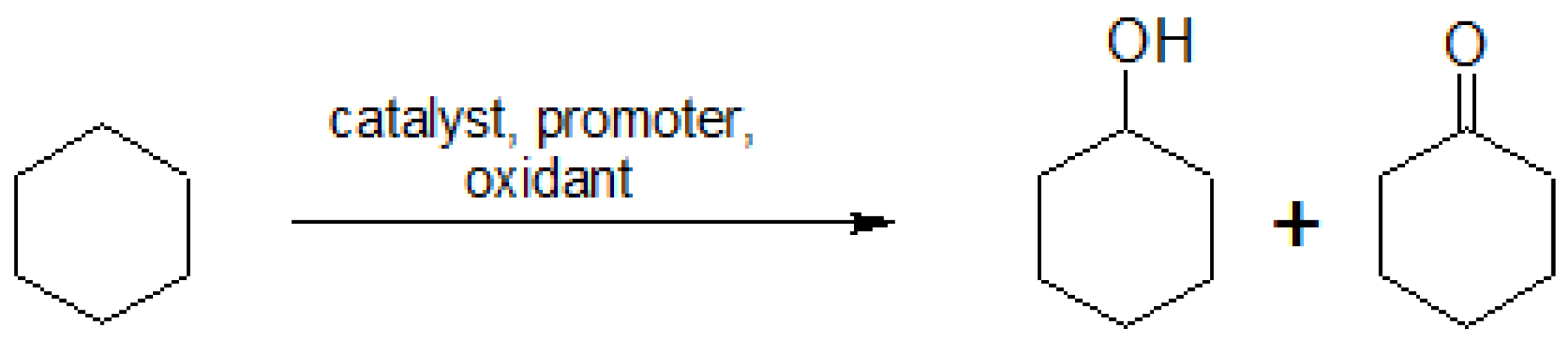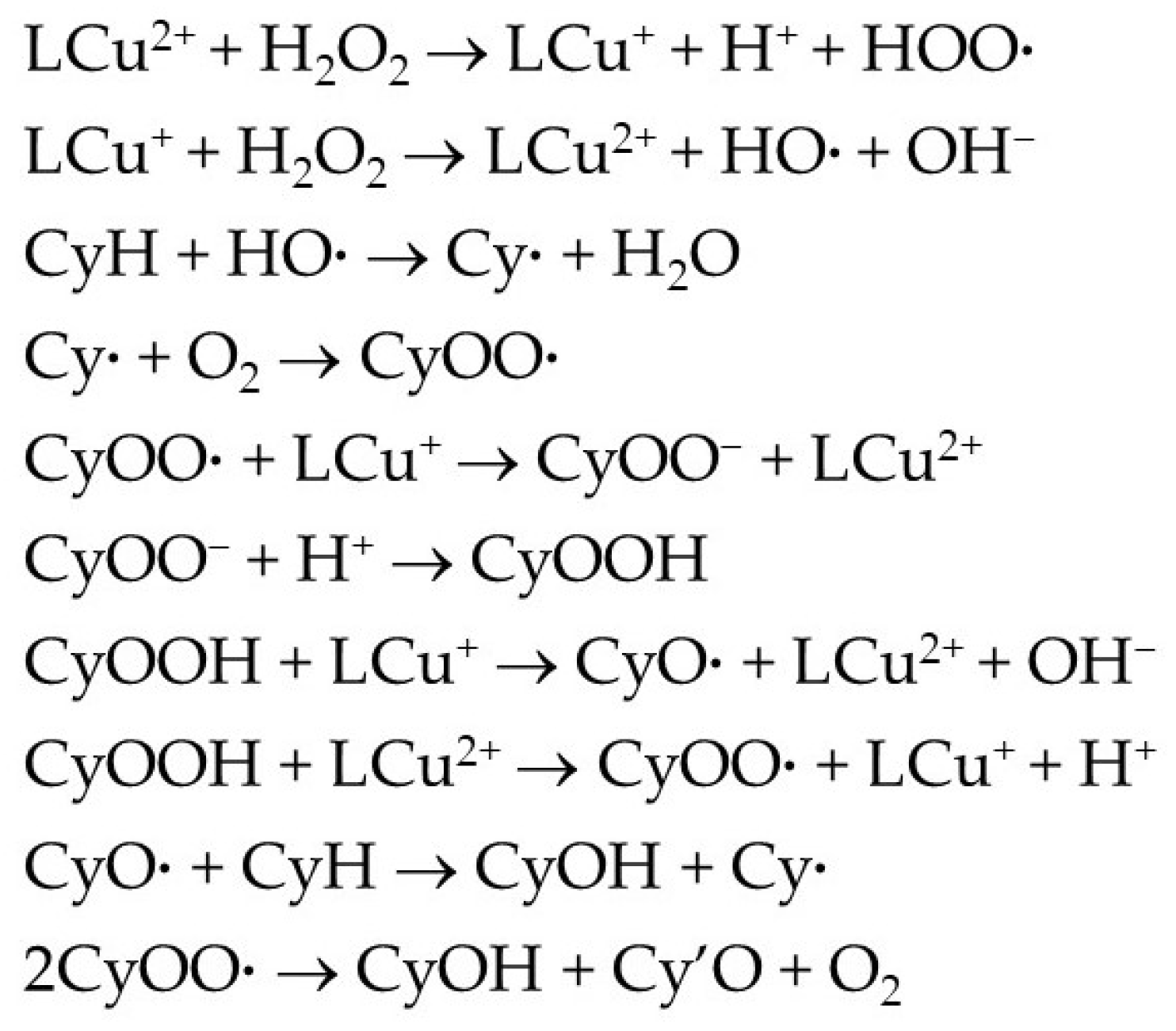Abstract
Two tetranuclear and two mononuclear Cu(II) complexes with arylhydrazones of malononitrile derived ligands (compounds 1–2 and 3–4, respectively), one trinuclear Co(II/III) complex with an arylhydrazone of acetoacetanilide (5) and one tetranuclear Zn(II) complex of 3-(2-carboxyphenyl-hydrazone)pentane-2,4-dione (6) were screened as potential catalysts in the peroxidative oxidation of cyclohexane by aqueous H2O2 in acetonitrile. The best results were attained in the presence of pyrazine-2-carboxylic acid (PCA) with 1 (26% yield, TON = 52.0) and with 2 (24%, TON = 48.0) after 4 h at 40 °C. In the presence of complexes 5 and 6, no oxygenated products were detected in the studied conditions. The employment of non-conventional conditions like supercritical carbon dioxide (scCO2) as reaction medium or microwave (MW) irradiation was assessed for complexes 1 and 2. After 6 h in acetonitrile–scCO2, at 50 °C and with HNO3 as promoter, only 17% yield was achieved using 1 as catalyst, and 21% using 2. Total yields of oxygenates up to 14 (with 1) and 13% (2) and TOFs of 56.0 and 52.0 h−1, respectively, were obtained working under MW irradiation at 70 °C and for the much shorter time of 0.5 h.
