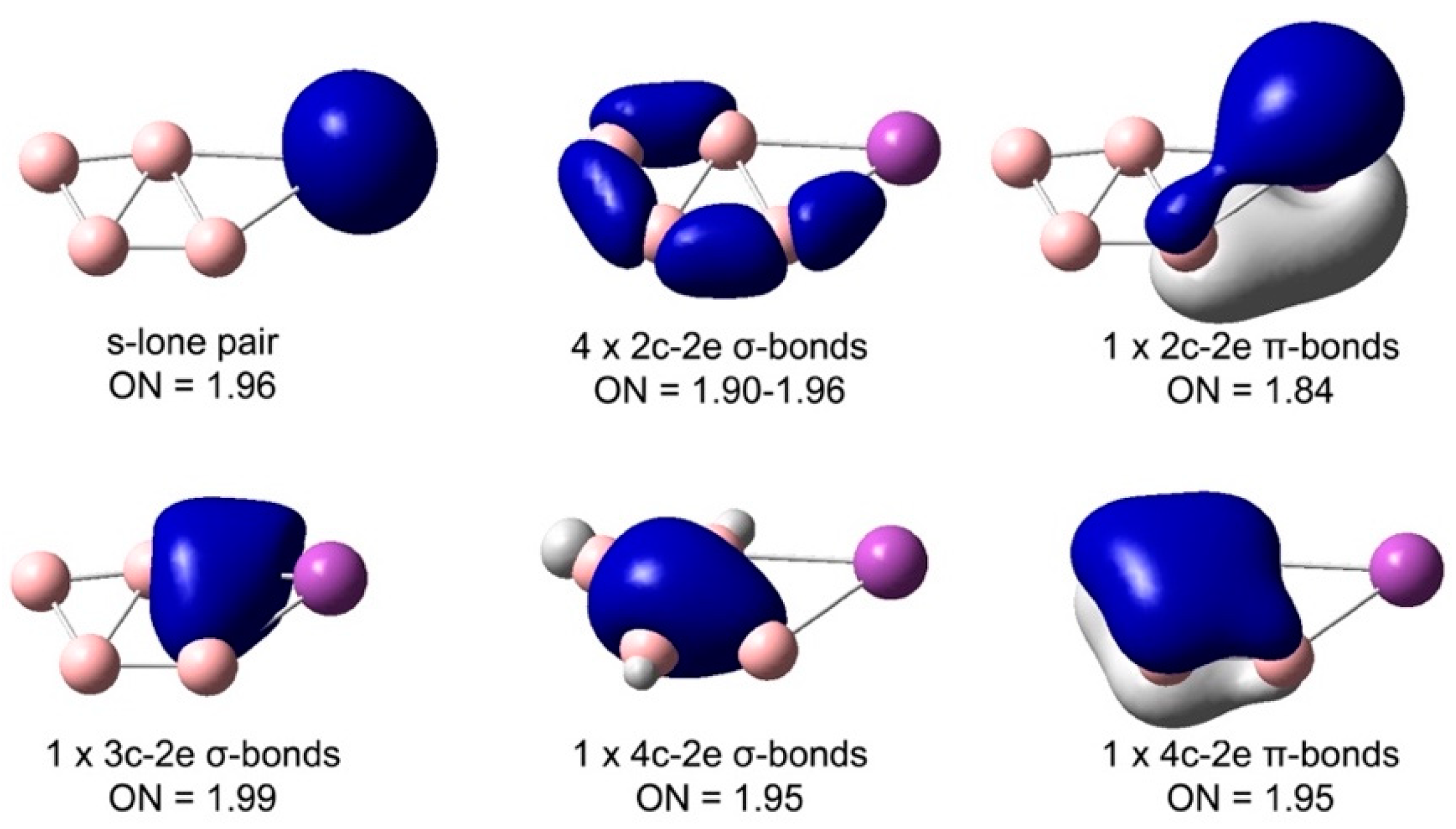
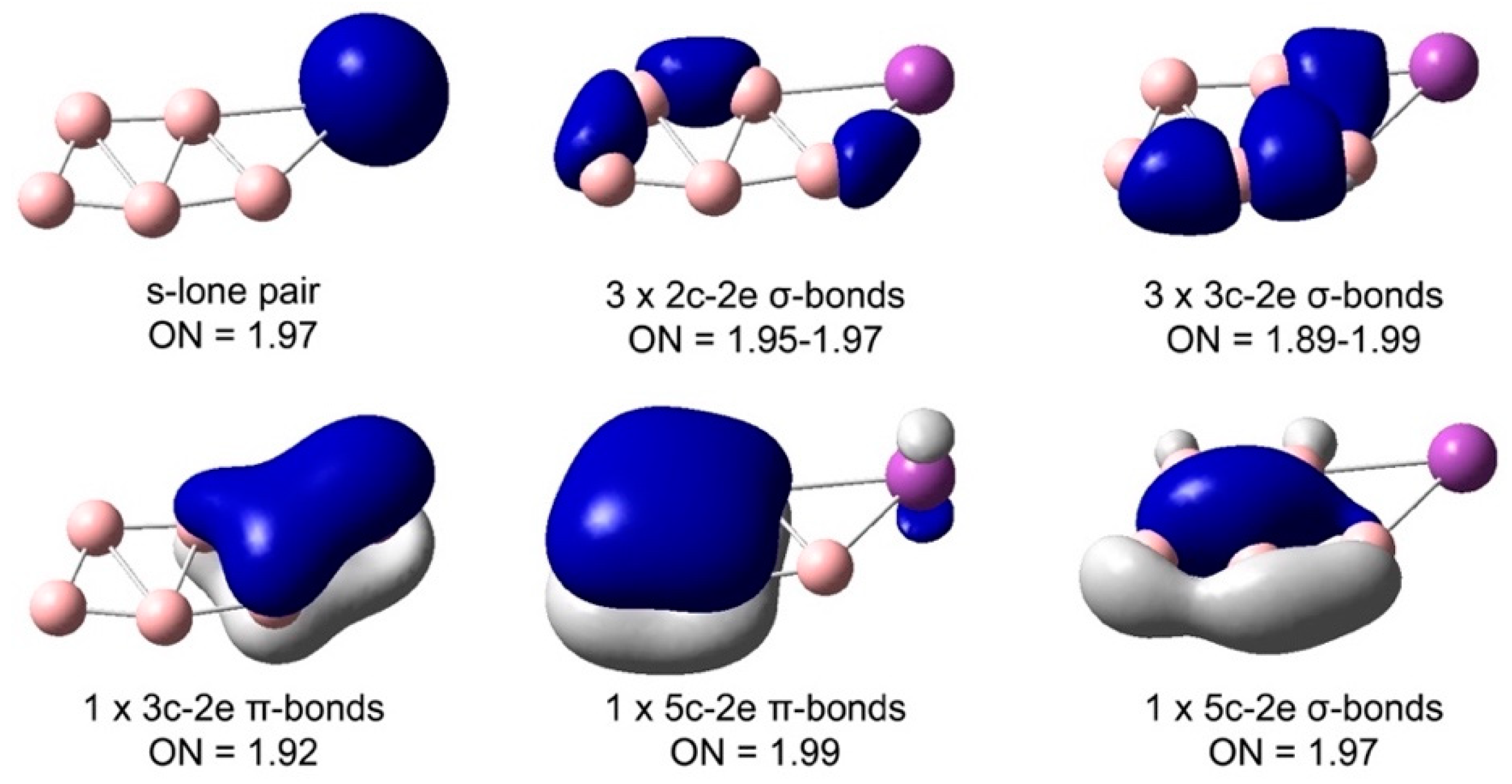
4.2. Chemical Bonding in the Bismuth–Boron Clusters
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipscomb, W.N. The boranes and their relatives. Science 1977, 196, 1047–1055. [Google Scholar] [CrossRef]
- Jemmis, E.D.; Prasad, D.L. Icosahedral B12, macropolyhedral boranes, β-rhombohedral boron and boron-rich solids. J. Solid State Chem. 2006, 179, 2768–2774. [Google Scholar] [CrossRef]
- Albert, B.; Hillebrecht, H. Boron: Elementary challenge for experimenters and theoreticians. Angew. Chem. Int. Ed. 2009, 48, 8640–8668. [Google Scholar] [CrossRef]
- Oger, E.; Crawford, N.R.; Kelting, R.; Weis, P.; Kappes, M.M.; Ahlrichs, R. Boron cluster cations: Transition from planar to cylindrical structures. Angew. Chem. Int. Ed. 2007, 46, 8503–8506. [Google Scholar] [CrossRef]
- Wang, L.S. Photoelectron spectroscopy of size-selected boron clusters: From planar structures to borophenes and borospherenes. Int. Rev. Phys. Chem. 2016, 35, 69–142. [Google Scholar] [CrossRef]
- Jian, T.; Chen, X.; Li, S.-D.; Boldyrev, A.I.; Li, J.; Wang, L.S. Probing the structures and bonding of size-selected boron and doped-boron clusters. Chem. Soc. Rev. 2019, 48, 3550–3591. [Google Scholar] [CrossRef]
- Pan, S.; Barroso, J.; Jalife, S.; Heine, T.; Asmis, K.R.; Merino, G. Fluxional boron clusters: From theory to reality. Acc. Chem. Res. 2019, 52, 2732–2744. [Google Scholar] [CrossRef]
