Low-Dimensional Compounds Containing Bioactive Ligands. Part XX: Crystal Structures, Cytotoxic, Antimicrobial Activities and DNA/BSA Binding of Oligonuclear Zinc Complexes with Halogen Derivatives of 8-Hydroxyquinoline
Abstract
1. Introduction
2. Results and Discussion
2.1. Syntheses
2.2. Infrared Spectroscopy
2.3. NMR Characterization and Stability Studies
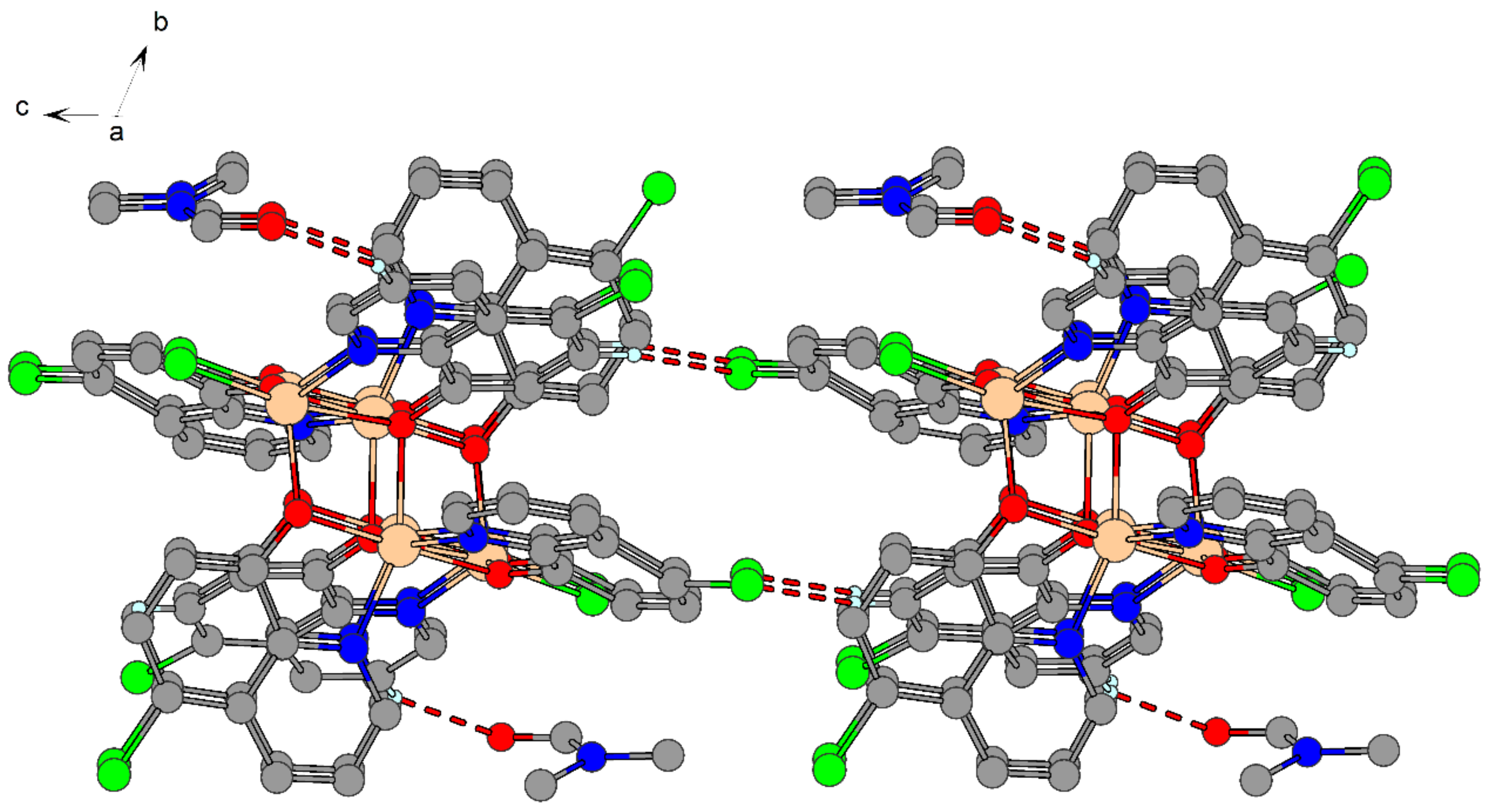
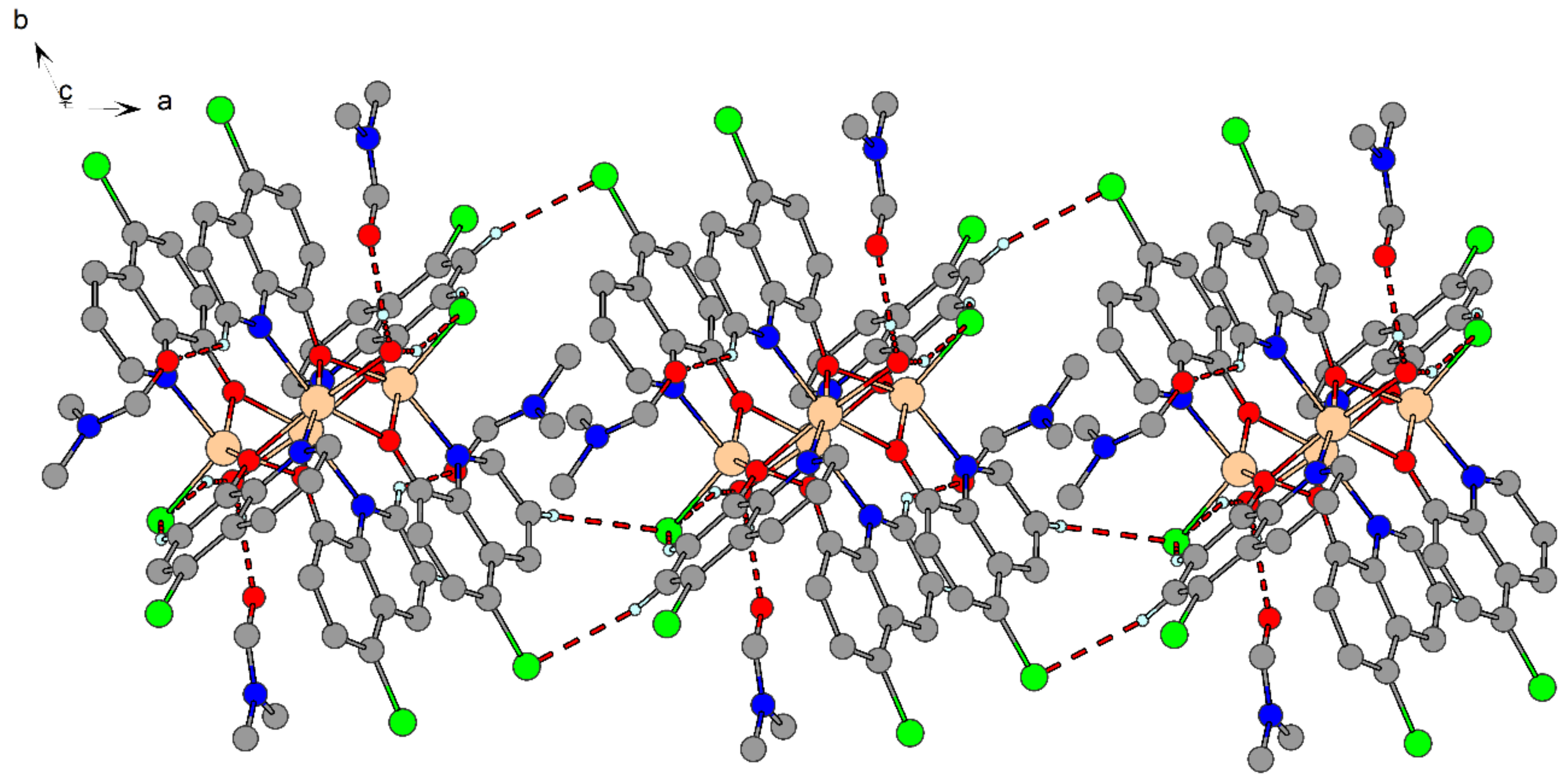
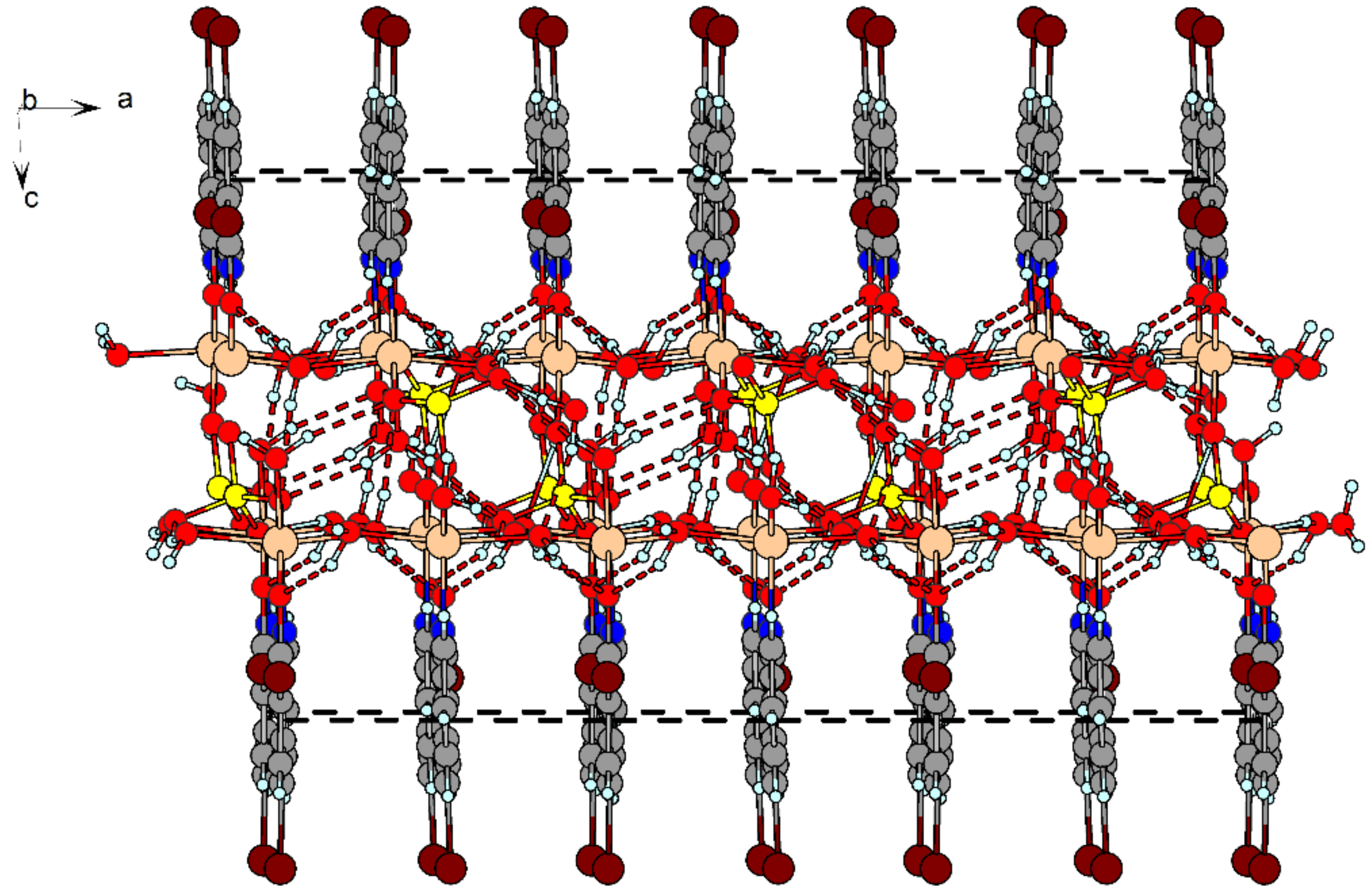
2.4. X-ray Structure Analysis
2.5. The DNA Binding Potency
2.6. BSA Interaction Study
2.7. Antiproliferative Activity
2.8. Antimicrobial Activity
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Syntheses
3.2.1. Synthesis of [Zn4Cl2(ClQ)6]·2DMF (1)
3.2.2. Synthesis of [Zn4Cl2(ClQ)6(H2O)2]·4DMF (2)
3.2.3. Synthesis of [Zn2(ClQ)3(HClQ)3]I3 (3)
3.2.4. Synthesis of [Zn2(dClQ)2(H2O)6(SO4)] (4)
3.2.5. Synthesis of [Zn2(dBrQ)2(H2O)6(SO4)] (5)
3.3. Physical Measurements
3.4. X-ray Structure Analysis
3.5. DNA/BSA Binding Studies
3.5.1. UV-vis Absorption Study
3.5.2. Ethidium Bromide Displacement Assay
3.5.3. BSA Binding Experiments
3.6. In Vitro Antitumor Activity
3.6.1. Cell Lines and Cell Culture
3.6.2. Assessment of Cytotoxicity
3.7. Antimicrobial Activity
3.7.1. Microorganisms Used
3.7.2. Agar Well-Diffusion Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Peng, K.; Liang, B.B.; Liu, W.; Mao, Z.W. What blocks more anticancer platinum complexes from experiment to clinic: Major problems and potential strategies from drug design perspectives. Coord. Chem. Rev. 2021, 449, 214210. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, B.P. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Afzal, O.; Kumar, S.; Haider, M.R.; Ali, M.R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef]
- Gupta, R.; Luxami, V.; Paul, K. Insights of 8-hydroxyquinolines: A novel target in medicinal chemistry. Bioorgan. Chem. 2021, 108, 104663. [Google Scholar] [CrossRef]
- Colson, P.; Rolain, J.M.; Lagier, J.C.; Brouqui, P.; Raoult, D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int. J. Antimicrob. Agents 2020, 55, 105932. [Google Scholar] [CrossRef]
- Amolegbe, S.A.; Adewuyi, S.; Akinremi, C.A.; Adediji, J.F.; Lawal, A.; Atayese, A.O.; Obaleye, J.A. Iron(III) and copper(II) complexes bearing 8-quinolinol with amino-acids mixed ligands: Synthesis, characterization and antibacterial investigation. Arab. J. Chem. 2015, 8, 742–747. [Google Scholar] [CrossRef]
- Scarim, C.B.; Lira de Farias, R.; Vieira de Godoy Netto, A.; Chin, C.M.; Leandro dos Santos, J.; Pavan, F.R. Recent advances in drug discovery against Mycobacterium tuberculosis: Metal-based complexes. Eur. J. Med. Chem. 2021, 214, 113166. [Google Scholar] [CrossRef]