1. Introduction
Alkanes, the main components of natural gas and oil [1,2,3,4], are highly abundant and relatively inexpensive potential carbon raw materials for the synthesis of added value organic chemicals [1,2,4,5,6,7,8], such as alcohols, ketones, aldehydes, carboxylic acids [1,8], amides, amines [1]. However, the intrinsic inertness of saturated hydrocarbons hampers their broad synthetic use under mild conditions [1,2,5,6,7,9]; such transformations commonly require the presence of (typically precious) metal catalysts and the use of strongly acidic reaction media [3,4,9] although resulting in low-product yields and selectivities [1].
Hence, the search for mild, efficient, selective and sustainable (environmentally benign [4]) methods for the functionalization of alkanes to industrially valuable products constitute a challenge to modern chemistry [1,2,3,4,6,7,9] and a subject of high relevance in various areas including catalysis [1,2,6,7,8], organic synthesis [1,6,7,8], bioinorganic [1,2,6,7,8], coordination [1,6,7] and green chemistries [1,2,6,7].
The current homogeneous cyclohexane oxidation process for the industrial production of nylon-6 and polyamide-6 intermediates bears serious limitations [1,2,5,10,11], being a highly complicated process [1], able to achieve only 5–12% yield of KA oil (the cyclohexanol and cyclohexanone mixture) to assure a selectivity of ca. 80–85% [5]. On a laboratory scale and in the presence of various metal catalysts or catalyst precursors, cyclohexane can be oxidized by aqueous H2O2 into a mixture of the corresponding alcohol and ketone (Scheme 1), resulting from the autodecomposition of the cyclohexyl hydroperoxide (CyOOH) or from its reduction with PPh3, following the method developed by Shul’pin [12,13,14]. This mild homogeneous catalytic oxidation typically proceeds in aqueous acetonitrile (CH3CN) medium, under atmospheric pressure, at room temperature (r.t.) or with slight heating (40–50 °C), and in the presence (optional) of an acid co-catalyst [2,15].
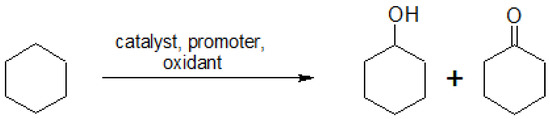
Scheme 1.
Catalytic oxidation of cyclohexane to yield a mixture of cyclohexanol and cyclohexanone (KA oil).
In the scope of the Green Chemistry, the replacement of conventional organic solvents by the so-called “green solvents” (e.g., water, biomass derivatives and “advanced” solvents, such as ionic liquids and supercritical fluids) is a common approach to more sustainable chemical processes [16].
Supercritical fluids (SCFs) have not only demonstrated their environmental benefits as useful solvents for extraction, chromatography [17,18] and other analytical methodologies [18], as cleaning solvents [18] and in some specialized reactions [17], but also offered substantial advantages in several aspects of catalytic processes. Indeed, SCFs proved to be attractive as mediums for chemical reactions [19] conferring surprisingly high levels of solubility of liquid and solid solutes, particularly when compressed to liquid-like densities [17]. The addition of reagents or co-solvents can also significantly change their characteristics. Since solvent properties play a key role in controlling the course and rate of reactions, changing the properties of SCFs with pressure and temperature is expected to drive chemical pathways and influence reaction rates, which could result in more selective and faster reactions [18], unlike what happens with conventional solvents [20].
Supercritical carbon dioxide (scCO2) has received considerable attention as a versatile and ecological medium for a variety of catalytic reactions [21,22] including polymerizations [18,21,22], hydroformylation, hydrogenation and partial oxidations [22], electrophilic reactions and enzymatic transformations [18], in addition to its use in materials processing and particle formation [21]. Its general lack of reactivity is essential for scCO2 success as a common substitute for more conventional solvents [17]. scCO2 is also an attractive solvent given the moderate critical conditions of pressure and temperature [23] (pc = 73.8 bar and tc = 31.1 °C [17]), allowing it to work with thermolabile compounds without causing degradation of the same. Another advantage is an easy separation because of pressure change [17,19,22,23]. In fact, a simple depressurization makes it possible to separate the CO2 from the solutes, as this becomes gaseous at atmospheric pressure, thus allowing solvent-free products to be obtained [17].
