Exploring the Interdependence between Electronically Unfavorable Situations and Pressure in a Chalcogenide Superconductor
Abstract
1. Introduction
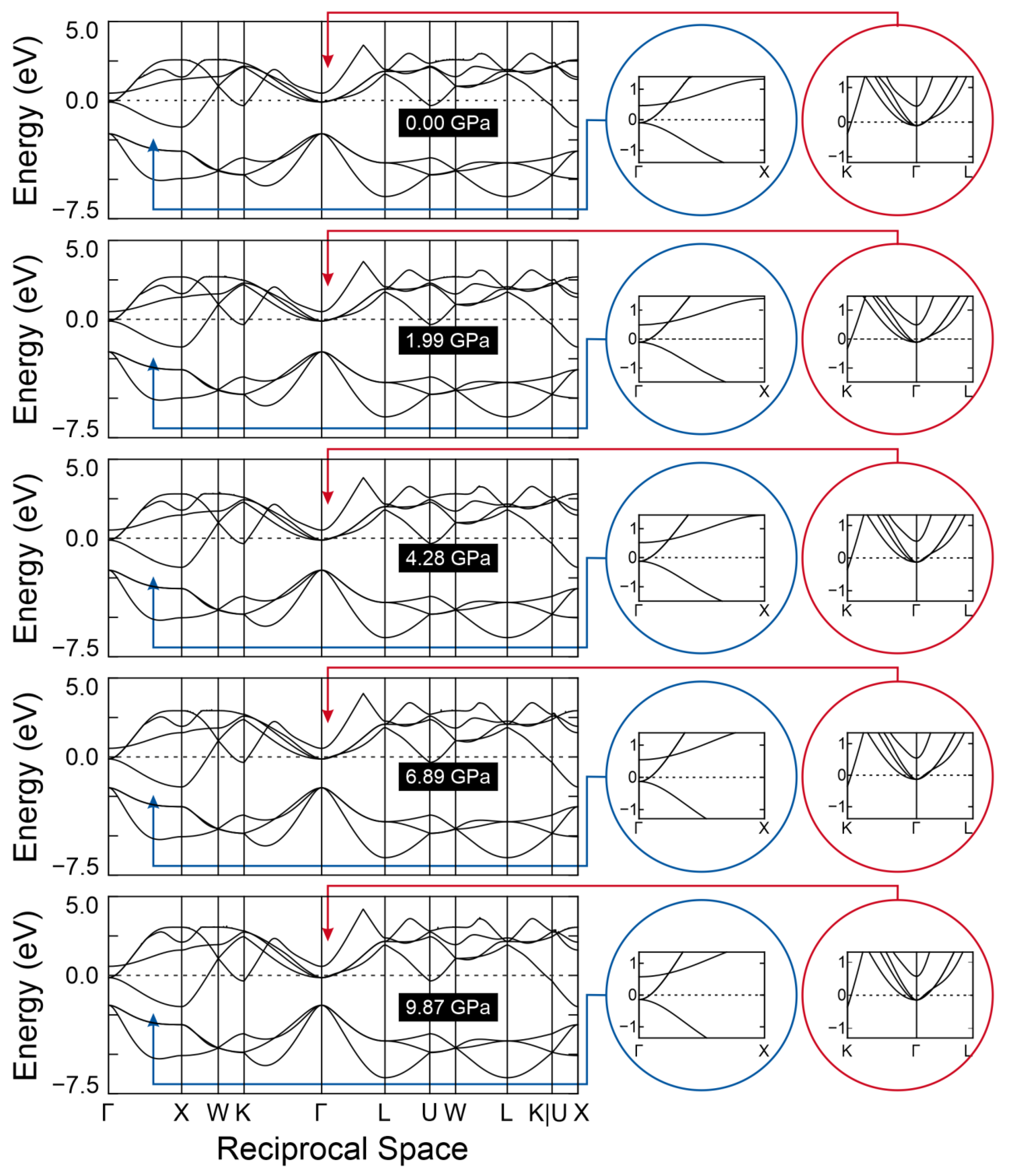
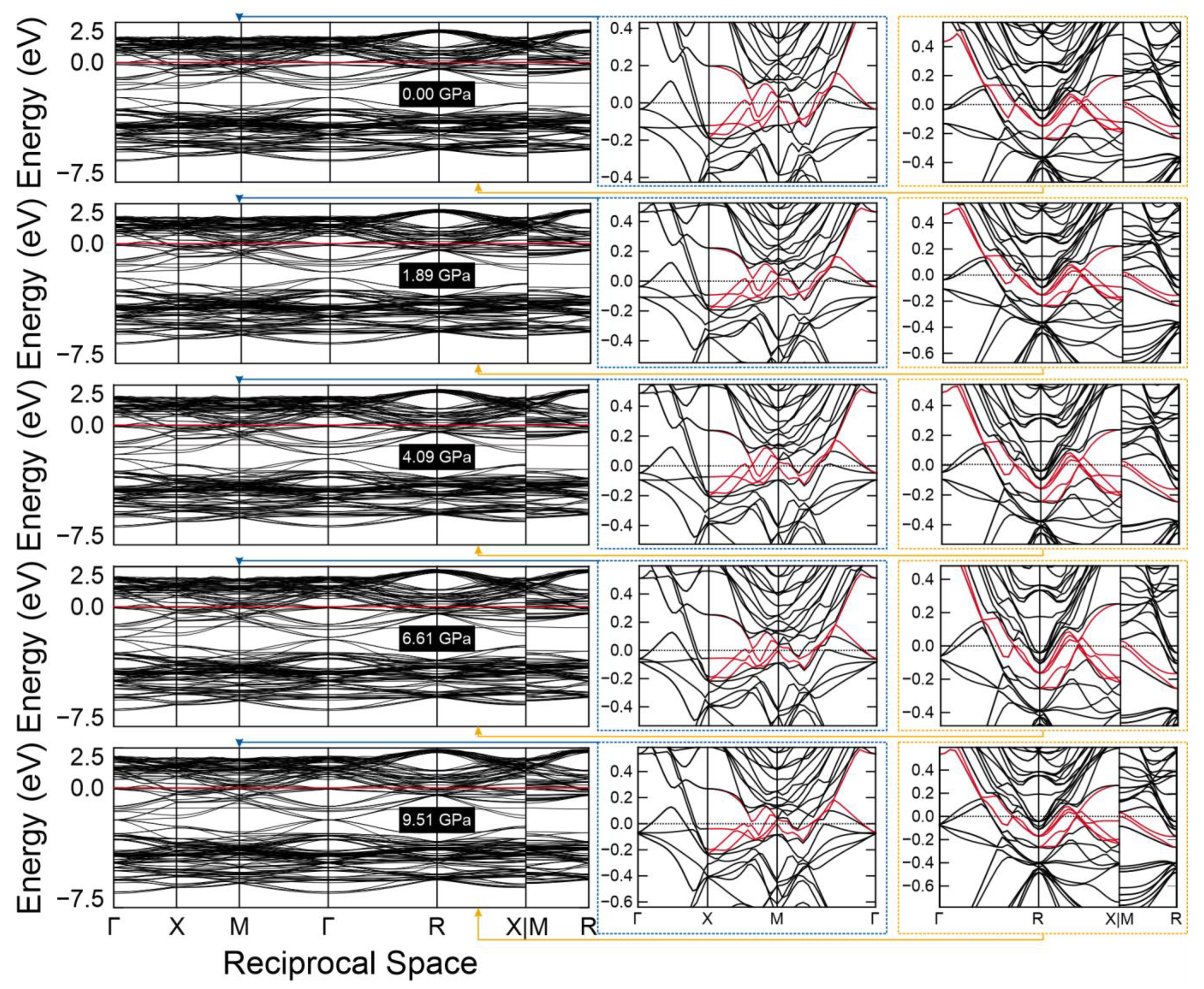
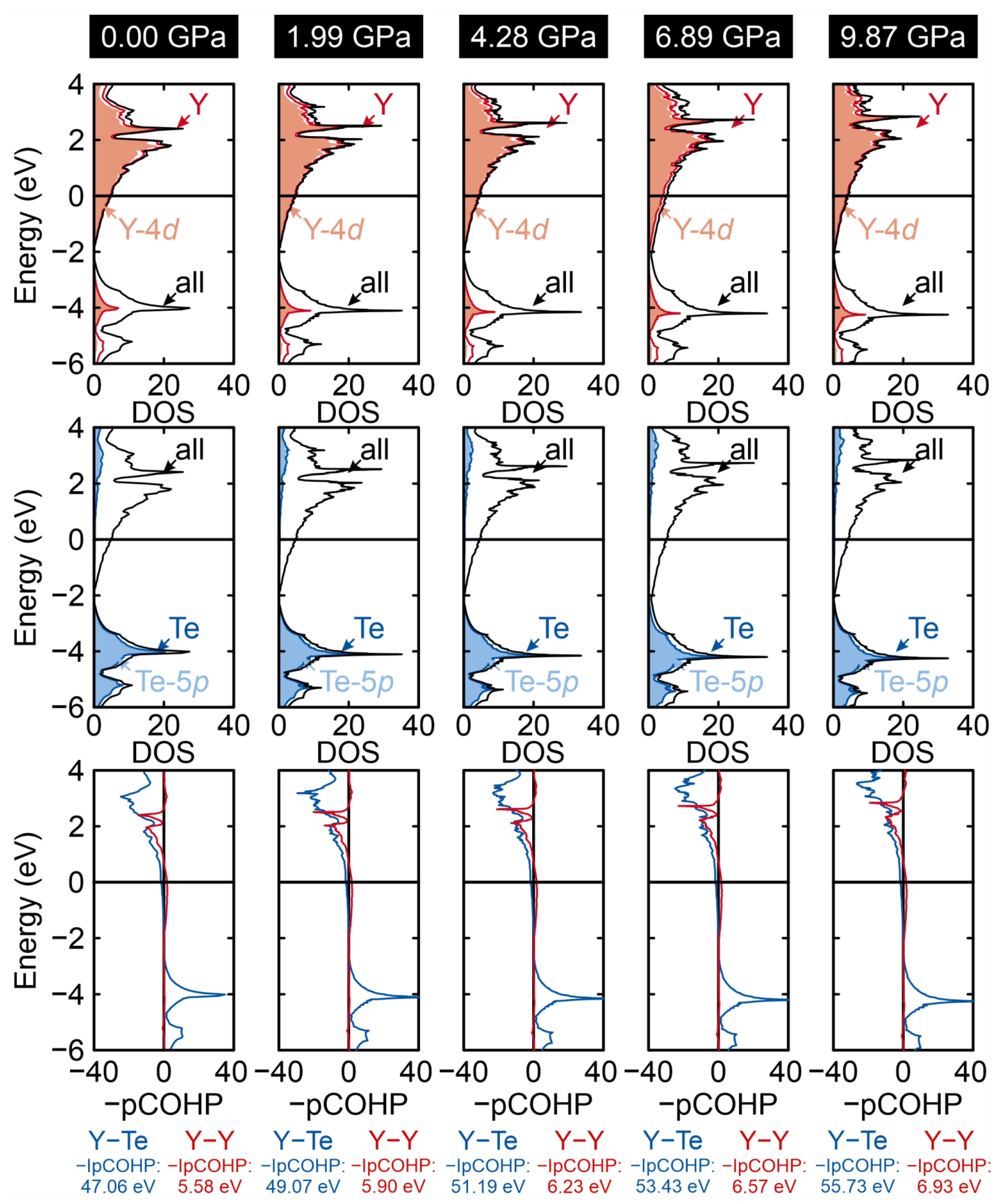
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schmitt, D.C.; Drake, B.L.; McCandless, G.T.; Chan, J.Y. Targeted Crystal Growth of Rare Earth Intermetallics with Synergistic Magnetic and Electrical Properties: Structural Complexity to Simplicity. Acc. Chem. Res. 2015, 48, 612–618. [Google Scholar] [CrossRef]
- Kanatzides, M.G. Discovery-Synthesis, Design, and Prediction of Chalcogenide Phases. Inorg. Chem. 2017, 56, 3158–3173. [Google Scholar] [CrossRef]
- Wuttig, M.; Yamada, N. Phase-change materials for rewriteable data storage. Nat. Mater. 2007, 6, 824–832. [Google Scholar] [CrossRef] [PubMed]
- Raoux, S.; Welnic, W.; Ielmini, D. Phase Change Materials and Their Application to Nonvolatile Memories. Chem. Rev. 2010, 110, 240–267. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Sootsman, J.R.; Chung, D.Y.; Kanatzidis, M.G. New and Old Concepts in Thermoelectric Materials. Angew. Chem. Int. Ed. 2009, 48, 8616–8639. [Google Scholar] [CrossRef] [PubMed]
- Hazan, M.Z.; Kane, C.L. Colloquium: Topological Insulators. Rev. Mod. Phys. 2010, 82, 3045–3067. [Google Scholar] [CrossRef]
- Nagata, S.; Atake, T. Survey of chalcogenide superconductors. J. Therm. Anal. Calorim. 1999, 57, 807–821. [Google Scholar] [CrossRef]
- Fries, K.S.; Steinberg, S. Fermi-Level Characteristics of Potential Chalcogenide Superconductors. Chem. Mater. 2018, 30, 2251–2261. [Google Scholar] [CrossRef]
- Miller, G.J. The “Coloring Problem” in Solids: How It Affects Structure, Composition and Properties. Eur. J. Inorg. Chem. 1998, 1998, 523–536. [Google Scholar] [CrossRef]
- Böttcher, P. Tellurium-Rich Tellurides. Angew. Chem. Int. Ed. Engl. 1988, 27, 759–772. [Google Scholar] [CrossRef]
- Papoian, G.A.; Hoffmann, R. Hypervalent Bonding in One, Two, and Three Dimensions: Extending the Zintl-Klemm Concept to Nonclassical Electron-Rich Networks. Angew. Chem. Int. Ed. 2000, 39, 2408–2448. [Google Scholar] [CrossRef]
