A Nanoscale Cobalt Functionalized Strandberg-Type Phosphomolybdate with β-Sheet Conformation Modulation Ability in Anti-Amyloid Protein Misfolding
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Single Crystal Structures
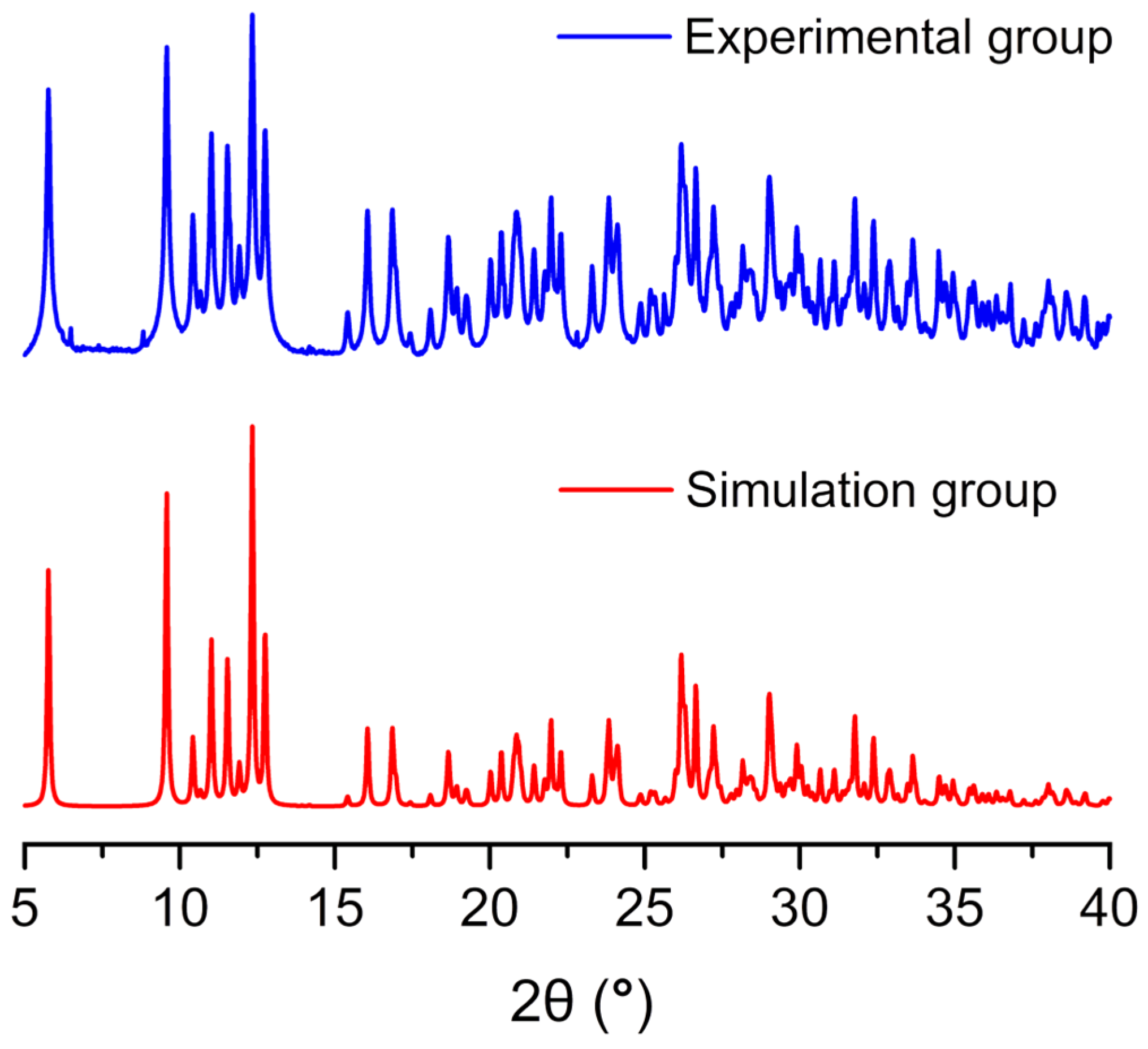
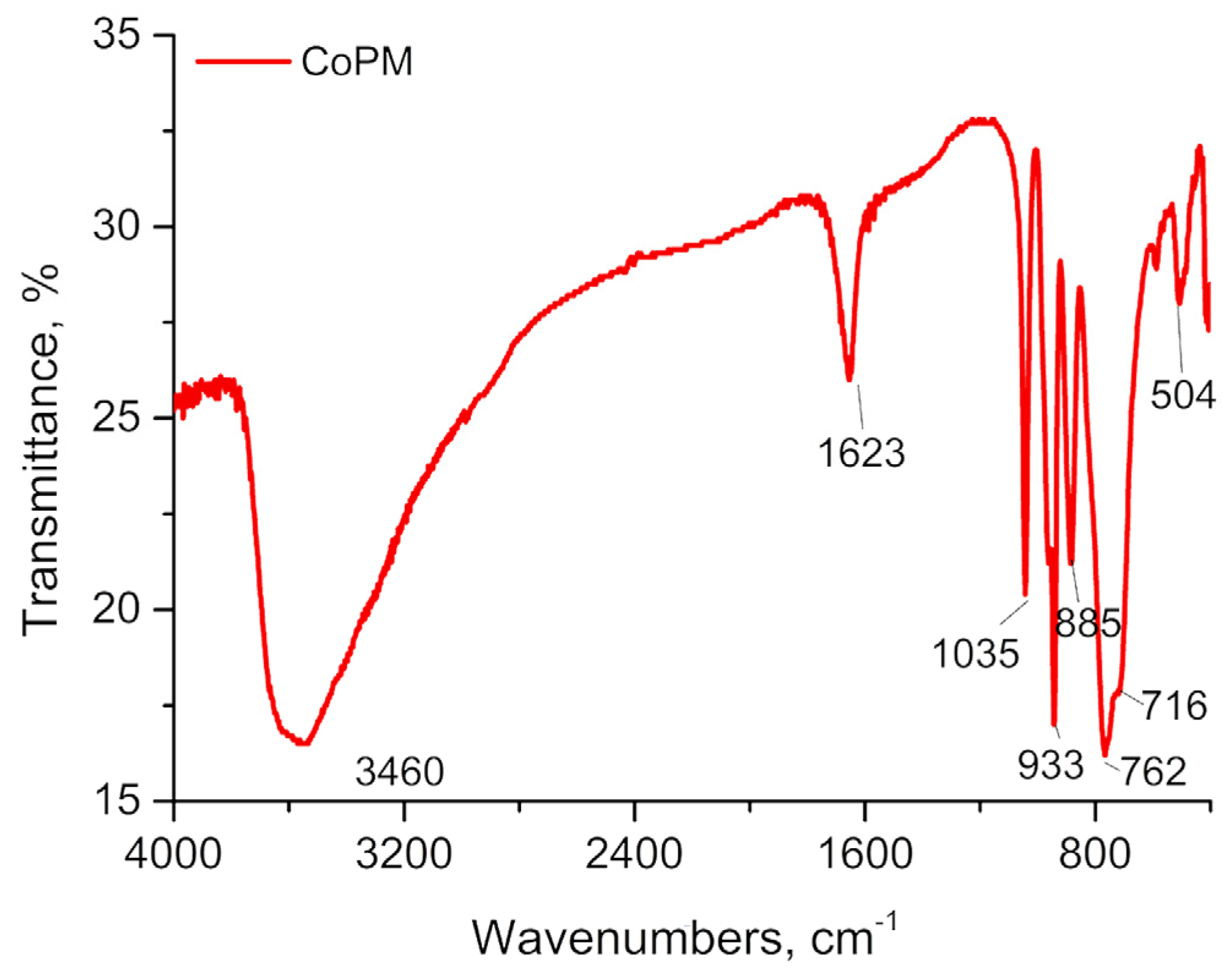
2.2. PXRD, IR and UV-Visible Spectrum
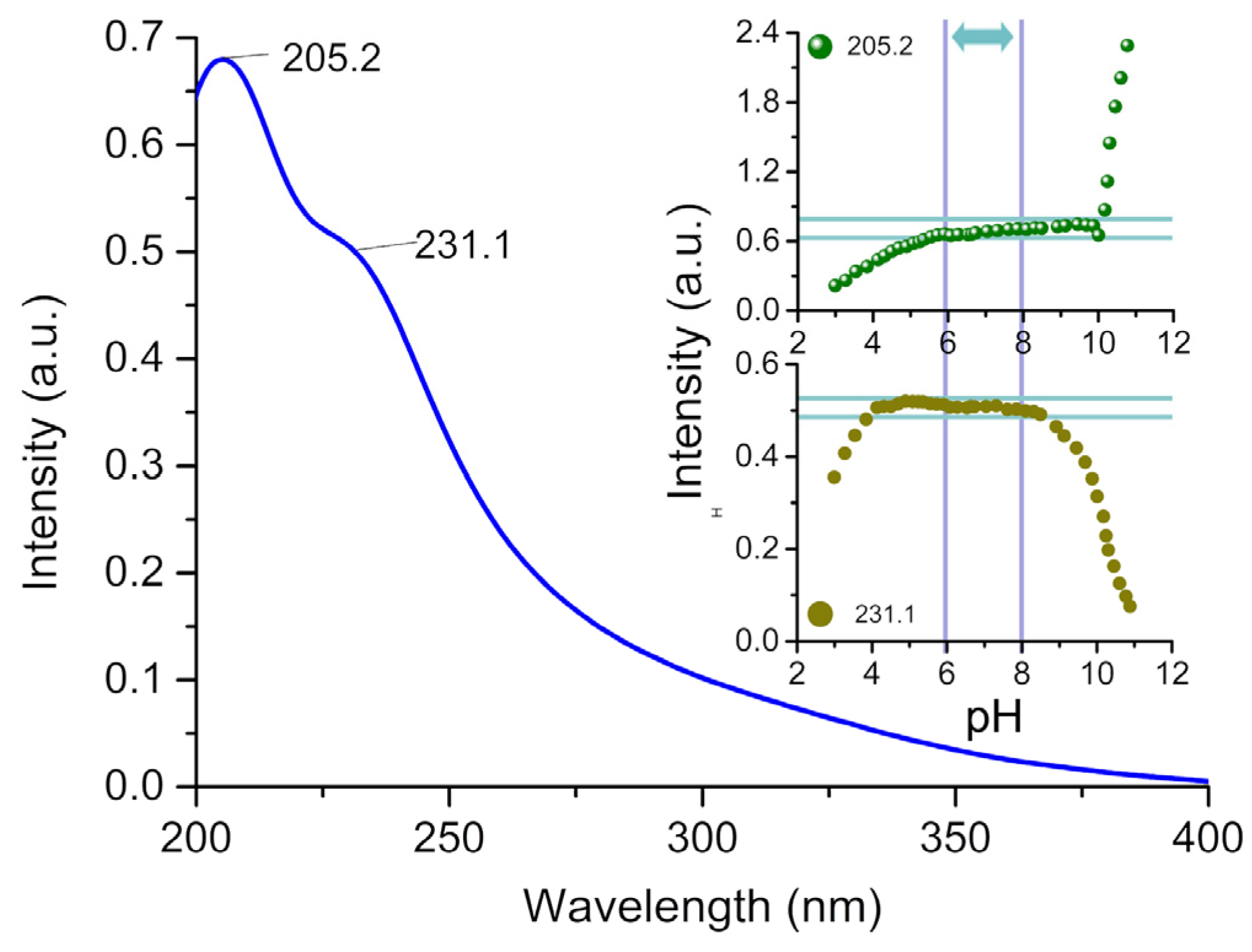
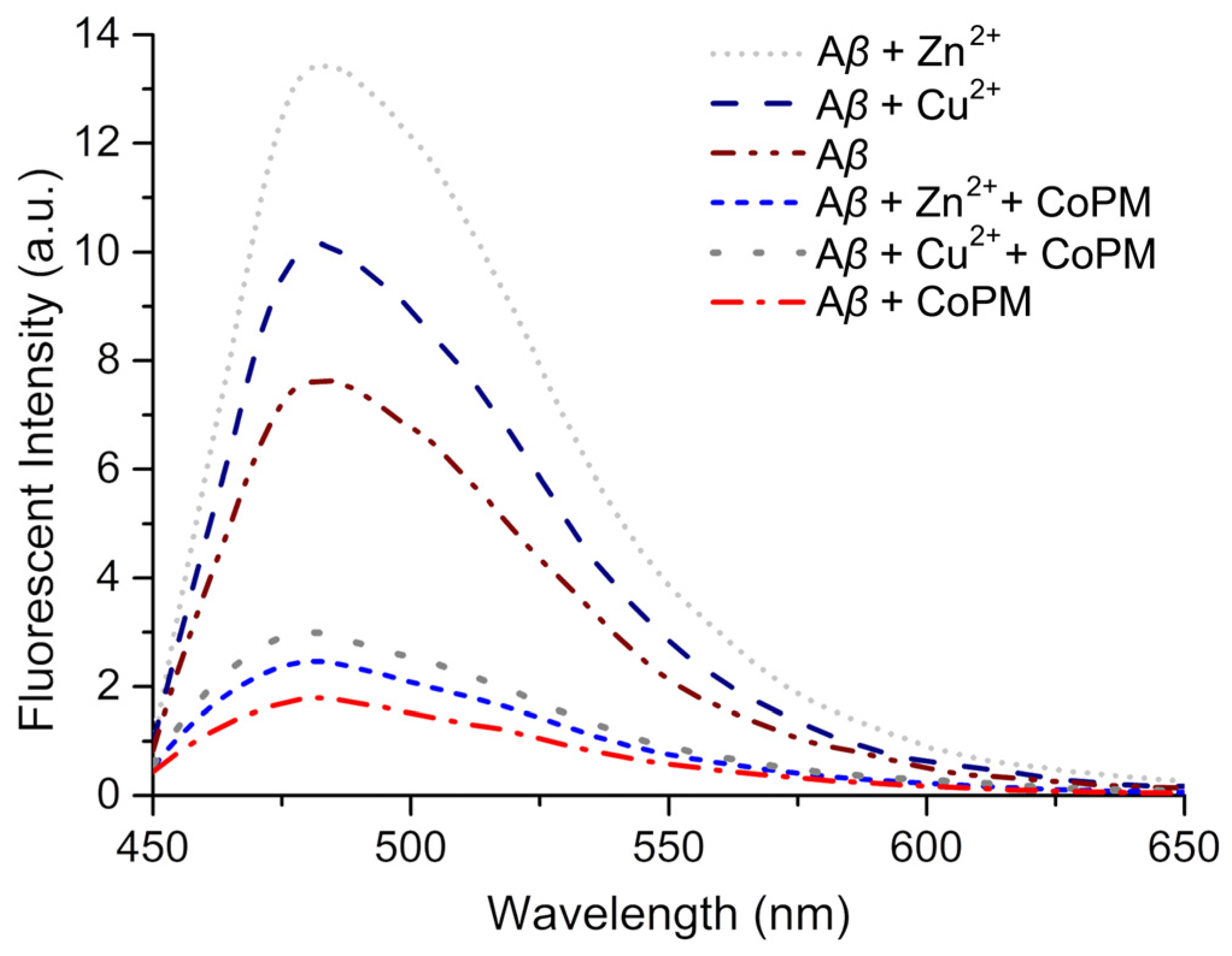
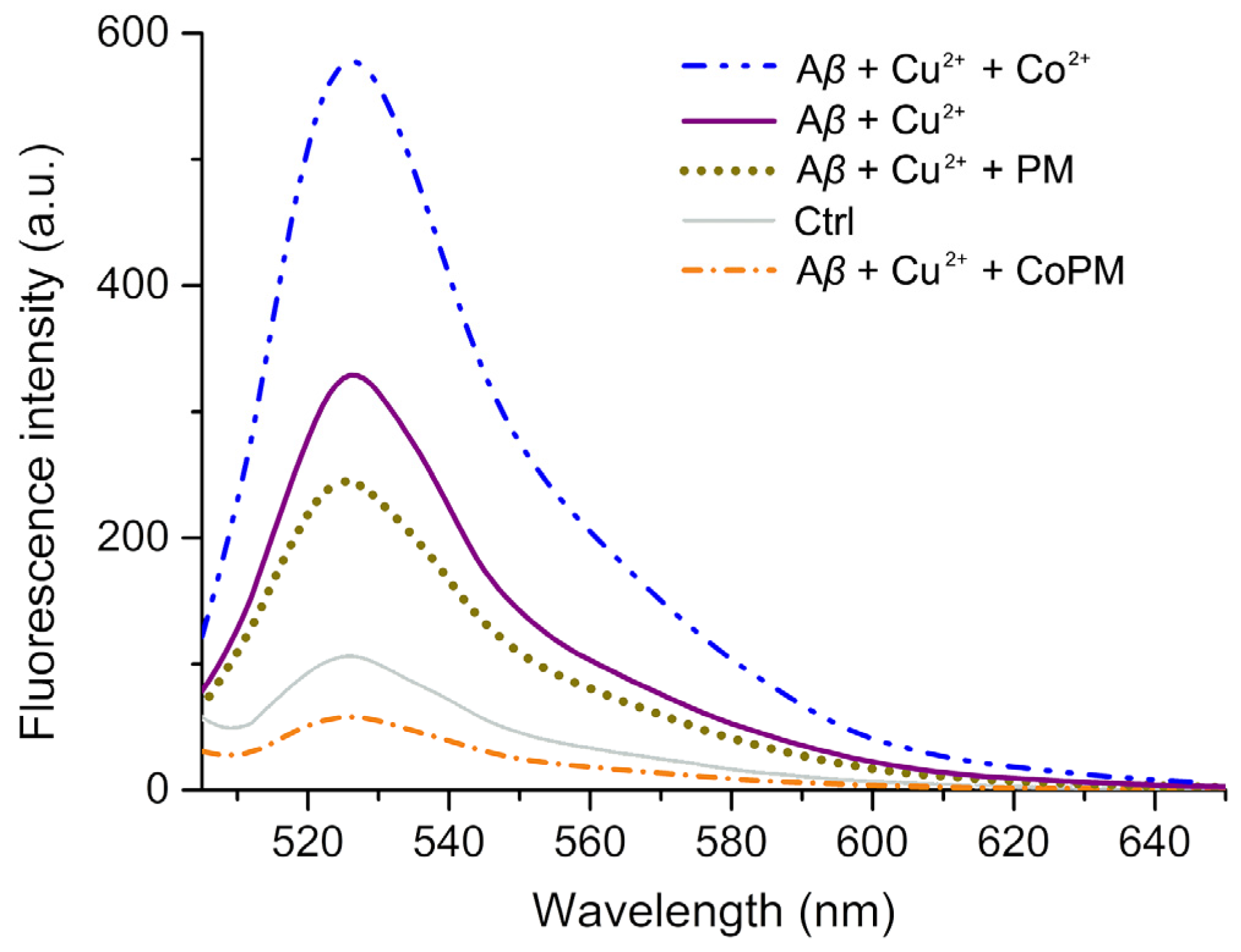
2.3. Modulation of Conformation
3. Materials and Methods
3.1. Synthesis
3.2. X-ray Crystallography
3.3. ThT Fluorescence Assay
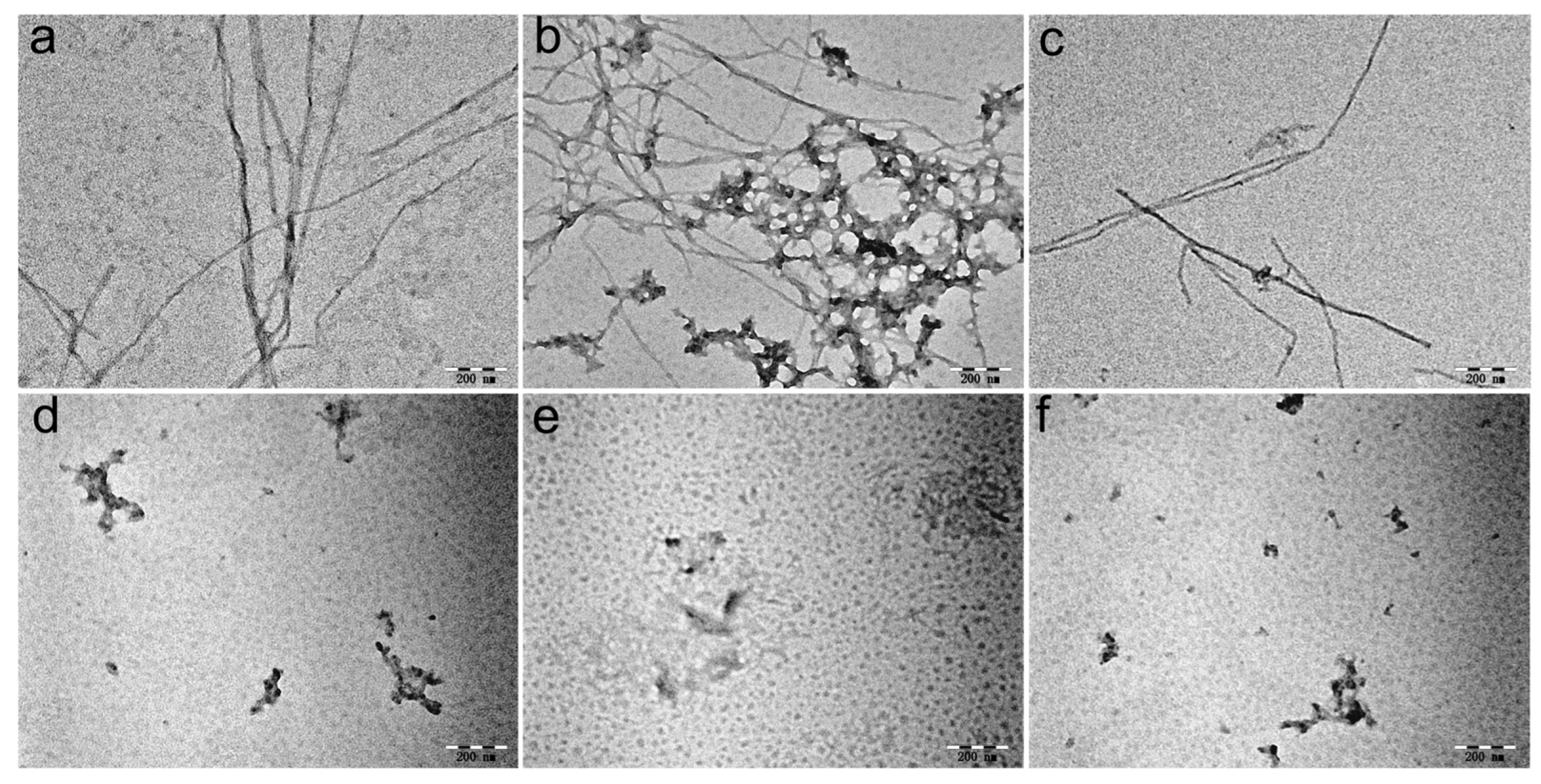
3.4. Morphological Analysis
3.5. Inhibition of ROS Generation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Silva, J.L. Ligand binding and hydration in protein misfolding: Insights from studies of prion and p53 tumor suppressor proteins. Acc. Chem. Res. 2010, 43, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.N.; Khan, R.H. Protein misfolding and related human diseases: A comprehensive review of toxicity, proteins involved, and current therapeutic strategies. Int. J. Biol. Macromol. 2022, 223, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Kepp, K.P. Bioinorganic chemistry of Alzheimer’s disease. Chem. Rev. 2012, 112, 5193–5239. [Google Scholar] [CrossRef]
- Vinters, H.V. Emerging concepts in Alzheimer’s disease. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 291–319. [Google Scholar] [CrossRef]
- Walsh, D.M.; Selkoe, D.J. Aβ Oligomers-a decade of discovery. J. Neurochem. 2007, 101, 1172–1184. [Google Scholar] [CrossRef]
- Saravanan, K.M.; Zhang, H.P.; Zhang, H.L.; Zhang, H.L.; Xi, W.H.; Wei, Y.J. On the conformational dynamics of β-amyloid forming peptides: A computational perspective. Front. Bioeng. Biotechnol. 2020, 8, 532. [Google Scholar] [CrossRef] [PubMed]
- Faller, P.; Hureau, C.; La Penna, G. Metal Ions and Intrinsically Disordered Proteins and Peptides: From Cu/Zn Amyloid-β to General Principles. Acc. Chem. Res. 2014, 47, 2252–2259. [Google Scholar] [CrossRef]