Another approach to a more sustainable chemistry consists in the employment of microwave (MW) irradiation—a form of electromagnetic energy with a frequency range of 300 MHz to 300 GHz—and the utilization of catalysts comprising 3d Earth-abundant metals [24].
MW-assisted organic transformations are effective, eco-friendly and economic since they allow an easy and good control of temperature and input parameters, faster reaction times and swift energy transfers (with selective heating for microwave-absorbing reagents and catalysts). Moreover, the employment of cheap tools and instruments, and a lesser dependence on solvents are clear advantages. The MW irradiation has been successfully applied to traditional cross-coupling reactions, such as Negishi and Kumada, Heck and Suzuki, as well as in diverse multi-step synthesis and solid phase reactions [24].
The arylhydrazones of active methylene compounds (AAMCs) exhibit interesting chemical and structural features, possessing several coordination modes, high flexibility and can exist in various tautomeric forms involving hydrogen bonds, thus providing a rich organic chemistry and versatility in complex formation [25,26,27]. Complexes containing these species or their analogs have found diverse applications, such as in the spectrophotometric determination of metal ions, as photoluminescent materials, light transmitters and sensors, in high-density recordable optical storage or spin-coating films [25,26], as molecular switches and in nanomotors [27].
AAMCs and their metal complexes have been in the spotlight due to their biological, analytical, and catalytic properties [25,28]. Indeed, some copper complexes with N,O-type or related ligands can typically act as catalysts for the peroxidative oxidation of cyclohexane to cyclohexanol and cyclohexanone thus mimicking multicopper enzymes such as the particulate methane monooxygenase (pMMO), which catalyzes the partial oxidation of alkanes to alcohols [26].
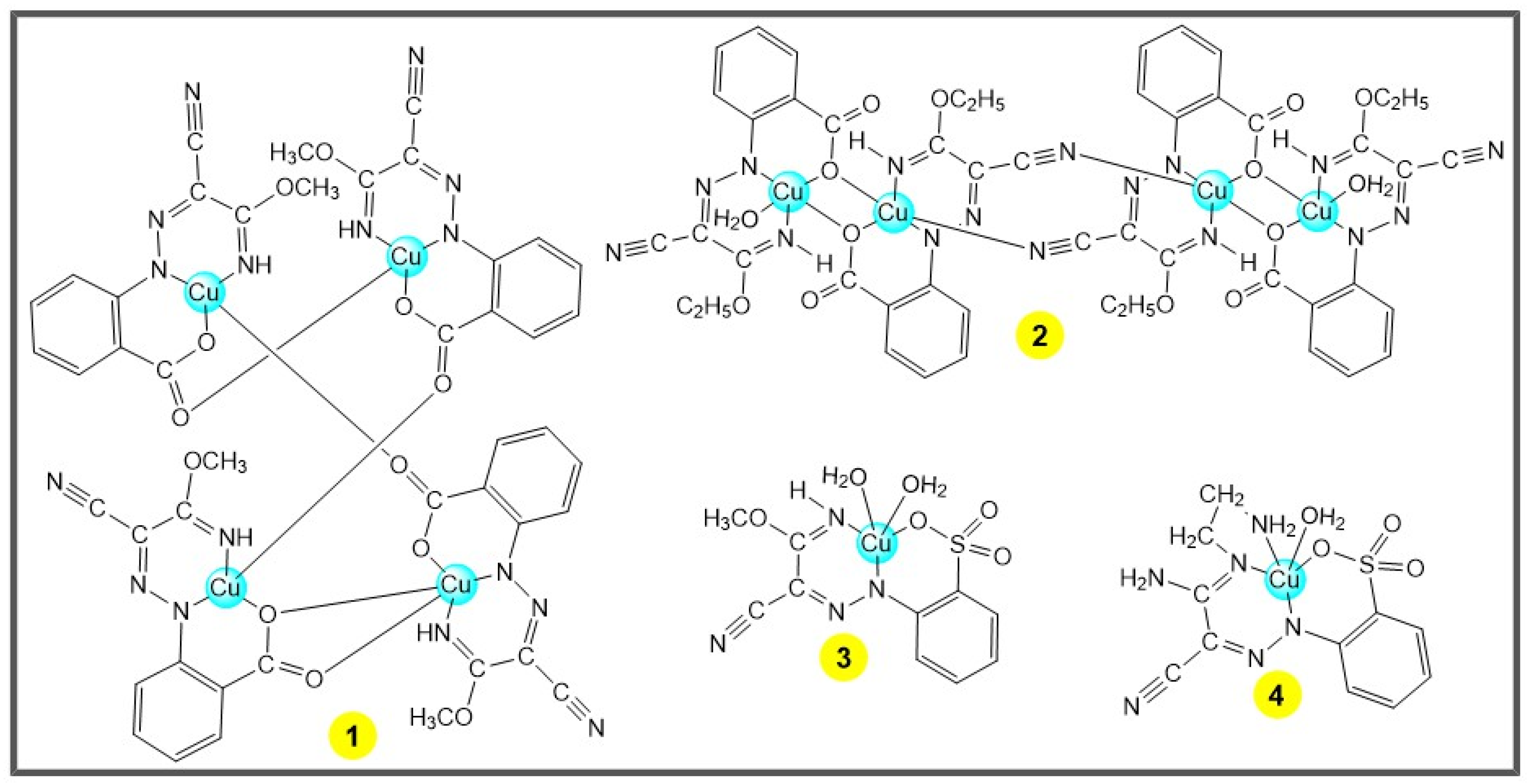
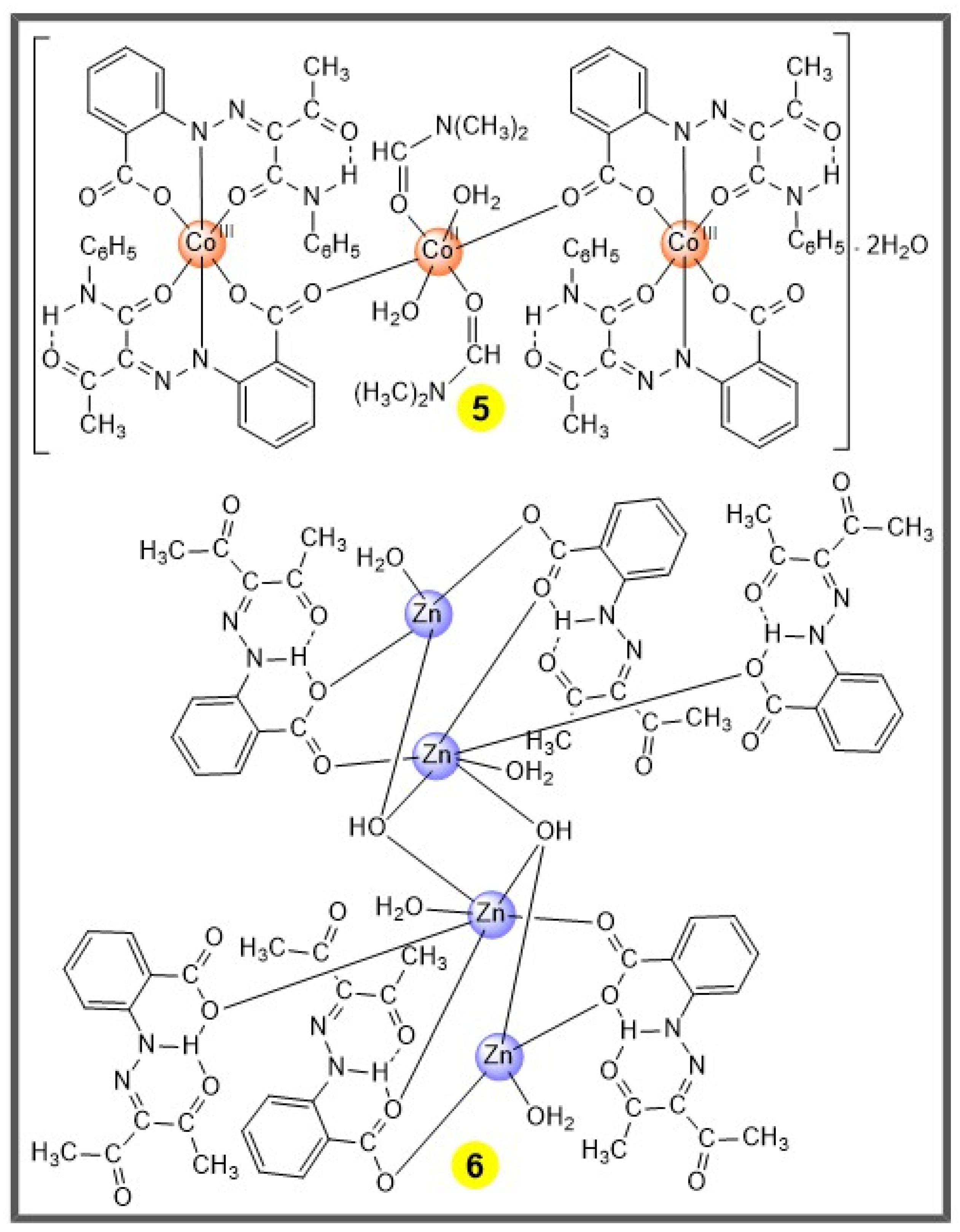
The present work focuses on the study of some 3d metal complexes comprising these organonitrogen ligands as potential catalysts for the transformation of cyclohexane into cyclohexanol and cyclohexanone, under mild conditions. For that purpose, two tetranuclear and two mononuclear Cu(II) complexes with ligands from arylhydrazones of malononitrile [29] (compounds 1–4, Scheme 2), one trinuclear Co(II/III) complex with an arylhydrazone of acetoacetanilide [28] (compound 5, Scheme 3) and one tetranuclear Zn(II) complex of 3-(2-carboxyphenyl-hydrazone)pentane-2,4-dione [27] (compound 6, Scheme 3) were employed as (pre-)catalysts for the aforementioned transformation. In addition, following our interest in exploring the potential of scCO2 as medium for catalytic reactions [19,21,23,30,31], some experiments were run in this supercritical fluid. The effect of experimental parameters, such as temperature, reaction time and type of added acid promoter, in the reaction yield was evaluated, as well as the effect of microwave irradiation.
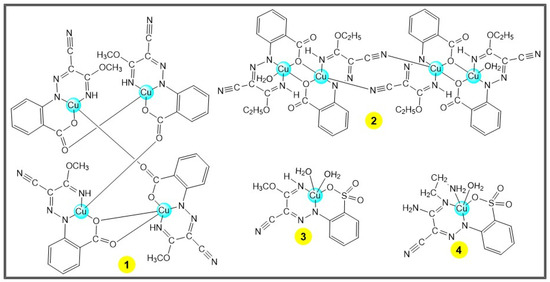
Scheme 2.
The tetra- (1–2) and mononuclear (3–4) Cu(II) complexes [29].