- Zintl, E. Intermetallische Phasen. Angew. Chem. 1939, 52, 1–6. [Google Scholar] [CrossRef]
- Schäfer, H.; Eisenmann, B.; Müller, W. Zintl Phases: Transitions between Metallic and Ionic Bonding. Angew. Chem. Int. Ed. 1973, 12, 694–712. [Google Scholar] [CrossRef]
- Janka, O.; Kauzlarich, S. Zintl Compounds. In Encyclopedia of Inorganic and Bioinorganic Chemistry; Scott, R.A., Ed.; WILEY-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Miller, G.J.; Schmidt, M.W.; Wang, F.; You, T.-S. Quantitative Advances in the Zintl-Klemm Formalism. Struct. Bond. 2011, 139, 1–55. [Google Scholar] [CrossRef]
- Nesper, R. The Zintl-Klemm Concept—A Historical Survey. Z. Anorg. Allg. Chem. 2014, 640, 2639–2648. [Google Scholar] [CrossRef]
- Gärtner, S.; Korber, N. Polyanions of Group 14 and Group 15 Elements in Alkali and Alkaline Earth Metal Solid State Compounds and Solvate Structures. Struct. Bond. 2011, 140, 25–57. [Google Scholar]
- Ertural, C.; Steinberg, S.; Dronskowski, R. Development of a robust tool to extract Mulliken and Löwdin charges from plane waves and its applications to solid-state materials. RSC Adv. 2019, 9, 29821–29830. [Google Scholar] [CrossRef]
- Wuttig, M.; Deringer, V.L.; Gonze, X.; Bichara, C.; Raty, J.-Y. Incipient Metals: Functional Materials with a Unique Bonding Mechanism. Adv. Mater. 2018, 30, 1803777. [Google Scholar] [CrossRef]
- Raty, J.-Y.; Schumacher, M.; Golub, P.; Deringer, V.L.; Gatti, C.; Wuttig, M. A Quantum-Mechanical Map for Bonding and Properties in Solids. Adv. Mater. 2019, 31, 1806280. [Google Scholar] [CrossRef]
- Cheng, Y.; Cojocaru-Mirédin, O.; Keutgen, J.; Yu, Y.; Küpers, M.; Schumacher, M.; Golub, P.; Raty, J.-Y.; Dronskowski, R.; Wuttig, M. Understanding the Structure and Properties of Sesqui-Chalcogenides (i.e., V2VI3 of Pn2Ch3 (Pn = Pnictogen, Ch = Chalcogen) Compounds) from a Bonding Perspective. Adv. Mater. 2019, 31, 1904316. [Google Scholar] [CrossRef]