- Pippi, B.; Lopes, W.; Reginatto, P.; Silva, F.É.K.; Joaquim, A.R.; Alves, R.J.; Silveira, G.P.; Vainstein, M.H.; Andrade, S.F.; Fuentefria, A.M. New insights into the mechanism of antifungal action of 8-hydroxyquinolines. Saudi Pharm. J. 2019, 27, 41–48. [Google Scholar] [CrossRef]
- Yadav, P.; Shah, K. Quinolines, a perpetual, multipurpose scaffold in medicinal chemistry. Bioorg. Chem. 2021, 109. [Google Scholar] [CrossRef] [PubMed]
- Saadeh, H.A.; Sweidan, K.A.; Mubarak, M.S. Recent advances in the synthesis and biological activity of 8-hydroxyquinolines. Molecules 2020, 25, 4321. [Google Scholar] [CrossRef] [PubMed]
- Zhiwei, R.; Songbai, X.; Qi, H. 5,7-Dihalo-8-quinolinol complex inhibits growth of ovarian cancer cells via the downregulation of expression of Wip1. Trop. J. Pharm. Res. 2020, 19, 1417–1422. [Google Scholar] [CrossRef]
- Mrozek-Wilczkiewicz, A.; Kuczak, M.; Malarz, K.; Cieślik, W.; Spaczyńska, E.; Musiol, R. The synthesis and anticancer activity of 2-styrylquinoline derivatives. A p53 independent mechanism of action. Eur. J. Med. Chem. 2019, 177, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Cherdtrakulkiat, R.; Boonpangrak, S.; Sinthupoom, N.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. Derivatives (halogen, nitro and amino) of 8-hydroxyquinoline with highly potent antimicrobial and antioxidant activities. Biochem. Biophys. Rep. 2016, 6, 135–141. [Google Scholar] [CrossRef]
- Rbaa, M.; Haida, S.; Tuzun, B.; Hichar, A.; Hassane, A.E.; Kribii, A.; Lakhrissi, Y.; Hadda, T.B.; Zarrouk, A.; Lakhrissi, B.; et al. Synthesis, characterization and bioactivity of novel 8-hydroxyquinoline derivatives: Experimental, molecular docking, DFT and POM analyses. J. Mol. Struct. 2022, 1258, 132688. [Google Scholar] [CrossRef]
- Budimir, A.; Humbert, N.; Elhabiri, M.; Osinska, I.; Biruš, M.; Albrecht-Gary, A.M. Hydroxyquinoline based binders: Promising ligands for chelatotherapy? J. Inorg. Biochem. 2011, 105, 490–496. [Google Scholar] [CrossRef]
- Farkasová, V.; Drweesh, S.A.; Lüköová, A.; Sabolová, D.; Radojević, I.D.; Čomić, L.R.; Vasić, S.M.; Paulíková, H.; Fečko, S.; Balašková, T.; et al. Low-dimensional compounds containing bioactive ligands. Part VIII: DNA interaction, antimicrobial and antitumor activities of ionic 5,7-dihalo-8-quinolinolato palladium(II) complexes with K+ and Cs+ cations. J. Inorg. Biochem. 2017, 167, 80–88. [Google Scholar] [CrossRef]
- Litecká, M.; Hreusová, M.; Kašpárková, J.; Gyepes, R.; Smolková, R.; Obuch, J.; David, T.; Potočňák, I. Low-dimensional compounds containing bioactive ligands. Part XIV: High selective antiproliferative activity of tris(5-chloro-8-quinolinolato)gallium(III) complex against human cancer cell lines. Bioorgan. Med. Chem. Lett. 2020, 30, 127206. [Google Scholar] [CrossRef]