- Bagheri, S.; Saboury, A.A.; Haertlé, T.; Rongioletti, M.; Saso, L. Probable reasons for neuron copper deficiency in the brain of patients with Alzheimer’s disease: The complex role of amyloid. Inorganics 2022, 10, 6. [Google Scholar] [CrossRef]
- Gaggelli, E.; Kozlowski, H.; Valensin, D.; Valensin, G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem. Rev. 2006, 106, 1995–2044. [Google Scholar] [CrossRef]
- Selkoe, D.J. Resolving controversies on the path to Alzheimer’s therapeutics. Nat. Med. 2011, 17, 1060–1065. [Google Scholar] [CrossRef]
- Chimon, S.; Shaibat, M.A.; Jones, C.R.; Calero, D.C.; Aizezi, B.; Ishii, Y. Evidence of fibril-like β-sheet structures in a neurotoxic amyloid intermediate of Alzheimer’s β-amyloid. Nat. Struct. Mol. Biol. 2007, 14, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, M.; Ren, J.S.; Qu, X.G. Current strategies for modulating Aβ aggregation with multifunctional agents. Acc. Chem. Res. 2021, 54, 2172–2184. [Google Scholar] [CrossRef]
- Matthews, R.G. Cobalamin-Dependent Methyltransferases. Acc. Chem. Res. 2001, 34, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Bandarian, V.; Matthews, R.G. Quantitation of Rate Enhancements Attained by the Binding of Cobalamin to Methionine Synthase. Biochemistry 2001, 40, 5056–5064. [Google Scholar] [CrossRef]
- Ma, X.; Hua, J.A.; Wang, K.; Zhang, H.M.; Zhang, C.L.; He, Y.F.; Guo, Z.J.; Wang, X.Y. Modulating conformation of Aβ-peptide: An effective way to prevent protein-misfolding disease. Inorg. Chem. 2018, 57, 13533–13543. [Google Scholar] [CrossRef]
- Du, D.Y.; Qin, J.S.; Li, S.L.; Su, Z.M.; Lan, Y.Q. Recent advances in porous polyoxometalate-based metal-organic framework materials. Chem. Soc. Rev. 2014, 43, 4615–4632. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.L.; Lin, L.Q.; Sun, D.H.; Chen, H.M.; Yang, D.P.; Li, Q.B. Bio-inspired synthesis of metal nanomaterials and applications. Chem. Soc. Rev. 2015, 44, 6330–6374. [Google Scholar] [CrossRef]
- Song, Y.F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef]
- Yang, K.; Ying, Y.X.; Cui, L.L.; Sun, J.C.; Luo, H.; Hu, Y.Y.; Zhao, J.W. Stable aqueous Zn−Ag and Zn−polyoxometalate hybrid battery driven by successive Ag+ cation and polyoxoanion redox reactions. Energy Storage Mater. 2021, 34, 203–210. [Google Scholar] [CrossRef]