- Maier, S.; Steinberg, S.; Cheng, Y.; Schön, C.-F.; Schumacher, M.; Mazzarello, R.; Golub, P.; Nelson, R.; Cojocaru-Mirédin, O.; Raty, J.-Y.; et al. Discovering Electron-Transfer Driven Changes in Chemical Bonding in Lead Chalcogenides (PbX, where X = Te, Se, S, O). Adv. Mater. 2020, 32, 2005533. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.; Hempelmann, J.; Fries, K.S.; Müller, P.C.; Dronskowski, R.; Steinberg, S. Bonding diversity in rock salt-type tellurides: Examining the interdependence between chemical bonding and materials properties. RSC Adv. 2021, 11, 20679–20686. [Google Scholar] [CrossRef]
- Hempelmann, J.; Müller, P.C.; Konze, P.M.; Stoffel, R.P.; Steinberg, S.; Dronskowski, R. Longe-Range Forces in Rock-Salt-Type Tellurides and How they Mirror the Underlying Chemical Bonding. Adv. Mater. 2021, 33, 2100163. [Google Scholar] [CrossRef]
- Gladisch, F.C.; Steinberg, S. Revealing the Nature of Bonding in Rare-Earth Transition-Metal Tellurides by Means of Methods Based on First Principles. Eur. J. Inorg. Chem. 2017, 2017, 3395–3400. [Google Scholar] [CrossRef]
- Göbgen, K.C.; Gladisch, F.C.; Steinberg, S. The Mineral Stützite: A Zintl-Phase or Polar Intermetallic? A Case Study Using Experimental and Quantum-Chemical Techniques. Inorg. Chem. 2018, 57, 412–421. [Google Scholar] [CrossRef]
- Göbgen, K.C.; Fries, K.S.; Gladisch, F.C.; Dronskowski, R.; Steinberg, S. Revealing the Nature of Chemical Bonding in an ALn2Ag3Te5-Type Alkaline-Metal (A) Lanthanide (Ln) Silver Telluride. Inorganics 2019, 7, 70. [Google Scholar] [CrossRef]
- Eickmeier, K.; Fries, K.S.; Gladisch, F.C.; Dronskowski, R.; Steinberg, S. Revisiting the Zintl-Klemm Concept for ALn2Ag3Te5-Type Alkaline-Metal (A) Lanthanide (Ln) Silver Tellurides. Crystals 2020, 10, 184. [Google Scholar] [CrossRef]
- Eickmeier, K.; Steinberg, S. Revealing the Bonding Nature in an ALnZnTe3-Type Alkaline-Metal (A) Lanthanide (Ln) Zinc Telluride by Means of Experimental and Quantum-Chemical Techniques. Crystals 2020, 10, 916. [Google Scholar] [CrossRef]
- Smid, S.; Steinberg, S. Probing the Validity of the Zintl-Klemm Concept for Alkaline-Metal Copper Tellurides by Means of Quantum-Chemical Techniques. Materials 2020, 13, 2178. [Google Scholar] [CrossRef]