- Kuchárová, V.; Kuchár, J.; Lüköová, A.; Jendželovský, R.; Majerník, M.; Fedoročko, P.; Vilková, M.; Radojević, I.D.; Čomić, L.R.; Potočňák, I. Low-dimensional compounds containing bioactive ligands. Part XII: Synthesis, structures, spectra, in vitro antimicrobial and cytotoxic activities of zinc(II) complexes with halogen derivatives of quinolin-8-ol. Polyhedron 2019, 170, 447–457. [Google Scholar] [CrossRef]
- Vranec, P.; Potočňák, I. Low-dimensional compounds containing bioactive ligands. Part III: Palladium(II) complexes with halogenated quinolin-8-ol derivatives. J. Mol. Struct. 2013, 1041, 219–226. [Google Scholar] [CrossRef]
- Vranec, P.; Potočňák, I.; Sabolová, D.; Farkasová, V.; Ipóthová, Z.; Pisarčíková, J.; Paulíková, H. Low-dimensional compounds containing bioactive ligands. V: Synthesis and characterization of novel anticancer Pd(II) ionic compounds with quinolin-8-ol halogen derivatives. J. Inorg. Biochem. 2014, 131, 37–46. [Google Scholar] [CrossRef]
- El-Dissouky, A.; Fahmy, A.; Amer, A. Complexing Ability of some r-Lactone Derivatives. Thermal, Magnetic and Spectral Studies on Cobalt(II), Nickel(I1) and Copper(I1) Complexes and their Base Adducts. Inorganica Chim. Acta 1987, 133, 311–316. [Google Scholar] [CrossRef]
- Ananthanarayanan, V. Studies on the vibrational spectrum of the so4- Ion in Crystalline M2′M″(SO4)2· 6H2O (M′ =K or NH4 and M″ =Mg, Zn, Ni, or Co): Observations on the symmetry of the sulfate ion in crystals. J. Chem. Phys. 1968, 48, 573–581. [Google Scholar] [CrossRef]
- Arjunan, V.; Mohan, S.; Ravindran, P.; Mythili, C.V. Vibrational spectroscopic investigations, ab initio and DFT studies on 7-bromo-5-chloro-8-hydroxyquinoline. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2009, 72, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Arici, K. Vibrational Spectra of 4-hydroxy-3-cyano-7-chloro-quinoline by Density Functional Theory and ab initio Hartree-Fock Calculations. Int. J. Chem Technol. 2017, 1, 24–29. Available online: http://dergipark.gov.tr/ijct (accessed on 26 October 2017). [CrossRef][Green Version]
- Zhang, H.R.; Liu, Y.C.; Chen, Z.F.; Guo, J.; Peng, Y.X.; Liang, H. Crystal Structures, Cytotoxicity, Cell Apoptosis Mechanism, and DNA Binding of Two 8-Hydroxylquinoline Zinc(II) Complexes. Russ. J. Coord. Chem. 2018, 44, 322–334. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; Van Rijn, J.; Verschoor, G.C. Synthesis, structure, and spectroscopic properties of copper(II) compounds containing nitrogen–sulphur donor ligands; the crystal and molecular structure of aqua [1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE, Version 2.1; Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Khakhlary, P.; Baruah, J.B. Studies on cluster, salt and molecular complex of zinc-quinolinate. Chem. Sci. J. 2015, 127, 215–223. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wei, J.H.; Chen, Z.F.; Liu, M.; Gu, Y.Q.; Huang, K.-B.; Li, Z.Q.; Liang, H. The antitumor activity of zinc(II) and copper(II) complexes with 5,7-dihalo-substituted-8-quinolinoline. Eur. J. Med. Chem. 2013, 69, 554–563. [Google Scholar] [CrossRef]