- Hua, J.A.; Wei, X.M.; Li, Y.F.; Li, L.Z.; Zhang, H.; Wang, F.; Zhang, C.L.; Ma, X. A cyclen-functionalized cobalt-substituted sandwich-type tungstoarsenate with versatility in removal of methylene blue and anti-ROS-sensitive tumor cells. Molecules 2022, 27, 6451. [Google Scholar] [CrossRef]
- Zheng, K.T.; Niu, B.X.; Lin, C.M.; Song, Y.Z.; Ma, P.T.; Wang, J.P.; Niu, J.Y. DL-Serine covalently modified multinuclear lanthanide-implanted arsenotungstates with fast photochromism. Chin. Chem. Lett. 2023, 34, 107238. [Google Scholar] [CrossRef]
- Mirzaei, M.; Eshtiagh-Hosseini, H.; Alipour, M.; Frontera, A. Recent developments in the crystal engineering of diverse coordination modes (0–12) for Keggin-type polyoxometalates in hybrid inorganic–organic architectures. Coord. Chem. Rev. 2014, 275, 1–18. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, K.; Wang, J.; Niu, B.; Ma, P.; Wang, J.; Niu, J. Discovery and isolation of two arsenotungastate species: [As4W48O168]36− and [As2W21O77(H2O)3]22−. Inorg. Chem. 2023, 62, 3338–3342. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.H.; Zeng, X.H.; Zhu, C.F.; Shu, J.H.; Xiao, P.X.; Xu, H.; Liu, L.C.; Zhang, J.Y.; Zeng, Q.D.; Xie, J.L. A series of organic–inorganic hybrid materials consisting of flexible organic amine modified polyoxomolybdates: Synthesis, structures and properties. RSC Adv. 2016, 6, 106248–106259. [Google Scholar] [CrossRef]
- Jin, H.J.; Zhou, B.B.; Yu, Y.; Zhao, Z.F.; Su, Z.H. Inorganic–organic hybrids constructed from heteropolymolybdate anions and copper–organic fragments: Syntheses, structures and properties. CrystEngComm 2011, 13, 585–590. [Google Scholar] [CrossRef]
- Weng, J.B.; Hong, M.C.; Liang, Y.C.; Shi, Q.; Cao, R. A nucleobase–inorganic hybrid polymer consisting of copper bis(phosphopentamolybdate) and cytosine. J. Chem. Soc. Dalton Trans. 2002, 3, 289–290. [Google Scholar] [CrossRef]
- Meng, J.X.; Lu, Y.; Li, Y.G.; Fu, H.; Wang, E.B. Controllable self-assembly of four new metal–organic frameworks based on different phosphomolybdate clusters by altering the molar ratio of H3PO4 and Na2MoO4. CrystEngComm 2011, 13, 2479–2486. [Google Scholar] [CrossRef]