- Piazza, Z.A.; Hu, H.S.; Li, W.L.; Zhao, Y.F.; Li, J.; Wang, L.S. Planar hexagonal B36 as a potential basis for extended single-atom layer boron sheets. Nat. Commun. 2014, 5, 3113. [Google Scholar] [CrossRef]
- Mannix, A.J.; Zhou, X.-F.; Kiraly, B.; Wood, J.D.; Alducin, D.; Myers, B.D.; Liu, X.; Fisher, B.L.; Santiago, U.; Guest, J.R.; et al. Synthesis of borophenes: Anisotropic, two-dimensional boron polymorphs. Science 2015, 350, 1513–1516. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, J.; Zhong, Q.; Li, W.; Li, S.; Li, H.; Cheng, P.; Meng, S.; Chen, L.; Wu, K. Experimental realization of two-dimensional boron sheets. Nat. Chem. 2016, 8, 563–568. [Google Scholar] [CrossRef]
- Xie, S.Y.; Wang, Y.; Li, X.B. Flat boron: A new cousin of graphene. Adv. Mater. 2019, 31, 1900392. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Benu, D.P.; Xu, X.; Yuliarto, B.; Yamauchi, Y.; Golberg, D. Borophene: Two-dimensional boron monolayers: Synthesis, properties, and potential applications. Chem. Rev. 2022, 122, 1000–1051. [Google Scholar] [CrossRef]
- Zhai, H.J.; Zhao, Y.F.; Li, W.L.; Chen, Q.; Bai, H.; Hu, H.S.; Piazza, Z.A.; Tian, W.J.; Lu, H.G.; Wu, Y.B.; et al. Observation of an all-boron fullerene. Nat. Chem. 2014, 6, 727–731. [Google Scholar] [CrossRef]
- Romanescu, C.; Galeev, T.R.; Li, W.L.; Boldyrev, A.I.; Wang, L.S. Transition-metal-centered monocyclic boron wheel clusters (M© Bn): A new class of aromatic borometallic compounds. Acc. Chem. Res. 2013, 46, 350–358. [Google Scholar] [CrossRef]
- Li, W.L.; Chen, X.; Jian, T.; Chen, T.T.; Li, J.; Wang, L.S. From planar boron clusters to borophenes and metalloborophenes. Nat. Rev. Chem. 2017, 1, 0071. [Google Scholar] [CrossRef]