- Zhang, H.R.; Liu, Y.C.; Meng, T.; Qin, Q.P.; Tang, S.F.; Chen, Z.F.; Zou, B.Q.; Liu, Y.N.; Liang, H. Cytotoxicity, DNA binding and cell apoptosis induction of a zinc(II) complex of HBrQ. MedChemComm 2015, 6, 2224–2231. [Google Scholar] [CrossRef]
- Drweesh, E.A.; Kuchárová, V.; Volarevic, V.; Miloradovic, D.; Ilic, A.; Radojević, I.D.; Raković, I.R.; Smolková, R.; Vilková, M.; Sabolová, D.; et al. Low-dimensional compounds containing bioactive ligands. Part XVII: Synthesis, structural, spectral and biological properties of hybrid organic-inorganic complexes based on [PdCl4]2− with derivatives of 8-hydroxyquinolinium. J. Inorg. Biochem. 2022, 228, 111697. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-Mello, M.V.; Caballero, A.B.; Lopez-Espinar, A.; Guedes, G.P.; Caubet de Souza, A.M.T.A.; Lanznaster, M.; Gamez, P. DNA-interacting properties of two analogous square-planar cis-chlorido complexes: Copper versus palladium. J. Biol. Inorg. Chem. 2021, 26, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Mansouri-Torshizi, H.; Khosravi, F.; Ghahghaei, A.; Shahraki, S.; Zareian-Jahromi, S. Investigation on the interaction of newly designed potential antibacterial Zn(II) complexes with CT-DNA and HSA. J. Biomol. Struct. Dyn. 2018, 36, 2713–2737. [Google Scholar] [CrossRef]
- Yallur, B.C.; Katrahalli, U.; Krishna, P.M.; Hadagali, M.D. BSA binding and antibacterial studies of newly synthesized 5,6-Dihydroimidazo [2,1-b]thiazole-2-carbaldehyde. Spectrochim. Acta A Mol. Biomol. 2019, 222, 117192. [Google Scholar] [CrossRef] [PubMed]
- Butkus, J.M.; O’Riley, S.; Chohan, B.S.; Basu, S. Interaction of Small Zinc Complexes with Globular Proteins and Free Tryptophan. Int. J. Spectrosc. 2016, 2016, 1378680. [Google Scholar] [CrossRef]
- Rigaku. CrysAlisPRO, Version 1.0.43; Rigaku Oxford Diffraction: Yarnton, UK, 2020. [Google Scholar]
- Petříček, V.; Dušek, M.; Palatinus, L. Crystallographic Computing System JANA2006: General features. Z. Krist.-Cryst. Mater. Online 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–885. [Google Scholar] [CrossRef]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, K. Diamond. Version 3.2K.; Crystal Impact GbR: Bonn, Germany, 2014. [Google Scholar]