- Xu, X.X.; Gao, X.; Lu, T.T.; Liu, X.X.; Wang, X.L. Hybrid material based on a coordination -complex-modified polyoxometalate nanorod (CC/POMNR) and PPy: A new visible light activated and highly efficient photocatalyst. J. Mater. Chem. A 2015, 3, 198–206. [Google Scholar] [CrossRef]
- Chen, K.; Liu, S.Q.; Zhang, Q.Y. Degradation and detection of endocrine disruptors by laccase-mimetic polyoxometalates. Front. Chem. 2022, 10, 854045. [Google Scholar] [CrossRef]
- Ma, Y.; Xue, Q.; Min, S.T.; Zhang, Y.P.; Hu, H.M.; Gao, S.L.; Xue, G.L. An unusual fan-type polyanion with a silver cation located at the axial center, [AgAsIII2(AsIIIAsVMo4O18(OH)2)3]11–. Dalton Trans. 2013, 42, 3410–3416. [Google Scholar] [CrossRef]
- Li, F.Y.; Xu, L. Coordination assemblies of polyoxomolybdate cluster framework: From labile building blocks to stable functional materials. Dalton Trans. 2011, 40, 4024–4034. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.X.; Wang, Z.; Zhang, R.R.; Xu, L.Q.; Sun, D. A novel polyoxometalate-based hybrid containing a 2D [CoMo8O26]∞ structure as the anode for lithium-ion batteries. Chem. Commun. 2017, 53, 10560–10563. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.A.; Wei, X.M.; Ma, X.; Jiao, J.Z.; Chai, B.H.; Wu, C.B.; Zhang, C.L.; Niu, Y.L. A {Cd4Cl2O14} cluster functionalized sandwich-type tungstoarsenate as a conformation modulator for misfolding Aβ peptides. CrystEngComm 2022, 24, 1171–1176. [Google Scholar] [CrossRef]
- Chaudhary, H.; Iashchishyn, I.A.; Romanova, N.V.; Rambaran, M.A.; Musteikyte, G.; Smirnovas, V.; Holmboe, M.; Ohlin, C.A.; Svedruzic, Z.M.; Morozova-Roche, L.A. Polyoxometalates as effective nano-inhibitors of amyloid aggregation of pro-inflammatory S100A9 protein involved in neurodegenerative diseases. ACS Appl. Mater. Interfaces 2021, 13, 26721–26734. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Yan, C.R.; Wang, C.; Wang, C.L.; Cao, Y.; Zhou, Y.; Guan, P.; Hu, X.L.; Zhu, W.L.; Ding, S.C. Advanced nanomaterials for modulating Alzheimer’s related amyloid aggregation. Nanoscale Adv. 2023, 5, 46–80. [Google Scholar] [CrossRef]