- Barroso, J.; Pan, S.; Merino, G. Structural transformations in boron clusters induced by metal doping. Chem. Soc. Rev. 2022, 51, 1098–1123. [Google Scholar] [CrossRef]
- Lindsay, L.; Broido, D.; Reinecke, T. First-principles determination of ultrahigh thermal conductivity of boron arsenide: A competitor for diamond? Phys. Rev. Lett. 2013, 111, 025901. [Google Scholar] [CrossRef]
- Tian, F.; Song, B.; Chen, X.; Ravichandran, N.K.; Lv, Y.; Chen, K.; Sullivan, S.; Kim, J.; Zhou, Y.; Liu, T.H. Unusual high thermal conductivity in boron arsenide bulk crystals. Science 2018, 361, 582–585. [Google Scholar] [CrossRef]
- Shin, J.; Gamage, G.A.; Ding, Z.; Chen, K.; Tian, F.; Qian, X.; Zhou, J.; Lee, H.; Zhou, J.; Shi, L. High ambipolar mobility in cubic boron arsenide. Science 2022, 377, 437–440. [Google Scholar] [CrossRef]
- Khairul, I.; Wang, Q.Q.; Jiang, Y.H.; Wang, C.; Naranmandura, H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget 2017, 8, 23905. [Google Scholar] [CrossRef]
- Byeon, E.; Kang, H.-M.; Yoon, C.; Lee, J.S. Toxicity mechanisms of arsenic compounds in aquatic organisms. Aquat. Toxicol. 2021, 237, 105901. [Google Scholar] [CrossRef] [PubMed]
- Mohan, R. Green bismuth. Nat. Chem. 2010, 2, 336. [Google Scholar] [CrossRef] [PubMed]
- Ramler, J.; Lichtenberg, C. Bismuth species in the coordination sphere of transition metals: Synthesis, bonding, coordination chemistry, and reactivity of molecular complexes. Dalton Trans. 