- Rojas, J.J.; Ochoa, V.J.; Ocampo, S.A.; Muñoz, J.F. Screening for antimicrobial activity of ten medicinal plants used in Colombian folkloric medicine: A possible alternative in the treatment of non-nosocomial infections. BMC Complement. Altern. Med. 2006, 6, 2. [Google Scholar] [CrossRef] [PubMed]










| δH (mult., J [Hz]) | ||||||
|---|---|---|---|---|---|---|
| H-2 | H-3 | H-4 | H-6 | H-7 | ||
| 1 * | ClQ | 8.74 (br s) | 7.75 (dd, 8.5, 4.5) | 8.54 (d, 8.5) | 7.50 (d, 8.5) | 6.73 (br s) |
| 2 * | ClQ | 8.74 (br s) | 7.75 (dd, 8.4, 4.4) | 8.54 (dd, 8.4, 1.5) | 7.50 (d, 8.4) | 6.74 (d, 8.4) |
| 3 | ClQ | 8.66 (br s) | 7.74 (br s) | 8.52 (br s) | 7.51 (br s) | 6.72 (br s) |
| HClQ | 8.95 (br s) | 7.74 (br s) | 8.52 (br s) | 7.60 (br s) | 7.08 (br s) | |
| 4 ** | dClQ | 8.55 (br s) 8.79 (br s) | 7.70 (m) | 8.50 (d, 8.4) | 7.70 (m) | - |
| 5 ** | dBrQ | 8.47 (br s) 8.76 (br s) | 7.70 (br s) 7.75 (br s) | 8.41 (d, 8.5) 8.44 (br s) | 7.92 (s) 7.92 (s) | - |
| 1 | 2 | 1 | 2 | ||
|---|---|---|---|---|---|
| Zn1-Cl1 | 2.2766 (5) | 2.3149 (5) | O3-Zn1-O1 | 106.79 (6) | 95.66 (5) |
| Zn1-O1 | 2.0153 (13) | 2.0388 (13) | O3-Zn1-N2i | 106.90 (6) | 93.30 (5) |
| Zn1i-O2 | 2.2779 (13) | 2.0789 (12) | O1-Zn1-N2i | 128.77 (6) | 148.68 (5) |
| Zn1-O3 | 2.0134 (12) | 2.0194 (12) | O3-Zn1-Cl1 | 109.17 (4) | 116.24 (4) |
| Zn1i-N2 | 2.1101 (17) | 2.1126 (16) | O1-Zn1-Cl1 | 106.28 (4) | 98.52 (4) |
| Zn2i-O1 | 2.0926 (13) | 2.0893 (12) | N2i-Zn1-Cl1 | 97.67 (5) | 104.23 (4) |
| Zn2-O2 | 2.0809 (12) | 2.0463 (13) | O3-Zn1-O2i | 78.82 (5) | 143.62 (5) |
| Zn2i-O2/O4i a | 2.2058 (12) | 2.2044 (13) | O1-Zn1-O2i | 75.39 (5) | 77.12 (5) |
| Zn2-O3 | 2.1016 (13) | 2.1054 (12) | N2i-Zn1-O2i | 74.63 (6) | 77.92 (5) |
| Zn2i-N1 | 2.0983 (15) | 2.1525 (16) | Cl1-Zn1-O2i | 170.49 (4) | 100.13 (4) |
| Zn2-N3 | 2.0791 (15) | 2.0791 (15) | O2-Zn2-N3 | 100.07 (5) | 108.62 (6) |
| O2-Zn2-O1i | 78.25 (5) | 76.73 (5) | |||
| N3-Zn2-O1i | 102.75 (6) | 170.93 (6) | |||
| O2-Zn2-N1i | 153.47 (6) | 150.34 (5) | |||
| N3-Zn2-N1i | 98.11 (6) | 99.11 (6) | |||
| O1i-Zn2-N1i | 79.01 (5) | 77.27 (5) | |||
| O2-Zn2-O3 | 99.92 (5) | 99.00 (5) | |||
| N3-Zn2-O3 | 78.85 (6) | 79.48 (5) | |||
| O1i-Zn2-O3 | 177.72 (5) | 92.56 (5) | |||
| N1i-Zn2-O3 | 102.44 (5) | 96.15 (5) | |||
| O2-Zn2-O2i/O4i a | 78.62 (5) | 81.48 (5) | |||
| N3-Zn2-O2i/O4 | 156.91 (6) | 92.26 (5) | |||
| O1i-Zn2-O2i/O4 | 99.55 (5) | 95.84 (5) | |||
| N1i-Zn2-O2i/O4 | 91.90 (5) | 87.24 (5) | |||
| O3-Zn2-O2i/O4 | 78.69 (5) | 171.46 (5) |
| Complex | D-H···A | d(D-H) | d(H···A) | d(D···A) | <(DHA) |
|---|---|---|---|---|---|
| 1 | C22-H22···O40 i | 0.95 | 2.32 | 3.127 | 149.9 |
| C26-H26···Cl1A ii | 0.95 | 2.77 | 3.633 | 151.6 | |
| 2 | C22-H22···Cl1 iii | 0.95 | 2.76 | 3.592 | 146.2 |
| C37-H37···Cl1 | 0.95 | 2.80 | 3.661 | 151.2 | |
| C11-H11···O5 | 0.95 | 2.38 | 3.158 | 138.3 | |
| C36-H36···Cl25 i | 0.95 | 2.71 | 3.631 | 162.3 | |