- Hua, J.A.; Wang, F.; Wei, X.M.; Qin, Y.X.; Lian, J.M.; Wu, J.H.; Ma, P.T.; Ma, X. A nanoenzyme constructed from manganese and Strandberg-type phosphomolybdate with versatility in antioxidant and modulating conformation of Aβ protein misfolding aggregates in vitro. Int. J. Mol. Sci. 2023, 24, 4317. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Hu, G.H.; Yan, X. Hydrothermal synthesis, structure and properties of a new phosphomolybdate based on novel [Co(H2O)4(HP2Mo5O23)]8- anions and [H8(H2O)16]8+ water cluster cations. Chem. Res. Chin. Univ. 2016, 32, 329–333. [Google Scholar] [CrossRef]
- Hua, J.A.; Wei, X.M.; Zhang, N.; Chen, P.J.; Ma, X. Synthesis of a linear cobalt-substituted strandberg-type polyphospolybdate with ability in modulating conformation of Aβ-peptide misfolding aggregation. Chin. J. Inorg. Chem. 2022, 38, 1908–1918. [Google Scholar]
- Zheng, S.T.; Zhang, J.; Juan, J.M.; Yuan, D.Q.; Yang, G.Y. Poly(polyoxotungstate)s with 20 nickel centers: From nanoclusters to one-dimensional chains. Angew. Chem. Int. Ed. Engl. 2009, 48, 7176–7179. [Google Scholar] [CrossRef]
- Brown, I.D.; Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. Acta Crystallogr. B Struct. Sci. 1985, 41, 244–247. [Google Scholar] [CrossRef]
- Shields, G.P.; Raithby, P.R.; Allen, F.H.; Motherwell, W.D.S. The assignment and validation of metal oxidation states in the Cambridge structural database. Acta Crystallogr. B Struct. Sci. 2000, 56, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Streb, C.; Ritchie, C.; Long, D.L.; Kögerler, P.; Cronin, L. Modular assembly of a functional polyoxometalate-based open framework constructed from unsupported AgI–AgI interactions. Angew. Chem. Int. Ed. Engl. 2007, 46, 7579–7582. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wei, X.M.; Wang, M.; Zhang, N.; Chen, P.J.; Hua, J.A. A hexa-Cu cluster sandwiched silicotungstate with reactive oxygen species catalytic ability and anti-tumor activity in PC12 cells. J. Mol. Struct. 2022, 1267, 133616. [Google Scholar] [CrossRef]