- Eickmeier, K.; Steinberg, S. Exploring the frontier between polar intermetallics and Zintl phases for the examples of the prolific ALnTnTe3-type alkali metal (A) lanthanide (Ln) late transition metal (Tn) tellurides. Z. Naturforsch. B 2021, 76, 635–642. [Google Scholar] [CrossRef]
- Gladisch, F.C.; van Leusen, J.; Passia, M.T.; Kögerler, P.; Steinberg, S. Rb3Er4Cu5Te10: Exploring the Frontier between Polar Intermetallics and Zintl-Phases via Experimental and Quantumchemical Approaches. Eur. J. Inorg. Chem. 2021, 2021, 4946–4953. [Google Scholar] [CrossRef]
- Gladisch, F.C.; Pippinger, T.; Meyer, J.; Pries, J.; Richter, J.; Steinberg, S. Examination of a Structural Preference in Quaternary Alkali-Metal (A) Rare-Earth (R) Copper Tellurides by Combining Experimental and Quantum-chemical Means. Inorg. Chem. 2022, 61, 9269–9282. [Google Scholar] [CrossRef] [PubMed]
- Eickmeier, K.; Poschkamp, R.; Dronskowski, R.; Steinberg, S. Exploring the Impact of Lone Pairs on the Structural Features of Alkaline-Earth (A) Transition-Metal (M,M’) Chalcogenides (Q) AMM’Q3. Eur. J. Inorg. Chem. 2022, 2022, e202200360. [Google Scholar] [CrossRef]
- Koch, P.; Steinberg, S. Exploring the subtle factors that control the structural preferences in Cu7Te4. J. Phys. Condens. Matter 2023, 35, 064003. [Google Scholar] [CrossRef] [PubMed]
- Simons, J.; Steinberg, S. Identifying the Origins of Vacancies in the Crystal Structures of Rock Salt-type Chalcogenide Superconductors. ACS Omega 2019, 4, 15721–15728. [Google Scholar] [CrossRef]
- Hulliger, F.; Hull, G.W. Superconductivity in rocksalt-type compounds. Solid State Sci. 1970, 8, 1379–1382. [Google Scholar] [CrossRef]
- Moodenbaugh, A.R.; Johnston, D.C.; Viswanathan, R.; Shelton, R.N.; DeLong, L.E.; Fertig, W.A. Superconductivity of Transition Metal Sulfides, Selenides, and Phosphides with the NaCI Structure. J. Low Temp. Phys. 1978, 33, 175–203. [Google Scholar] [CrossRef]
- Zaanen, J.; Chakravarty, S.; Senthil, T.; Anderson, P.; Lee, P.; Schmalian, J.; Imada, M.; Pines, D.; Randeria, M.; Varma, C.; et al. Towards a complete theory of high Tc. Nat. Phys. 2006, 2, 138–143. [Google Scholar] [CrossRef]