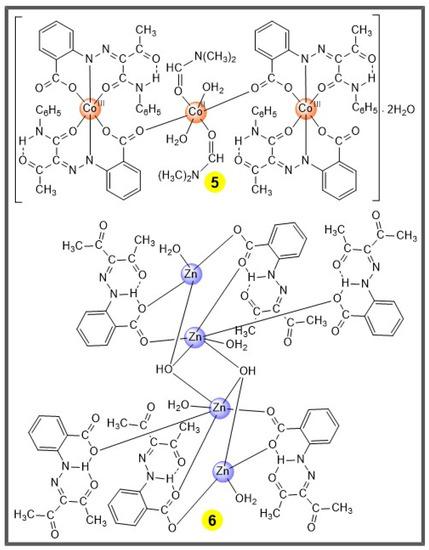
Scheme 3.
The trinuclear Co(II/III) 5 [28] and tetranuclear Zn(II) 6 [27] complexes.
2. Results and Discussion
2.1. Catalytic Studies under Conventional Conditions
The results obtained under conventional conditions for the peroxidative oxidation of cyclohexane with hydrogen peroxide and employing the tetranuclear Cu(II) complex 1 as catalyst are summarized in Table 1; those using the tetranuclear Cu(II) complex 2 are shown in Table 2, and in Table 3 the ones utilizing the mononuclear Cu(II) complexes 3 and 4 or the polynuclear Co(II/III) and Zn(II) complexes 5 and 6.

Table 1.
Peroxidative oxidation of cyclohexane catalyzed by the tetranuclear Cu(II) complex 1.

Table 2.
Peroxidative oxidation of cyclohexane catalyzed by the tetranuclear Cu(II) complex 2.

Table 3.
Peroxidative oxidation of cyclohexane catalyzed by complexes 3–6.
The experiments performed at room temperature with nitric acid as promoter and catalyst 1 (Table 1, entries 1 to 4) have shown a slight increase in the total yield of oxygenated products upon increasing of the reaction time from 1 to 4 h; a further increase to 6 h had nearly no influence. Using PCA instead of HNO3 led to an evident decrease in the yield, at room temperature (Table 1, entry 5).
When the reaction temperature was increased to 40 °C in the presence of HNO3 an overall rise in the total yield of products was observed with no significant changes associated with higher reaction times (Table 1, entries 6–9) or higher temperature (Table 1, entry 10). With PCA as promoter, a reaction time of 4 h at 40 °C gave the best results for this system (Table 1, compare entries 11–13; total yield of 26% and TON = 52.0 in entry 13) but a marked decrease in the yield with a further increase of temperature to 50 °C (Table 1, entry 14). Without any added acid promoter, the results were similar to those obtained with nitric acid (Table 1, entries 15 and 16; compare with entries 9 and 10).
A study concerning the effect of the amount of H2O in the system was carried out (Table 1, entries 17–21). The successive replacement of CH3CN by water resulted in lower total yields of oxygenated products from 16–18% to 10% although with a slight increase in the cyclohexanone production.
No significant changes were observed in the outcome of the system CyH/H2O2/HNO3 at 50 °C when performing the reaction in uncapped glass tubes (thus with more O2 available) or upon extending the reaction time from 4 to 6 h (Table 1, entry 10 versus 22 and 23, respectively).
Typical copper(II) salts such as CuCl2 and Cu(NO3)2·3H2O worked only marginally as catalysts for the reaction under study, whatever the acid promoter was used (Table 1, entries 24–27). The comparatively better results obtained with CuCl2 were somehow expected since this Cu(II) salt has been widely employed as catalyst for oxidative coupling and oxygenation reactions in organic synthesis due to the formation of active multinuclear copper species in the presence of common coordinating solvents (such as acetonitrile) [32,33].
Concerning the tetranuclear Cu(II) complex 2 as catalyst (Table 2, entries 1 to 8), PCA proved to be better acid promoter than HNO3 both at 40 °C and 50 °C; at room temperature, only HNO3 was effective (Table 2, entry 2) although the amount of oxygenated products was still very limited (only 5% of cyclohexanol). The beneficial effect of increasing the temperature on the reaction yield was again observed.
The influence of H2O amount in the outcome of the peroxidative oxidation of cyclohexane with hydrogen peroxide was similar to what was recorded for complex 1: as the amount of water in the system increased, there was a decrease in the total yield of oxygenated products (cyclohexanol + cyclohexanone) but, in contrast, the production of cyclohexanone slightly increased (Table 2, entries 7 and 9–13).
Both mononuclear Cu(II) complexes 3 and 4 exhibited comparatively lower catalytic activities (Table 3, entries 1–7 and 8–14, respectively); the best results were obtained with HNO3 as acid promoter (PCA did not fulfill a promoting role) after 4 h at 50 °C (certify entries 6 and 13 in Table 3: 8% total yield, TON = 16.0 and TOF = 4.0 h−1 for 3, and 4% total yield, TON = 8.0 and TOF = 2.0 h−1 for 4). A temperature effect on the reaction yield was marginally observed. Considering the catalytic activity per metal center (Cu2+ ion), the obtained results are in the same order of magnitude as the ones obtained with the tetranuclear copper(II) complexes 1 and 2. No oxygenated products were detected when using the trinuclear Co(II/III) complex 5 or the tetranuclear Zn(II) complex 6 as catalysts (in this order, Table 3 entries 15–16 and 17–18).