- Ma, X.; Sun, Y.S.; Lu, Q.L.; Bai, X.Q.; Zang, Q.J.; Yan, X.W.; Wang, F.; Hua, J.A. A nanoscale ferromagnetic nickel-supercluster functionalized giant heterometallic arsenototungstate with ability in catalyzing reactive oxygen species generation. J. Mol. Sturct. 2023, 1291, 136010. [Google Scholar] [CrossRef]
- Feng, S.L.; Lu, Y.; Zhang, Y.X.; Su, F.; Sang, X.J.; Zhang, L.C.; You, W.S.; Zhu, Z.M. Three new Strandberg-type phenylphosphomolybdate supports for immobilizing horseradish peroxidase and their catalytic oxidation performances. Dalton. Trans. 2018, 47, 14060–14069. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chen, Y.; Wang, C.Y.; Yin, A.P.; Li, H.L.; Zhao, J.S. Polyoxometalates encapsulated into hollow periodic mesoporous organosilica as nanoreactors for extraction oxidation desulfurization. Catalysts 2023, 13, 747. [Google Scholar] [CrossRef]
- Yang, D.H.; Li, S.Z.; Ma, P.T.; Wang, J.P.; Niu, J.Y. Carboxylate-functionalized phosphomolybdates: Ligand-directed conformations. Inorg. Chem. 2013, 52, 8987–8992. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Y.Q.; Hua, J.A.; Xu, C.Y.; Yang, T.; Yuan, J.; Chen, G.Q.; Guo, Z.J.; Wang, X.Y. A β-sheet-targeted theranostic agent for diagnosing and preventing aggregation of pathogenic peptides in Alzheimer’s disease. Sci. China Chem. 2020, 63, 73–82. [Google Scholar] [CrossRef]
- Barnham, K.J.; Bush, A.I. Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem. Soc. Rev. 2014, 43, 6727–6749. [Google Scholar] [CrossRef]
- Gao, N.; Liu, Z.; Zhang, H.; Liu, C.; Yu, D.; Ren, J.; Qu, X. Site-directed chemical modification of amyloid by polyoxometalates for inhibition of protein misfolding and aggregation. Angew. Chem. Int. Ed. 2022, 61, e202115336. [Google Scholar] [CrossRef]
- Wang, X.H.; Wang, X.Y.; Zhang, C.L.; Jiao, Y.; Guo, Z.J. Inhibitory action of macrocyclic platiniferous chelators on metal-induced Aβ aggregation. Chem. Sci. 2012, 3, 1304–1312. [Google Scholar] [CrossRef]
- Kozlowski, H.; Janicka-Klos, A.; Brasun, J.; Gaggelli, E.; Valensin, D.; Valensin, G. Copper, iron, and zinc ions homeostasis and their role in neurodegenerative disorders (metal uptake, transport, distribution and regulation). Coordin. Chem. Rev. 2009, 253, 2665–2685. [Google Scholar] [CrossRef]