| O4-H1O···O6 | 0.88 | 1.81 | 2.685 | 171.2 | |
| O4-H2O···Cl1 iv | 0.86 | 2.44 | 3.247 | 155.6 |
| Zn1-O1 | 2.097 (3) | O1-Zn1-O2 | 88.54 (11) |
| Zn1-O2 | 2.101 (3) | O1-Zn1-O3 | 91.48 (10) |
| Zn1-O3 | 2.171 (3) | O1-Zn1-N1 | 77.03 (12) |
| Zn1-N1 | 2.142 (3) | O1-Zn1-N2 | 165.42 (12) |
| Zn1-N2 | 2.098 (3) | O1-Zn1-N3 | 93.46 (12) |
| Zn1-N3 | 2.089 (3) | O2-Zn1-N1 | 93.25 (12) |
| O2-Zn1-N2 | 78.93 (12) | ||
| O2-Zn1-N3 | 161.62 (12) | ||
| O3-Zn1-O2 | 84.24 (11) | ||
| O3-Zn1-N1 | 168.32 (12) | ||
| O3-Zn1-N2 | 94.61 (11) | ||
| O3-Zn1-N3 | 77.46 (11) | ||
| N1-Zn1-N2 | 88.3 (3) | ||
| N3-Zn1-N1 | 169.4 (3) | ||
| N3-Zn1-N2 | 84.5 (3) |
| D-H···A | d (D-H) | d (H···A) | d (D···A) | <(DHA) |
|---|---|---|---|---|
| O1-H1-O1 i | 1.202 | 1.202 | 2.399 | 172 |
| O2-H2···O3 i | 1.20 | 1.24 | 2.435 | 174 |
| Zn1-O1w | 2.079 (6) | O1-Zn1-O2 | 172.5 (2) |
| Zn1-O2w | 2.053 (6) | N1-Zn1-O1 | 79.5 (3) |
| Zn1-O3w | 2.078 (6) | N1-Zn1-O2 | 93.0 (3) |
| Zn1-N1 | 2.094 (7) | O1w-Zn1-N1 | 93.5 (3) |
| Zn1-O1 | 2.118 (6) | O1w-Zn1-O1 | 93.1 (2) |
| Zn1-O2 | 2.214 (6) | O1w-Zn1-O2 | 87.9 (2) |
| Zn2-O5 | 2.060 (6) | O2w-Zn1-O2 | 87.4 (2) |
| Zn2-O4w | 2.061 (7) | O2w-Zn1-O3w | 82.8 (2) |
| Zn2-O6 | 2.088 (6) | O2w-Zn1-O1w | 167.9 (2) |
| Zn2-O6w | 2.095 (7) | O2w-Zn1-N1 | 97.8 (3) |
| Zn2-O5w | 2.112 (7) | O2w-Zn1-O1 | 93.1 (3) |
| Zn2-N6 | 2.135 (7) | O3w-Zn1-O1w | 86.4 (3) |
| O3w-Zn1-N1 | 173.8 (3) | ||
| O3w-Zn1-O1 | 94.3 (2) | ||
| O3w-Zn1-O2 | 93.2 (2) | ||
| O4w-Zn2-O6 | 96.0 (3) | ||
| O4w-Zn2-N6 | 94.2 (3) | ||
| O4w-Zn2-O5w | 169.4 (3) | ||
| O4w-Zn2-O6w | 84.5 (3) | ||
| O5-Zn2-O4w | 88.3 (3) | ||
| O5-Zn2-O5w | 87.6 (3) | ||
| O5-Zn2-O6 | 86.6 (2) | ||
| O5-Zn2-O6w | 101.3 (3) | ||
| O5-Zn2-N6 | 165.6 (3) | ||
| O5w-Zn2-N6 | 92.1 (3) | ||
| O6-Zn2-O5w | 93.5 (3) | ||
| O6w-Zn2-O5w | 86.7 (3) | ||
| O6-Zn2-O6w | 172.1 (3) | ||
| O6-Zn2-N6 | 79.1 (3) | ||
| O6w-Zn2-N6 | 93.0 (3) |
| D-H···A | d (D-H) | d (H···A) | d (D···A) | <(DHA) |
|---|---|---|---|---|
| O1w-H1O2···O6 ii | 0.87 | 1.89 | 2.740 | 165.6 |
| O1w-H2O2···O2 ii | 0.87 | 2.16 | 3.021 | 173.0 |
| O2w-H2O4···O6 i | 0.88 | 1.96 | 2.788 | 157.2 |
| O2w-H1O4···O4 | 0.88 | 1.97 | 2.738 | 146.1 |
| O3w-H1O3···O2 ii | 0.87 | 2.05 | 2.879 | 159.3 |
| O3w-H2O3···O4 i | 0.87 | 2.21 | 2.932 | 139.3 |
| O4w-H2O8···O1 ii | 0.87 | 2.05 | 2.789 | 141.7 |
| O4w-H1O8···O3 iv | 0.87 | 2.08 | 2.886 | 153.6 |
| O5w-H1O7···O1 i | 0.87 | 1.87 | 2.707 | 160.7 |
| O5w-H2O7···O3 iii | 0.87 | 1.99 | 2.787 | 152.3 |
| O6-H1O9···O5w iii | 0.87 | 2.01 | 2.855 | 161.2 |
| O6w-H2O9···O4 iii | 0.87 | 2.03 | 2.825 | 151.5 |
| Complex | λmax1 [nm] | λmax2 [nm] | Δλmax2 [nm] | hypochromism at λmax2 [%] | KsvEB ×104 [M−1] | KsvBSA ×106 [M−1] |
|---|---|---|---|---|---|---|