The employment of CH3CN/H2O solvent mixtures is essential to solubilize both substrate and catalyst and the mild alkane oxidation does not proceed effectively only in water or acetonitrile as sole solvents. The acetonitrile was chosen due to: (i) its miscibility with water and ability to solubilize substrate, catalysts and products; (ii) the high stability towards oxidation under the applied reaction conditions (contrasting with solvents like methanol, ethanol or acetone); (iii) its coordination ability; and (iv) the best results previously achieved upon utilization of this solvent [2,15].
Complexes 1 and 2 are readily soluble in the acidic CH3CN/H2O medium, yielding green and greenish yellow solutions, respectively, with a slight solid residue in suspension; throughout the oxidation process, a minor change in the color of the solutions was observed and upon reaction completion greenish solutions with a small amount of a dark flocculated precipitate were obtained.
The mononuclear Cu(II) compounds 3 and 4 exhibit a higher solubility in the chosen medium thus affording almost clear yellow and greenish yellow solutions, respectively, that became paler and completely clear after the peroxidative oxidation reaction.
Finally, regarding the multinuclear Co(II/III) and Zn(II) complexes 5 and 6, there was a shift from dark green and yellow solutions, comprising a slight solid residue in suspension, to green (with a residual amount of a flocculated precipitate) and completely clear yellow solutions, respectively.
The presence of an acid promoter proved to be essential for achieving better results. Its role as co-catalyst can be associated with: (i) promotion of proton transfer steps; (ii) catalyst activation by unsaturation of the Cu(II) centers, upon ligand protonation; (iii) enhancement of oxidative properties of the catalysts and H2O2 (acids can also be oxidants themselves); (iv) facilitation of the formation of peroxo complexes; and (v) preventing the decomposition of H2O2 to water and oxygen (thus suppressing eventual catalase activity in acidic medium) [2,15].
As reported for other copper(II) catalysts [1,2,10], the oxidation of cyclohexane is expected to follow a radical mechanism (Scheme 4) via metal-promoted formation of the hydroxyl radical (HO·) from H2O2, which acts as H-abstractor from cyclohexane (CyH) to give cyclohexyl radical (Cy·). The reaction of this radical with O2 forms the peroxyl radical CyOO· which can further react with a LCu(I) species to yield the corresponding anion which is converted into the main primary product—cyclohexyl hydroperoxide—by protonation. CyOOH undergoes copper-assisted decomposition giving the corresponding alkoxyl CyO· and alkylperoxyl CyOO· radicals; the former is converted to the alcohol (CyOH) by H-abstraction from the substrate, and the latter dismutates to yield both the alcohol and the ketone (Cy′O).
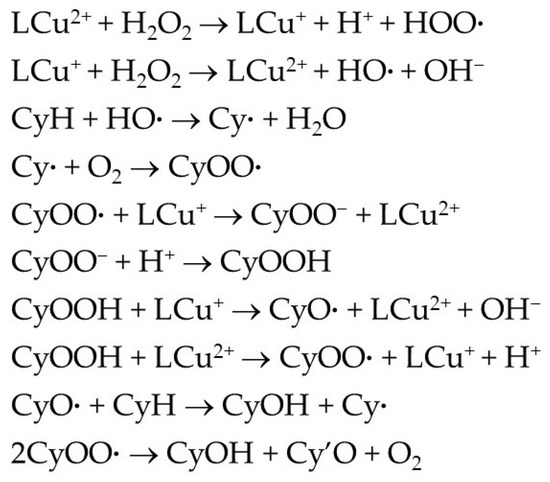
Scheme 4.
Simplified mechanism for the Cu-catalyzed oxidation of cyclohexane (CyH) by H2O2 to cyclohexyl hydroperoxide, cyclohexanol and cyclohexanone.
2.2. Catalytic Studies in scCO2 or with MW Irradiation
The results obtained in supercritical carbon dioxide medium or under microwave irradiation are summarized in Table 4 and Table 5, respectively.

Table 4.
Peroxidative oxidation of cyclohexane catalyzed by complexes 1–2 in acetonitrile/water–scCO2 medium.

Table 5.
Peroxidative oxidation of cyclohexane catalyzed by complexes 1–2 under MW irradiation.
The use of scCO2 in the system cyclohexane/H2O2/HNO3/complex 1 at 40 °C led to the total yield of oxygenated compounds of 7% for the reaction time of 4 h (it was only 1% for 2 h) (Table 4, entries 3 and 2, respectively). Increasing the temperature to 50 °C and reaction time to 6 h led to a further enhancement of the yield of total oxygenated compounds to 17%. These values are slightly below those obtained under conventional conditions and similar reaction parameters (e.g., compare with Table 1, entry 9: 18% after 6 h at 40 °C). A lower CO2 pressure led to a worse yield of products (Table 4, compare entries 6 and 7).
The introduction of scCO2 in the system cyclohexane/H2O2/PCA/complex 2 at 50 °C and 4 h did not result in a better performance, since no oxygenated products were detected (Table 4, entry 9 versus Table 2, entry 8—20%, TON = 40.0 and TOF = 10.0 h−1). However, the use of nitric acid in combination with scCO2 resulted in a slight increase in the reaction yield and catalytic parameters (21%, TON = 42.0, TOF = 10.5 h−1—Table 4, entry 8 vs. 16%, TON = 32.0, TOF = 8.0 h−1—Table 2, entry 7).