2021, 50, 7120–7138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Xu, Z.; Ye, H.; Yang, Z.; You, J.; Liu, M.; He, Y.; Kanatzidis, M.G.; Liu, S. Nucleation-controlled growth of superior lead-free perovskite Cs3Bi2I9 single-crystals for high-performance X-ray detection. Nat. Commun. 2020, 11, 2304. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.; Figueiredo, S.A.; Pinto, R.M.; Silvestre, S.M. Bismuth compounds in medicinal chemistry. Future Med. Chem. 2012, 4, 1495–1523. [Google Scholar] [CrossRef]
- Madouri, D.; Ferhat, M. How do electronic properties of conventional III–V semiconductors hold for the III–V boron bismuth BBi compound? Phys. Status Solidi B 2005, 242, 2856–2863. [Google Scholar] [CrossRef]
- Cui, S.; Feng, W.; Hu, H.; Feng, Z.; Wang, Y. First principles studies of phase stability, electronic and elastic properties in BBi compound. Comput. Mater. Sci. 2010, 47, 968–972. [Google Scholar] [CrossRef]
- Bagci, S.; Yalcin, B.G. Structural, mechanical, electronic and optical properties of BBi, BP and their ternary alloys BBi1−xPx. J. Phys. D Appl. Phys. 2015, 48, 475304. [Google Scholar] [CrossRef]
- Chen, W.J.; Kulichenko, M.; Choi, H.W.; Cavanagh, J.; Yuan, D.F.; Boldyrev, A.I.; Wang, L.S. Photoelectron spectroscopy of size-selected bismuth–boron clusters: BiBn– (n = 6–8). J. Phys. Chem. A 2021, 125, 6751–6760. [Google Scholar] [CrossRef]
- Jian, T.; Cheung, L.F.; Chen, T.T.; Wang, L.S. Bismuth–boron multiple bonding in BiB2O− and Bi2B−. Angew. Chem. Int. Ed. 2017, 56, 9551–9555. [Google Scholar] [CrossRef]