| 1 | 215 | 275 | 2 | 16.32 | 1.06 | 2.04 |
| 2 | 218 | 275 | 1 | 21.72 | 0.62 | 0.68 |
| 3 | 219 | 259 | 4 | 46.54 | 0.34 | 5.44 |
| HeLa | A549 | MCF-7 | MDA-MB-231 | HCT116 | Caco-2 | Cos-7 | |
|---|---|---|---|---|---|---|---|
| 1 | 71.2 | 39.5 | 8.6 | 29.9 | 7.9 | 33.9 | 26.5 |
| 2 | 33.6 | 30.3 | 7.1 | 25.5 | 6.9 | 28.6 | 25.2 |
| 3 | 8.3 | 5.8 | 5.9 | 8.1 | 5.5 | 6.2 | 6.9 |
| 4 | 14.88 | 5.81 | 5.93 | 6.92 | 5.49 | 6.30 | 6.53 |
| 5 | 31.83 | 25.70 | 5.93 | 8.10 | 6.89 | 17.63 | 36.76 |
| HClQ | 39.60 | 18.17 | 6.56 | 9.51 | 5.93 | 103.68 | 20.82 |
| HdClQ | 33.12 | 31.23 | 6.57 | 9.85 | 7.10 | 38.86 | 62.95 |
| HdBrQ | 59.91 | 79.72 | 12.03 | 47.15 | 28.66 | 85.05 | 88.17 |
| cisplatin | 18.82 | 13.50 | 21.60 | 73.40 | 3.28 | 23.20 | 18.30 |
| RIZD (%) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| E. coli | NA | NA | NA | NA | NA |
| S. aureus | NA | NA | NA | NA | 66.97 |
| Compound | 1 | 2 | 3 | 4 a | 5 |
|---|---|---|---|---|---|
| Empirical formula | C60H44Cl8N8O8Zn4 | C66H62Cl8N10O12Zn4 | C54H33Cl6I3N6O6Zn2 | C18H20Cl4N2O12S1Zn2 | C18H20Br4N2O12S1Zn2 |
| Formula weight [g·mol−1] | 1550.11 | 1732.33 | 1586.1 | 761.01 | 938.8 |
| Temperature [K] | 95 (2) | 95 (2) | 95 (2) | 95 (2) | 100 (2) |
| Wavelength [Å] | 1.54184 | 1.54184 | 1.54184 | 1.54184 | 1.54184 |
| Crystal system | Triclinic | Triclinic | Monoclinic | Triclinic | Triclinic |
| Space group | P1 | P1 | I2/c | P1 | P1 |
| Unit cell dimensions [Å, °] | a = 10.5958 (2) b = 11.9402 (2) c = 13.4286 (2) α = 110.555 (1) β = 106.214 (1) γ = 97.530 (1) | a = 11.5637 (1) b = 12.8889 (1) c = 13.9496 (2) α = 108.460 (1) β = 103.392 (1) γ = 107.652 (1) | a = 16.1712 (2) b = 15.5676 (2) c = 22.0518 (3) β = 99.646 (1) | a = 10.2395 (2) b = 18.4516 (5) c = 27.6232 (11) α = 75.983 (3) β = 86.842 (2) γ = 89.267 (2) | a = 7.1417 (2) b = 10.3496 (4) c = 17.6888 (8) α = 89.915 (3) β = 86.591 (3) γ = 88.077 (2) |
| Volume [Å3] | 1477.08 (4) | 1751.46 (4) | 5472.98 (12) | 5055.9 (3) | 1304.40 (9) |
| Z; density (calculated) [g cm−3] | 1; 1.743 | 1; 1.642 | 4; 1.9249 | 8; 1.999 | 2; 2.390 |
| Absorption coefficient [mm−1] | 5.708 | 4.935 | 17.53 | 7.616 | 10.790 |
| F(000) | 780 | 880 | 3072 | 3056 | 908 |
| Crystal shape, color | prism, orange | prism, yellow | prism, dark brown | plate, yellow | plate, yellow |
| Crystal size [mm3] | 0.187 × 0.119 × 0.058 | 0.154 × 0.113 × 0.088 | 0.20 × 0.18 × 0.12 | 0.016 × 0.029 × 0.204 | 0.042 × 0.056 × 0.086 |
| θ range for data collection [°] | 3.753 to 76.298 | 3.584 to 76.466 | 3.45 to 74.56 | 3.285 to 74.115 | 2.502 to 77.357 |
| Index ranges | −12 ≤ h ≤ 13, −15 ≤ k ≤ 15, −16 ≤ l ≤ 16 | −14 ≤ h ≤ 14, −16 ≤ k ≤ 16, −17 ≤ l ≤ 17 | −20 ≤ h ≤ 20, −18 ≤ k ≤ 19, −27 ≤ l ≤ 27 | −12 ≤ h ≤ 10, −23 ≤ k ≤ 21, −30 ≤ l ≤ 34 | −8 ≤ h ≤ 8, −13 ≤ k ≤ 13, −22 ≤ l ≤ 22 |