- Bardeen, J.; Cooper, L.N.; Schrieffer, J.R. Theory of Superconductivity. Phys. Rev. 1957, 108, 1175–1204. [Google Scholar] [CrossRef]
- Gabovich, A.M.; Voitenko, A.I.; Ausloss, M. Charge- and spin-density waves in existing superconductors: Competition between Cooper pairing and Peierls or excitonic instabilities. Phys. Rep. 2002, 367, 583–709. [Google Scholar] [CrossRef]
- Simon, A. Superconductivity and Chemistry. Angew. Chem. Int Ed. Engl. 1997, 36, 1788–1806. [Google Scholar] [CrossRef]
- Simon, A. Superconductivity—A source of surprises. Solid State Sci. 2005, 7, 1451–1455. [Google Scholar] [CrossRef]
- Vaitheeswaran, G.; Kanchana, V.; Svane, A.; Christensen, N.E.; Olsen, J.S.; Jorgensen, J.-E.; Gerward, L. High-pressure stuctural study of yttrium monochalcogenides from experiment and theory. Phys. Rev. B Condens. Matter Mater. Phys. 2011, 83, 184108. [Google Scholar] [CrossRef]
- Flahaut, J.; Domange, L.; Guittard, M.; Pardo, M.-P.; Partie, M. Nouveaux résultats relatifs à l’etude cristallographique des sulfures, séléniures et tellurures L2 × 3 des éléments des terres rares, de l’yttrium et du scandium. CR Hebd. Séances Acad. Sci. 1963, 257, 1530–1533. [Google Scholar]
- Dronskowski, R. Computational Chemistry of Solid State Materials; WILEY-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Hoffmann, R. How Chemistry and Physics Meet in the Solid State. Angew. Chem. Int. Ed. 1987, 26, 846–878. [Google Scholar] [CrossRef]
- Rustige, C.; Brühmann, M.; Steinberg, S.; Meyer, E.; Daub, K.; Zimmermann, S.; Wolberg, M.; Mudring, A.-V.; Meyer, G. The Prolific {ZR6}X12R and {ZR6}X10 Structure Types with Isolated Endohedrally Stabilized (Z) Rare-Earth Metal (R) Cluster Halide (X) Complexes. Z. Anorg. Allg. Chem. 2012, 638, 1922–1931. [Google Scholar] [CrossRef]
- Steinberg, S.; Brgoch, J.; Miller, G.J.; Meyer, G. Identifying a Structural Preference in Reduced Rare-Earth Metal Halides by Combining Experimental and Computational Techniques. Inorg. Chem. 2012, 51, 11356–11364. [Google Scholar] [CrossRef]