- Cheung, L.F.; Czekner, J.; Kocheril, G.S.; Wang, L.S. High resolution photoelectron imaging of boron-bismuth binary clusters: Bi2Bn− (n = 2–4). J. Chem. Phys. 2019, 150, 064304. [Google Scholar] [CrossRef]
- Gao, H.W.; Choi, H.W.; Hui, J.; Chen, W.J.; Kocheril, G.S.; Wang, L.S. On the electronic structure and spin-orbit coupling of BiB from photoelectron imaging of cryogenically-cooled BiB− anion. J. Chem. Phys. 2023, 159, 114301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the density functional ladder: Nonempirical meta–generalized gradient approximation designed for molecules and solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef]
- Peterson, K.A. Systematically convergent basis sets with relativistic pseudopotentials. I. Correlation consistent basis sets for the post-d group 13–15 elements. J. Chem. Phys. 2003, 119, 11099–11112. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H., Jr.; Harrison, R.J. Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of electronic excitations within the adiabatic approximation of time dependent density functional theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Bannwarth, C.; Grimme, S. A simplified time-dependent density functional theory approach for electronic ultraviolet and circular dichroism spectra of very large molecules. Comp. Theor. Chem. 2014, 1040–1041, 45–53. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Boldyrev, A.I. Developing paradigms of chemical bonding: Adaptive natural density partitioning. Phys. Chem. Chem. Phys. 2008, 10, 5207–5217. [Google Scholar] [CrossRef] [PubMed]