When microwave irradiation was employed as alternative to conventional heating, it was possible to achieve total yields of oxygenates up to 14% (for the tetranuclear Cu(II) complex 1—Table 5, entry 2) and 13% (for compound 2—Table 5, entry 6), although at a higher temperature (70 °C) but in quite a shorter reaction time (0.5 h), thus leading to the highest TOFs of 56.0 and 52.0 h−1, respectively. These values, however, are below the best ones recorded for the experiments held in conventional conditions when using nitric acid as co-catalyst, namely: 20% for catalyst 1, at 40 °C and 1 h reaction time (Table 1, entry 6) and 16% for catalyst 2, at 40 or 50 °C and 4 h reaction time (Table 2, entries 4 and 7). On the other hand, the increase of the reaction time to 1 h or the temperature to 80 °C (Table 5, entries 3–4 and 7–8) did not lead to higher yields of oxygenated products.
Therefore, a higher temperature under MW irradiation (70 °C) or a longer reaction time under conventional heating are essential to achieve better results concerning the peroxidative oxidation of cyclohexane into a mixture of cyclohexanol and cyclohexanone.
2.3. Comparison with Other Copper Catalytic Systems
The most relevant results concerning other Cu(II) catalytic systems employed in the peroxidative oxidation of cyclohexane by hydrogen peroxide are summarized in Table 6.

Table 6.
Peroxidative oxidation of cyclohexane with H2O2 catalyzed by several copper complexes in acetonitrile/water medium.
The highest total yields of oxygenates obtained in this work when using the tetranuclear Cu(II) complexes 1 and 2 as catalysts (0.005 mmol) and HNO3 as co-catalyst (0.025 mmol), under conventional conditions, namely: (i) 20% (with TON = 40.0) at 40 °C and 1 h for 1 (Table 1, entry 6); (ii) 16% (with TON = 32.0) at 40 °C and 4 h for 2 (Table 2, entry 4), are slightly below the one recorded for the dicopper(II) complex [Cu2(H2O)2(μ-L)2], containing the basic form of the hydrazone-derived species H2L = 3-(2-hydroxy-4-nitrophenylhydrazo)pentane-2,4-dione, in CH3CN/aqueous H2O2, which afforded a total yield of 27% under mild conditions (25 °C, 6 h) (Table 6, entry 1) although employing higher catalyst, promoter and oxidant loadings (0.02 mmol [Cu2(H2O)2(μ-L)2], 0.2 mmol HNO3 and 10 mmol H2O2); therefore a lower TON of 13.3 was recorded [26].
However, taking into consideration non-polymeric homometallic Cu(II) complexes with other types of ligands, the performance of the compounds 1 and 2 in the peroxidative oxidation of cyclohexane by aqueous H2O2 is quite different. Indeed, several tested tri- and tetra-copper(II) compounds with aminopolyalcoholates ligands exhibited total product yields up to 39% [2] after 6 h reaction time at room temperature in acetonitrile, being the highest values achieved with [Cu4(O)(tea)4(BOH)4][BF4]2 (39%) and [Cu3(H2tea)2(poba)2(H2O)]·4H2O (37%) (where H3tea = triethanolamine; H2poba = 4-oxybenzoic acid) [34] (Table 6, entries 2 and 3). The dinuclear copper(II) compound [{Cu(Hdea)(H2dea)}2(H2pma)]·3H2O (H2dea = diethanolamine; H4pma = pyromellitic acid), encompassing a carboxylate ligand as well, proved to be an efficient catalyst concerning the mild peroxidative oxidation of cyclohexane in acetonitrile/water medium at 50 °C with a maximum yield of products of 34%, in the presence of trifluoroacetic acid (TFA) as a co-catalyst [10,35] (Table 6, entry 4).
Two tetranuclear Cu(II) complexes having carboxylate ligands and Schiff bases, instead of aminoalcoholates, also provided an interesting outcome concerning the peroxidative oxidation of cyclohexane (Table 6, entries 5 and 6): [Cu4(O)(L3)2(CH3COO)4] (where HL3 = 4-methyl-2,6-bis(2-bromoethyliminomethyl)phenol) yielded 36% of oxygenates upon reaction for 6 h at room temperature in an aqueous acidic (HNO3) medium [36]; [(CuL7)2(cdc)]2·2H2O (HL7 = 2-(2-pyridylmethyleneamino)benzenesulfonic acid; H2cdc = cyclohexane-1,4-dicarboxylic acid) afforded yields in the 29–31% range in the absence of the promoter TFA or in its presence, in aqueous CH3CN medium at 50 °C [10,37].
These remarkable results may arise from the presence of a good number of hydrophilic carboxylate (and sulfonate, in the case of [(CuL7)2(cdc)]2·2H2O) groups which are able to activate water molecules towards its significant role as a promoter for proton-shift steps involved in the formation of hydroxyl radicals from H2O2. Moreover, the multinuclear character of these complexes allows the presence, in proximity, of copper units in the oxidized (Cu2+) and reduced (Cu+) forms, thus favoring that key stage in the mechanism of the cyclohexane oxidation [37].
In the absence of carboxylate ligands, some Schiff base Cu(II) complexes also displayed an interesting catalytic activity towards the peroxidative oxidation of C6H12 in acetonitrile/water medium, under mild conditions, although longer reaction times were employed. The dinuclear Cu(II) complex [Cu2(L1)2(μ2-Cl)Cl]·2.5H2O (being HL1 = 1-((2-hydroxyethylimino)methyl)naphthalen-2-ol) gave yields of oxygenates varying from 9 to 30.4% depending on the n(H2O2)/n(catalyst) ratio (from 100 to 500) upon reaction at 35 °C for 8 h in CH3CN; however, when the reaction time was extended to 48 h, the yields increased from 15.5 to 48.2%, respectively [38] (Table 6, entry 7). With the mononuclear aroylhydrazone Cu(II) complex [Cu(H2L)(NO3)(H2O)], comprising the deprotonated keto form of the Schiff base (3,5-di-tert-butyl-2-hydroxybenzylidene)-2-hydroxybenzohydrazide (H3L), a maximum overall yield of 30% was attained after 24 h (at room temperature and without any additive) [1,39] (Table 6, entry 8).