- Steinberg, S.; Bell, T.; Meyer, G. Electron Counting Rules and Electronic Structure in Tetrameric Transition-Metal (T)-Centered Rare-Earth (R) Cluster Complex Halides (X). Inorg. Chem. 2015, 54, 1026–1037. [Google Scholar] [CrossRef]
- Deringer, V.L.; Lumeij, M.-W.; Stoffel, R.P.; Dronskowski, R. Mechanisms of Atomic Motion Through Crystalline GeTe. Chem. Mater. 2013, 25, 2220–2226. [Google Scholar] [CrossRef]
- Pyykkö, P. Strong Closed-Shell Interactions in Inorganic Chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef] [PubMed]
- Gladisch, F.C.; Steinberg, S. Revealing Tendencies in the Electronic Structures of Polar Intermetallic Compounds. Crystals 2018, 8, 80. [Google Scholar] [CrossRef]
- Steinberg, S.; Dronskowski, R. The Crystal Orbital Hamilton Population (COHP) Method as a Tool to Visualize and Analyze Chemical Bonding in Intermetallic Compounds. Crystals 2018, 8, 225. [Google Scholar] [CrossRef]
- Burdett, J.K.; Hughbanks, T. Niobium oxide (NbO) and titanium oxide (TiO): A study of the structural and electronic stability of structures derived from rock salt. J. Am. Chem. Soc. 1984, 106, 3101–3113. [Google Scholar] [CrossRef]
- Burdett, J.K.; Mitchell, J.F. Electronic Origin of Nonstoichiometry in Early-Transition-Metal Chalcogenides. Chem. Mater. 1993, 5, 1465–1473. [Google Scholar] [CrossRef]
- Gladisch, F.C.; Maier, S.; Steinberg, S. Eu2CuSe3 Revisited by Means of Experimental and Quantum-Chemical Techniques. Eur. J. Inorg. Chem. 2021, 2021, 1510–1517. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented wave-method. Phys. Rev. B Condens. Matter Mater. Phys. 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Marsman, M.; Furthmüller, J. Vienna Ab Initio Simulation Package (VASP), The User Guide; Department of Computational Materials Physics, Faculty of Physics, University of Vienna: Vienna, Austria, 2010. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. J. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter Mater. Phys. 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B Condens. Matter Mater. Phys. 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- von Appen, J. Quantenchemische Untersuchungen an Nitriden der Platingruppenmetalle. PhD Thesis, RWTH Aachen University, Aachen, Germany, 2006. [Google Scholar]