- Zubarev, D.Y.; Boldyrev, A.I. Revealing intuitively assessable chemical bonding patterns in organic aromatic molecules via adaptive natural density partitioning. J. Org. Chem. 2008, 73, 9251–9258. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Pyykkö, P. Relativistic effect in structural chemistry. Chem. Rev. 1988, 88, 563–594. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Boldyrev, A.I. Comprehensive analysis of chemical bonding in boron clusters. J. Comp. Chem. 2007, 28, 251–268. [Google Scholar] [CrossRef] [PubMed]
- Pyykkö, P. Additive covalent radii for single-, double-, and triple-bonded molecules and tetrahedrally bonded crystals: A summary. J. Phys. Chem. A 2015, 119, 2326–2337. [Google Scholar] [CrossRef] [PubMed]
- Cheung, L.F.; Kocheril, G.S.; Czekner, J.; Wang, L.S. Observation of Möbius aromatic planar metallaborocycles. J. Am. Chem. Soc. 2020, 142, 3356–3360. [Google Scholar] [CrossRef] [PubMed]
- Mauksch, M.; Tsogoeva, S.B. Demonstration of “Möbius” aromaticity in planar metallacycles. Chem. Eur. J. 2010, 16, 7843–7851. [Google Scholar] [CrossRef]

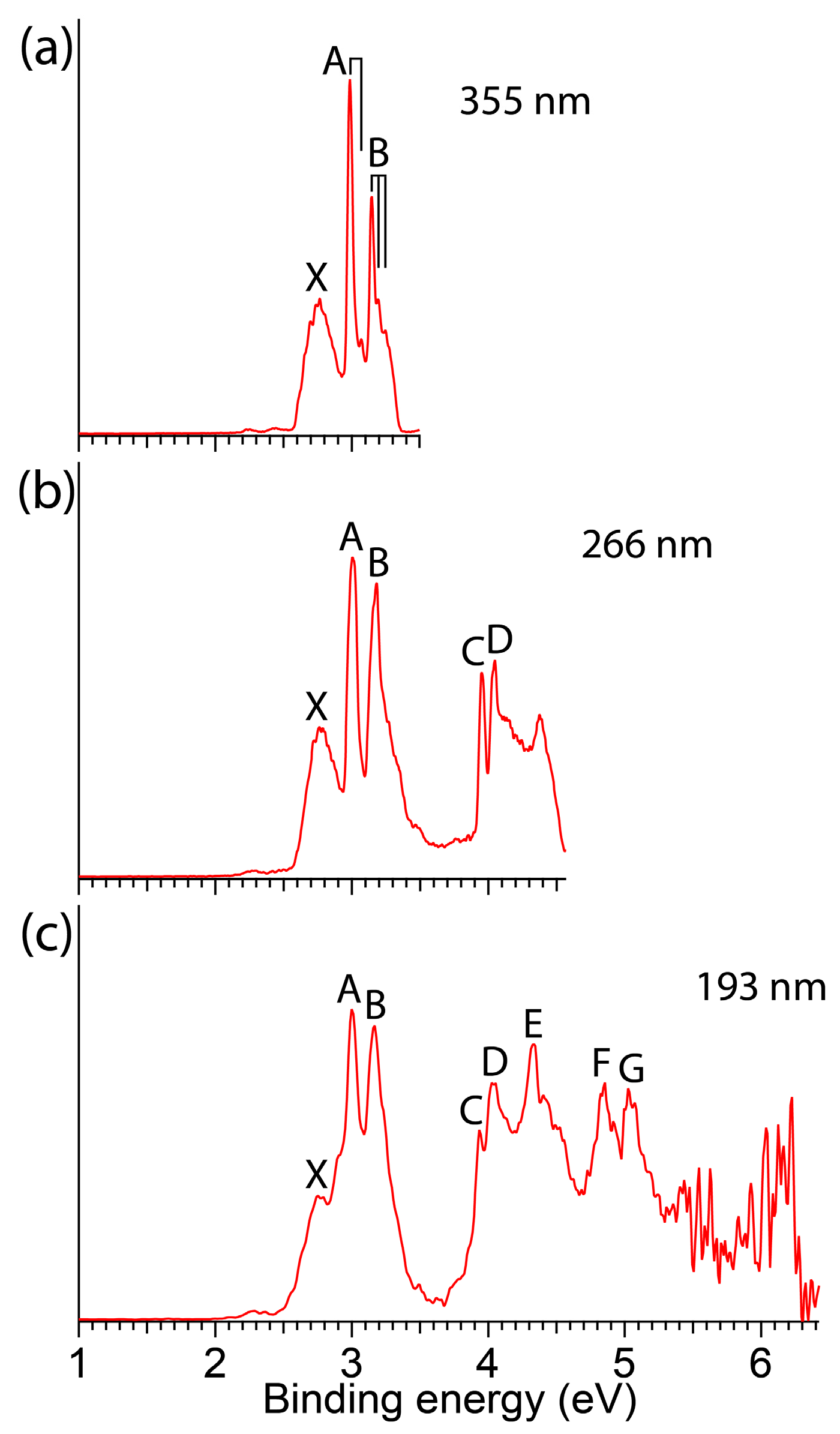
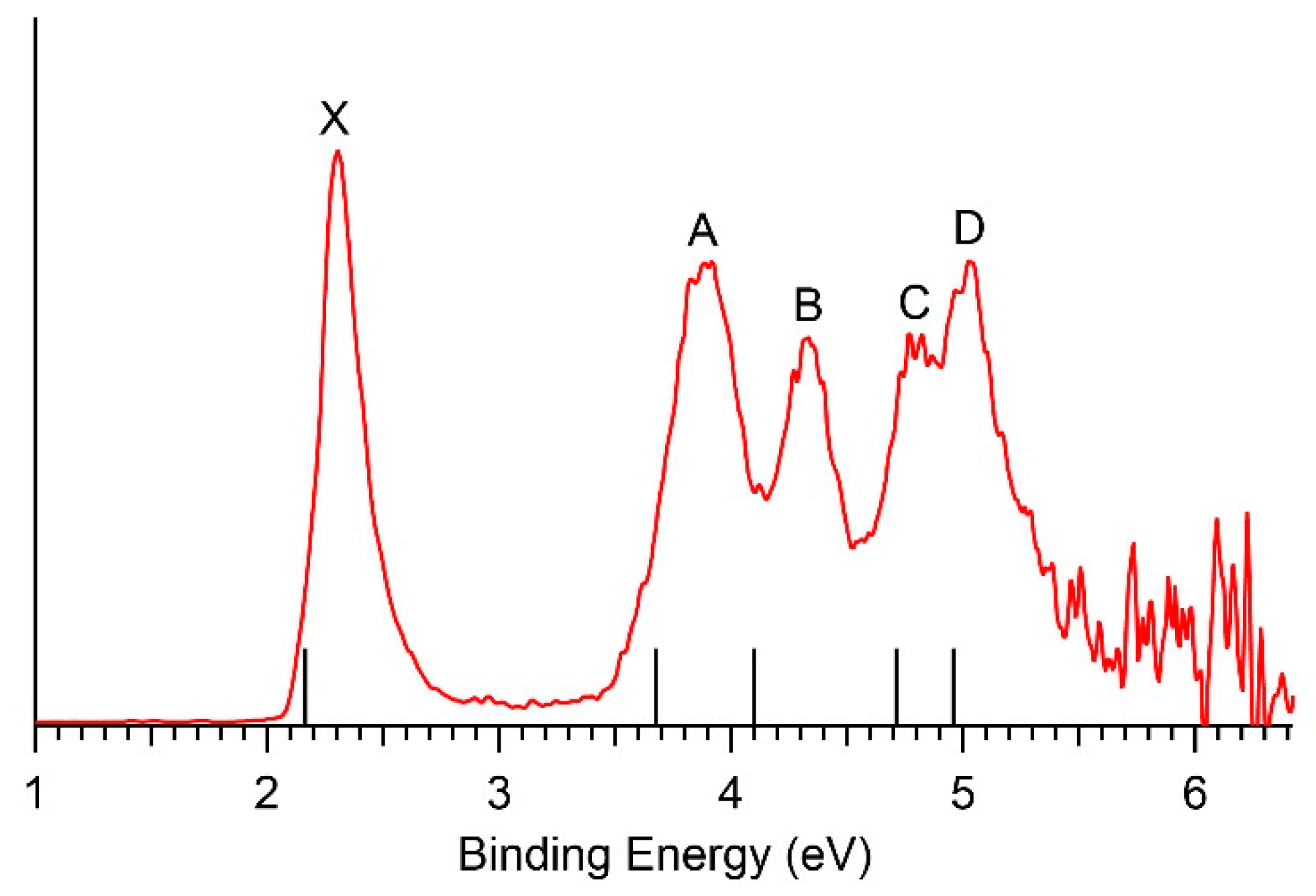

| VDE/ADE (exp) a | Final State and Electron Configuration | VDE/ADE (theo) | |
|---|---|---|---|
| X | 2.27/2.22 | 2A {…(18a)2(19a)2(20a)2(21a)2(22a)1} | 2.16/2.11 |
| A | 3.88 | 2A {…(18a)2(19a)2(20a)2(21a)1(22a)2} | 3.68 |
| B | 4.31 | 2A {…(18a)2(19a)2(20a)1(21a)2(22a)2} | 4.10 |
| C | 4.77 | 2A {…(18a)2(19a)1(20a)2(21a)2(22a)2} | 4.71 |
| D | 4.97 | 2A {…(18a)1(19a)2(20a)2(21a)2(22a)2} | 4.96 |
| VDE/ADE (exp) a | Final State and Electron Configuration | VDE/ADE (theo) | |