- Noël, S.; Cadet, S.; Gras, E.; Hureau, C. The benzazole scaffold: A SWAT to combat Alzheimer’s disease. Chem. Soc. Rev. 2013, 42, 7747–7762. [Google Scholar] [CrossRef]
- Palanimuthu, D.; Wu, Z.; Jansson, P.J.; Braidy, N.; Bernhardt, P.V.; Richardson, D.R.; Kalinowski, D.S. Novel chelators based on adamantane-derived semicarbazones and hydrazones that target multiple hallmarks of Alzheimer’s disease. Dalton Trans. 2018, 47, 7190–7205. [Google Scholar] [CrossRef] [PubMed]
- SAINT; Bruker AXS Inc.: Madison, WI, USA, 2007.
- Brese, N.E.; O’Keeffe, M. Bond-valence parameters for solids. Acta Crystallogr. B Struct. Sci. 1991, 47, 192–197. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHEXTL-97; Programs for crystal structure refinements; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Li, M.; Howson, S.E.; Dong, K.; Gao, N.; Ren, J.S.; Scott, P.; Qu, X.G. Chiral metallohelical complexes enantioselectively target amyloid-β for treating Alzheimer’s disease. J. Am. Chem. Soc. 2014, 136, 11655–11663. [Google Scholar] [CrossRef] [PubMed]

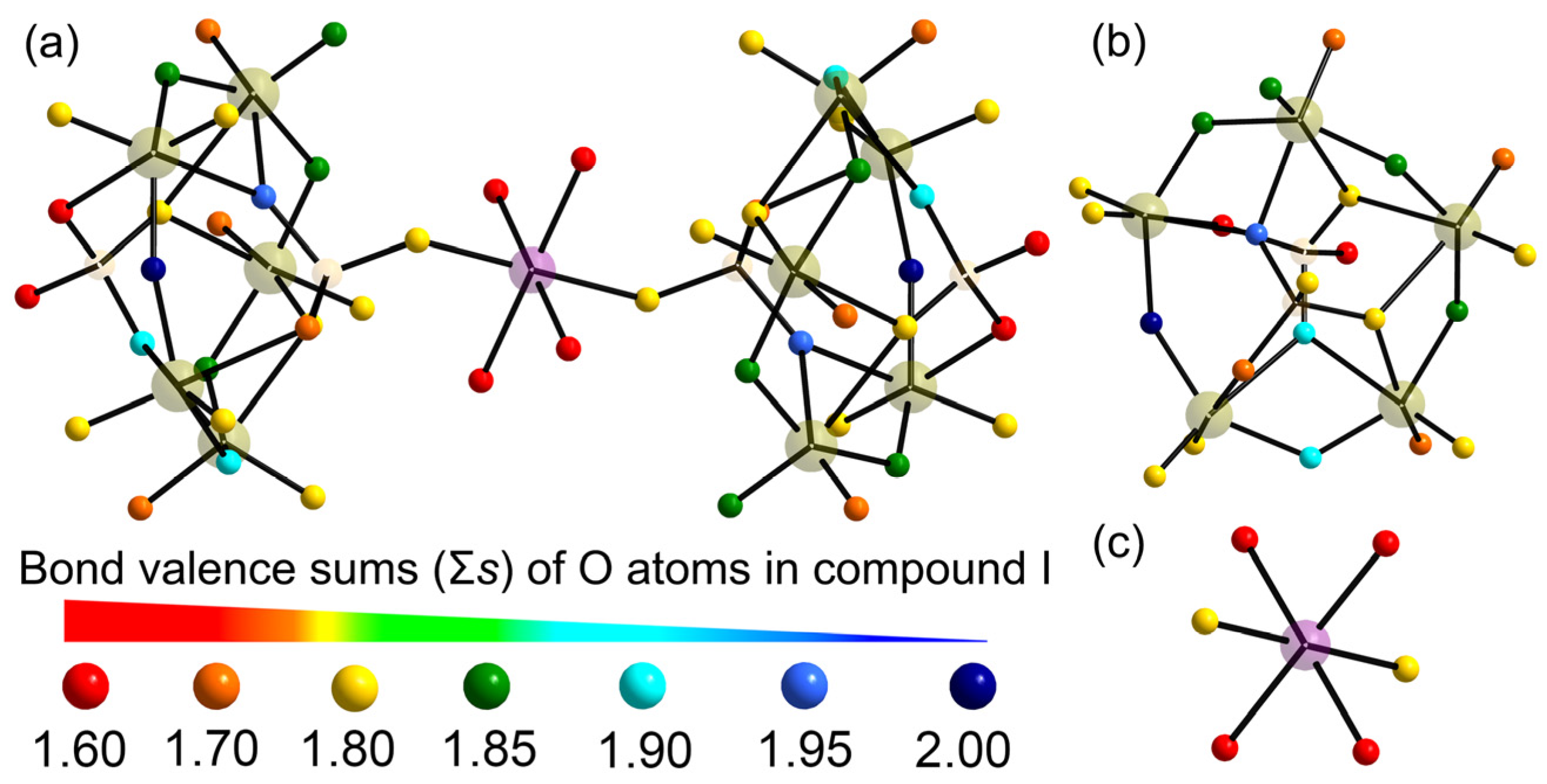
| Bond | Valence | Bond | Valence | Bond | Valence | Atom | Σs |
|---|---|---|---|---|---|---|---|
| Mo(1)-O(1) | 1.764 | Mo(1)-O(15) | 0.997 | Mo(1)-O(16) | 0.298 | ||
| Mo(1)-O(6) | 1.689 | Mo(1)-O(11) | 0.910 | Mo(1)-O(21) | 0.262 | Mo(1) | 5.919 |
| Mo(2)-O(2) | 1.804 | Mo(2)-O(12) | 0.920 | Mo(2)-O(19) | 0.468 | ||
| Mo(2)-O(7) | 1.709 | Mo(2)-O(11) | 0.912 | Mo(2)-O(16) | 0.183 | Mo(2) | 5.997 |
| Mo(3)-O(8) | 1.743 | Mo(3)-O(13) | 1.012 | Mo(3)-O(19) | 0.295 | ||
| Mo(3)-O(3) | 1.719 | Mo(3)-O(12) | 0.905 | Mo(3)-O(17) | 0.268 | Mo(3) | 5.942 |
| Mo(4)-O(4) | 1.769 | Mo(4)-O(13) | 0.963 | Mo(4)-O(20) | 0.360 | ||
| Mo(4)-O(9) | 1.719 | Mo(4)-O(14) | 0.879 | Mo(4)-O(18) | 0.252 | Mo(4) | 5.941 |
| Mo(5)-O(5) | 1.734 | Mo(5)-O(14) | 0.994 | Mo(5)-O(18) | 0.381 | ||
| Mo(5)-O(10) | 1.694 | Mo(5)-O(15) | 0.867 | Mo(5)-O(21) | 0.310 | Mo(5) | 5.979 |
| Co(1)-O(23) | 0.408 | Co(1)-O(1W) | 0.308 | Co(1)-O(2W)#5 | 0.278 | ||
| Co(1)-O(23)#5 | 0.408 | Co(1)-O(1W)#5 | 0.308 | Co(1)-O(2W) | 0.278 | Co(1) | 1.988 |