- Stoffel, R.P.; Wessel, C.; Lumey, M. Ab Initio Thermochemistry of Solid-State Materials. Angew. Chem. Int. Ed. 2010, 49, 5242–5266. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Curtarolo, S.; Setyawan, W.; Hart, G.L.; Jahnatek, M.; Chepulskii, R.V.; Taylor, R.H.; Wang, S.; Xue, J.; Yang, K.; Levy, O.; et al. AFLOW: An automatic framework for high-throughput materials discovery. Comput. Mater. Sci. 2012, 58, 218–226. [Google Scholar] [CrossRef]
- Ong, S.P.; Richards, W.D.; Jain, A.; Hautier, G.; Kocher, M.; Cholia, S.; Gunter, D.; Chevrier, V.L.; Persson, K.; Ceder, G. Python Materials Genomics (pymatgen): A Robust, Open-Source Python Library for Materials Analysis. Comput. Mater. Sci. 2013, 68, 314–319. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Savin, A.; Nesper, R.; Wengert, S.; Fässler, T.F. ELF: The Electron Localization Function. Angew. Chem. Int Ed. Engl. 1997, 36, 1808–1832. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. LOBSTER: A Tool to Extract Chemical Bonding from Plane-Wave Based DFT. J. Comput. Chem. 2016, 37, 1030–1035. [Google Scholar] [CrossRef]
- Maintz, S.; Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Analytic Projection from Plane-Wave and PAW Wavefunctions and Application to Chemical-Bonding Analysis in Solids. J. Comput. Chem. 2013, 34, 2557–2567. [Google Scholar] [CrossRef]
- Deringer, V.L.; Tchougréeff, A.L.; Dronskowski, R. Crystal Orbital Hamilton Population (COHP) Analysis as Projected from Plane-Wave Basis Sets. J. Phys. Chem. A 2011, 115, 5461–5466. [Google Scholar] [CrossRef] [PubMed]
- Dronskowski, R.; Blöchl, P.E. Crystal Orbital Hamilton Populations (COHP). Energy-Resolved Visualization of Chemical Bonding in Solids Based on Density-Functional Calculations. J. Phys. Chem. 1993, 97, 8617–8624. [Google Scholar] [CrossRef]
- Eck, B. wxDragon 2.2.3; RWTH Aachen University: Aachen, Germany, 2020. [Google Scholar]