|---|---|---|---|
| X | 2.74/2.61 | 1A′ {…(17a′)2(18a′)2(19a′)2(5a″)2(20a′)0} | 2.92/2.70 |
| A | 2.99 | 3A″ {…(17a′)2(18a′)2(19a′)2(5a″)1(20a′)1} | 2.93 |
| B | 3.15 | 1A″ {…(17a′)2(18a′)2(19a′)2(5a″)1(20a′)1} | 3.25 |
| C | 3.95 | 3A′ {…(17a′)2(18a′)2(19a′)1(5a″)2(20a′)1} | 3.85 |
| D | 4.05 | 3A′ {…(17a′)2(18a′)1(19a′)2(5a″)2(20a′)1} | 3.99 |
| E | 4.33 | 1A′ {…(17a′)2(18a′)2(19a′)1(5a″)2(20a′)1} | 4.43 |
| 3A′ {…(17a′)1(18a′)2(19a′)2(5a″)2(20a′)1} | 4.63 | ||
| F | 4.86 | 1A′ {…(17a′)2(18a′)1(19a′)2(5a″)2(20a′)1} | 5.03 |
| G | 5.02 | 1A′ {…(17a′)1(18a′)2(19a′)2(5a″)2(20a′)1} | 5.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.W.; Chen, W.-J.; Kocheril, G.S.; Yuan, D.-F.; Wang, L.-S. The Structures and Bonding of Bismuth-Doped Boron Clusters: BiB4− and BiB5−. Inorganics 2023, 11, 405. https://doi.org/10.3390/inorganics11100405
Choi HW, Chen W-J, Kocheril GS, Yuan D-F, Wang L-S. The Structures and Bonding of Bismuth-Doped Boron Clusters: BiB4− and BiB5−. Inorganics. 2023; 11(10):405. https://doi.org/10.3390/inorganics11100405
Chicago/Turabian StyleChoi, Hyun Wook, Wei-Jia Chen, G. Stephen Kocheril, Dao-Fu Yuan, and Lai-Sheng Wang. 2023. "The Structures and Bonding of Bismuth-Doped Boron Clusters: BiB4− and BiB5−" Inorganics 11, no. 10: 405. https://doi.org/10.3390/inorganics11100405
APA StyleChoi, H. W., Chen, W.-J., Kocheril, G. S., Yuan, D.-F., & Wang, L.-S. (2023). The Structures and Bonding of Bismuth-Doped Boron Clusters: BiB4− and BiB5−. Inorganics, 11(10), 405. https://doi.org/10.3390/inorganics11100405