| Reflections collected/ independent | 54196/6102 [R (int) = 0.0240] | 64538/7280 [R (int) = 0.0348] | 52620/5599 [R (int) = 0.0319] | 30212/19479 [R (int) = 0.0375] | 12316/12316 [R (int) = 0.0705] |
| Data/restraints/ parameters | 6102/0/449 | 7280/0/455 | 5590/0/390 | 19479/0/745 | 12316/0/361 |
| Goodness-of-fit on F2 | 1.022 | 1.047 | 3.579 | 1.078 | 1.091 |
| Final R indices [I>2σ(I)] | R1 = 0.0276, wR2 = 0.0683 | R1 = 0.0282, wR2 = 0.0721 | R1 = 0.0567, wR2 = 0.1826 | R1 = 0.1206, wR2 = 0.3232 | R1 = 0.0527, wR2 = 0.1657 |
| R indices (all data) | R1 = 0.0292, wR2 = 0.0692 | R1 = 0.0316, wR2 = 0.0748 | R1 = 0.0601, wR2 = 0.1854 | R1 = 0.1362, wR2 = 0.3334 | R1 = 0.0567, wR2 = 0.1696 |
| Largest diff. peak and hole [e Å−3] | 0.528 and −0.779 | 0.491 and −0.565 | 2.64 and −1.26 | 3.824 and −1.557 | 1.502 and −0.960 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harmošová, M.; Kello, M.; Goga, M.; Tkáčiková, Ľ.; Vilková, M.; Sabolová, D.; Sovová, S.; Samoľová, E.; Litecká, M.; Kuchárová, V.; et al. Low-Dimensional Compounds Containing Bioactive Ligands. Part XX: Crystal Structures, Cytotoxic, Antimicrobial Activities and DNA/BSA Binding of Oligonuclear Zinc Complexes with Halogen Derivatives of 8-Hydroxyquinoline. Inorganics 2023, 11, 60. https://doi.org/10.3390/inorganics11020060
Harmošová M, Kello M, Goga M, Tkáčiková Ľ, Vilková M, Sabolová D, Sovová S, Samoľová E, Litecká M, Kuchárová V, et al. Low-Dimensional Compounds Containing Bioactive Ligands. Part XX: Crystal Structures, Cytotoxic, Antimicrobial Activities and DNA/BSA Binding of Oligonuclear Zinc Complexes with Halogen Derivatives of 8-Hydroxyquinoline. Inorganics. 2023; 11(2):60. https://doi.org/10.3390/inorganics11020060
Chicago/Turabian StyleHarmošová, Michaela, Martin Kello, Michal Goga, Ľudmila Tkáčiková, Mária Vilková, Danica Sabolová, Simona Sovová, Erika Samoľová, Miroslava Litecká, Veronika Kuchárová, and et al. 2023. "Low-Dimensional Compounds Containing Bioactive Ligands. Part XX: Crystal Structures, Cytotoxic, Antimicrobial Activities and DNA/BSA Binding of Oligonuclear Zinc Complexes with Halogen Derivatives of 8-Hydroxyquinoline" Inorganics 11, no. 2: 60. https://doi.org/10.3390/inorganics11020060
APA StyleHarmošová, M., Kello, M., Goga, M., Tkáčiková, Ľ., Vilková, M., Sabolová, D., Sovová, S., Samoľová, E., Litecká, M., Kuchárová, V., Kuchár, J., & Potočňák, I. (2023). Low-Dimensional Compounds Containing Bioactive Ligands. Part XX: Crystal Structures, Cytotoxic, Antimicrobial Activities and DNA/BSA Binding of Oligonuclear Zinc Complexes with Halogen Derivatives of 8-Hydroxyquinoline. Inorganics, 11(2), 60. https://doi.org/10.3390/inorganics11020060