- Huppertz, H.; Heymann, G.; Schwarz, U.; Schwarz, M.R. High-Pressure Methods in Solid-State Chemistry. In Handbook of Solid State Chemistry; Dronskowski, R., Kikkawa, S., Stein, A., Eds.; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Schwarz, K.; Blaha, P. DFT Calculations for Real Solids. In Handbook of Solid State Chemistry; Dronskowski, R., Kikkawa, S., Stein, A., Eds.; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Hafner, J. Ab-Initio Simulations of Materials Using VASP: Density-Functional Theory and Beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef] [PubMed]
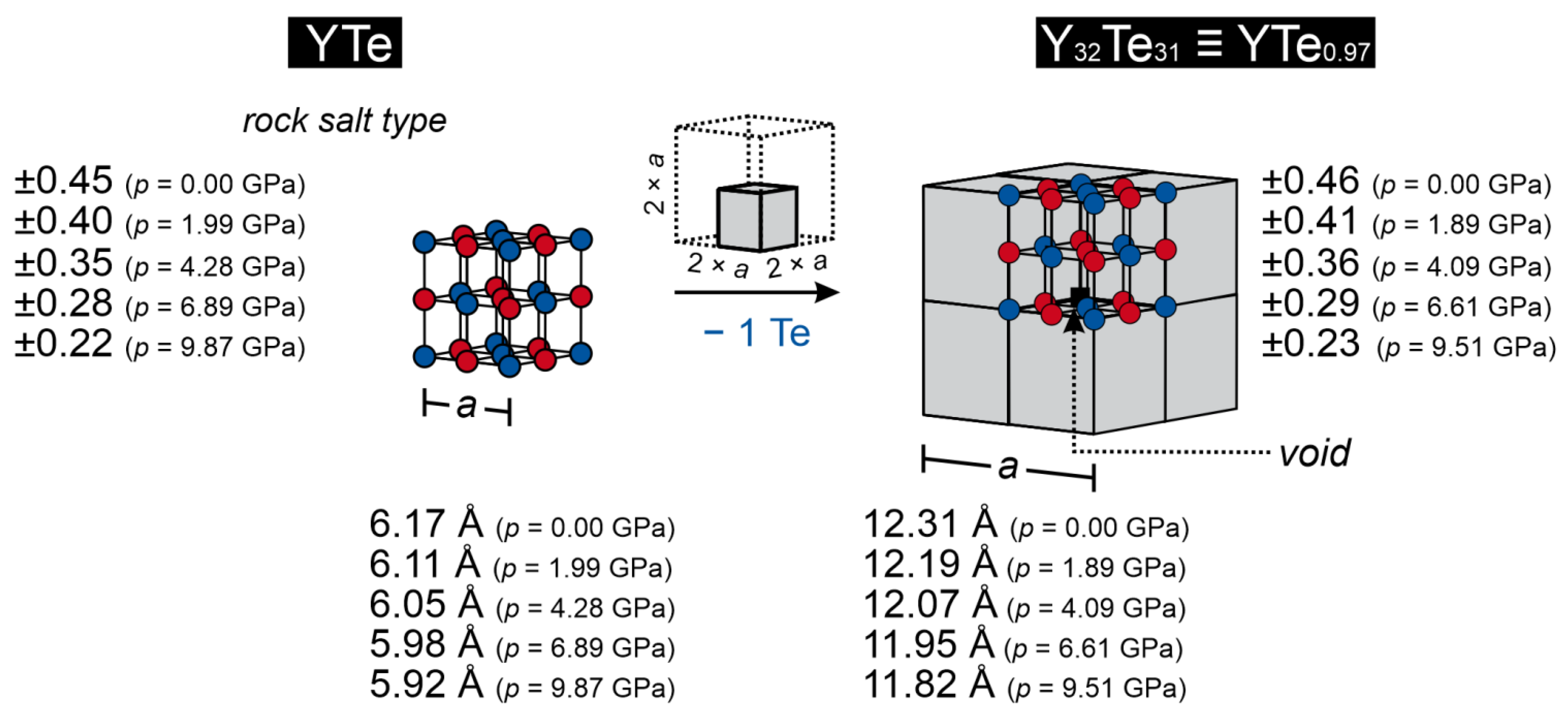
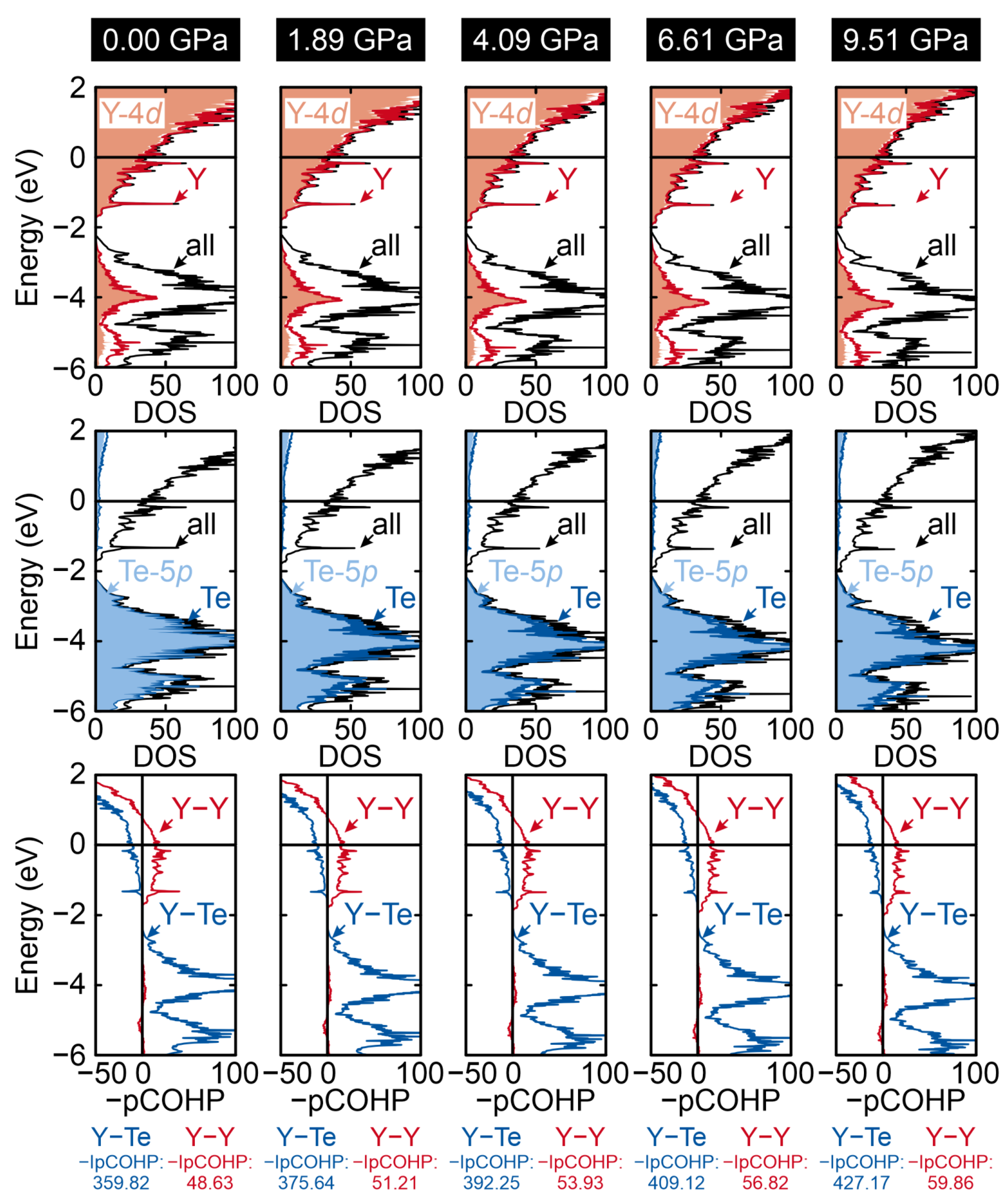
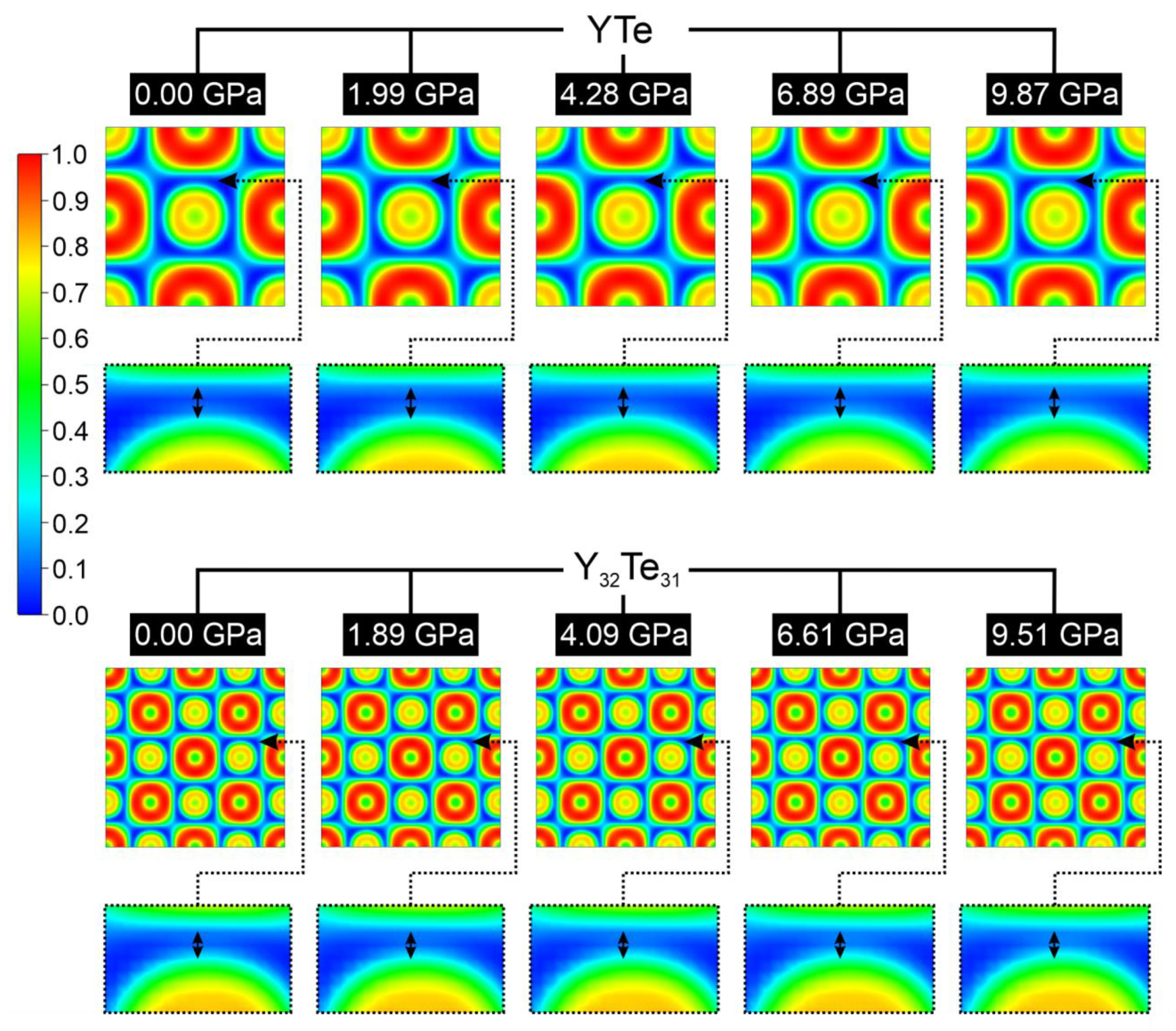
| YTe | Y32Te31 ≡ YTe0.97 | ||||||
|---|---|---|---|---|---|---|---|
| p [GPa] | Etot [eV/f.u.] | % (Y–Te) | % (Y–Y) | p [GPa] | Etot [eV/f.u.] | % (Y–Te) | % (Y–Y) |
| 0.00 | −12.290 | 89.40 | 10.60 | 0.00 | −12.013 | 88.09 | 11.91 |
| 1.99 | −12.198 | 89.27 | 10.73 | 1.89 | −12.003 | 88.00 | 12.00 |
| 4.28 | −12.165 | 89.15 | 10.85 | 4.09 | −11.972 | 87.91 | 12.09 |
| 6.89 | −12.107 | 89.05 | 10.95 | 6.61 | −11.917 | 87.81 | 12.19 |
| 9.87 | −12.022 | 88.94 | 11.06 | 9.51 | −11.835 | 87.71 | 12.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fries, K.S.; Steinberg, S. Exploring the Interdependence between Electronically Unfavorable Situations and Pressure in a Chalcogenide Superconductor. Inorganics 2023, 11, 61. https://doi.org/10.3390/inorganics11020061
Fries KS, Steinberg S. Exploring the Interdependence between Electronically Unfavorable Situations and Pressure in a Chalcogenide Superconductor. Inorganics. 2023; 11(2):61. https://doi.org/10.3390/inorganics11020061
Chicago/Turabian StyleFries, Kai S., and Simon Steinberg. 2023. "Exploring the Interdependence between Electronically Unfavorable Situations and Pressure in a Chalcogenide Superconductor" Inorganics 11, no. 2: 61. https://doi.org/10.3390/inorganics11020061
APA StyleFries, K. S., & Steinberg, S. (2023). Exploring the Interdependence between Electronically Unfavorable Situations and Pressure in a Chalcogenide Superconductor. Inorganics, 11(2), 61. https://doi.org/10.3390/inorganics11020061