| P(1)-O(17) | 1.308 | P(1)-O(18) | 1.231 | P(1)-O(16) | 1.272 | ||
| P(1)-O(22) | 1.100 | P(1) | 4.911 | ||||
| P(2)-O(23) | 1.331 | P(2)-O(21) | 1.156 | P(2)-O(20) | 1.301 | ||
| P(2)-O(19) | 1.151 | P(2) | 4.939 |
| Empirical Formula | H24CoK8Mo10O58P4 |
|---|---|
| Formula weight | 2407.20 |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/Å | 9.486(3) |
| b/Å | 10.270(3) |
| c/Å | 15.597(4) |
| α/deg | 94.275(4) |
| β/deg | 97.073(4) |
| γ/deg | 114.840(4) |
| V/Å3 | 1355.0(6) |
| Z | 1 |
| Dc/g cm−3 | 2.950 |
| μ/mm−1 | 3.391 |
| T/K | 296(2) |
| Limiting indices | −11 ≤ h ≤ 11 |
| −9≤ k ≤ 12 | |
| −18 ≤ l ≤ 16 | |
| Measured reflections | 6895 |
| Independent reflections | 4721 |
| Rint | 0.0149 |
| Data/restraints/parameters | 4721/0/376 |
| GOF on F2 | 1.051 |
| Final R indices [I > 2σ(I)] | R1 = 1.051 |
| wR2 = 0.0686 | |
| R indices (all data) | R1 = 0.0264 |
| wR2 = 0.0698 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Hua, J.; Zheng, P.; Tian, Y.; Kang, S.; Chen, J.; Duan, Y.; Ma, X. A Nanoscale Cobalt Functionalized Strandberg-Type Phosphomolybdate with β-Sheet Conformation Modulation Ability in Anti-Amyloid Protein Misfolding. Inorganics 2023, 11, 442. https://doi.org/10.3390/inorganics11110442
Wang M, Hua J, Zheng P, Tian Y, Kang S, Chen J, Duan Y, Ma X. A Nanoscale Cobalt Functionalized Strandberg-Type Phosphomolybdate with β-Sheet Conformation Modulation Ability in Anti-Amyloid Protein Misfolding. Inorganics. 2023; 11(11):442. https://doi.org/10.3390/inorganics11110442
Chicago/Turabian StyleWang, Man, Jiai Hua, Pei Zheng, Yuanzhi Tian, Shaodan Kang, Junjun Chen, Yifan Duan, and Xiang Ma. 2023. "A Nanoscale Cobalt Functionalized Strandberg-Type Phosphomolybdate with β-Sheet Conformation Modulation Ability in Anti-Amyloid Protein Misfolding" Inorganics 11, no. 11: 442. https://doi.org/10.3390/inorganics11110442
APA StyleWang, M., Hua, J., Zheng, P., Tian, Y., Kang, S., Chen, J., Duan, Y., & Ma, X. (2023). A Nanoscale Cobalt Functionalized Strandberg-Type Phosphomolybdate with β-Sheet Conformation Modulation Ability in Anti-Amyloid Protein Misfolding. Inorganics, 11(11), 442. https://doi.org/10.3390/inorganics11110442