On the other hand, the Cu(II) compound with phenanthroline [CuCl(phen)2]Cl·5H2O (Table 6, entry 9) afforded a remarkable total yield of 67% of oxygenated products at 70 °C in acetonitrile:water (3.5:1) solution; however, at a higher temperature range (50–70 °C), adipic acid was also formed in low quantities [1,40].
Recently, the heptanuclear complex [(Ph5Si5O10)2][PhCOO]4Cu7(EtOH)6, comprising four benzoate ligands, was evaluated as a catalyst for the peroxidative oxidation of alkanes with H2O2 at 50 °C in acetonitrile (Table 6, entry 10): using cyclohexane as substrate, a maximum yield of oxygenated products as high as 32% was achieved after 2 h [41], thus stressing the key role of a higher nuclearity and the presence of activating ligands (such as carboxylates) for a better catalytic performance.
Another important factor related to a higher activity is the presence of the very strong basic O2- ion in the complexes structure—due to its high affinity towards protons, the activation of the peroxide oxidant through proton abstraction would be enhanced resulting in more active systems. Indeed, the tetranuclear copper compounds [Cu4(O)(tea)4(BOH)4][BF4]2 and [Cu4(O)(L3)2(CH3COO)4] (Table 6, entries 2 and 5, respectively) are amongst the most active ones (and under milder conditions—reactions at r.t.).
In some cases, the total yields of oxygenates (CyOH and Cy’O) were similar to the ones obtained for the tetranuclear Cu(II) complexes 1 and 2 (20 and 16%, respectively): (i) the C-scorpionate complex [CuCl2{κ3-HOCH2C(pz)3}] (Hpz = pyrazole) led to 23% of the yield of ketone + alcohol at room temperature, in an acidic-CH3CN medium [1] (Table 6, entry 11); (ii) the compound [Cu(L)(bipy)]·MeOH, where H2L = o-[(o-hydroxyphenyl)methylideneamino]benzenesulfonic acid and bipy = 2,2′-bipyridine, led to a 20% yield, after 2 h of reaction time at 60 °C in acetonitrile [42] (Table 6, entry 12); (iii) several Cu(II) complexes with barbiturate derivatives as ligands (formed upon reaction of 5-formylbarbiturates with primary aryl amines) afforded yields of the oxidation products in the range 11.5–22.1% after 6 h of reaction at 50 °C in acidic acetonitrile medium [43] (Table 6, entry 13).
Finally, concerning the employment of microwave irradiation, the CuI/II pyrazolate compounds [Cu2(N,N-3,5-(NO2)2pz)2(PPh3)2] and [trans-Cu6(OH)6(3,5-(CF3)2pz)6] were successfully tested as catalysts for the MW-assisted neat oxidation of cyclohexane, affording significantly high yields (up to 58%) in rather short reaction times, but a different oxidant—tert-butylhydroperoxide (TBHP)—was used [1,44].
3. Materials and Methods
3.1. General
All reagents and solvents (p.a. grade) were obtained from commercial sources (cyclohexane >99.5% and pyrazine-2-carboxylic acid >98%, TCI (Zwijndrecht, Belgium); cyclohexanone 99.8% and cyclohexanol 99%, Aldrich (Darmstadt, Germany); nitric acid aqueous solution ≥65%, Sigma-Aldrich (Darmstadt, Germany); hydrogen peroxide aqueous solution 50% and triphenylphosphine 99%, Acros Organics (Geel, Belgium); nitromethane >98%, Alfa Aesar (Kandel, Germany); acetonitrile ≥99.9%, Fisher Chemical (Porto Salvo, Portugal)) and used as received. Complexes 1−6 were synthesized according to the reported procedures [25,26,27]. Liquid CO2 (99.995% purity) was supplied from Air Liquide (Algés, Portugal) and used without further purification. The MW assisted experiments were performed on a focused microwave Anton Paar monowave 300 reactor (Anton Paar GmbH, Graz, Austria) (10 W) fitted with a rotational system and an IR temperature detector. A Perkin-Elmer Clarus 500 gas chromatograph (PerkinElmer, Waltham, MA, USA) equipped with a BP-20 capillary column (SGE; 30 m × 0.22 mm × 25 μm) and an FID detector was used for quantitative analyses of the reaction mixtures; helium was used as the carrier gas; 230 °C was the temperature of the injector.
3.2. Catalytic Studies under Conventional Conditions
The peroxidative oxidation reactions were carried out in 10 mL capped glass tubes under vigorous stirring, according to the following protocol: CH3CN (4.016 mL, or 4.066 mL when no promoter or PCA was used) and CH3NO2 [GC internal standard] (1.0 mmol; 0.541 mL of stock solution 1.85 M in CH3CN) were added to the solid metal complexes (typically 5 μmol). The solution of the promoter was then added (0.025 mmol of HNO3, 0.050 mL of stock solution 0.50 M in CH3CN; due to its limited solubility, 3.10 mg of PCA were directly placed in the vial) followed by 1.0 mmol (0.109 mL) of cyclohexane. Hydrogen peroxide was then added dropwise (5.0 mmol; 0.284 mL of stock 50% aqueous solution), and reaction was left for 1, 2, 4 or 6 h at room temperature (r.t.), 40 or 50 °C. In the final 5 mL reaction volume the initial conditions were thus the following: [CyH] = 0.2 M; [H2O2] = 1 M; [promoter] = 5 × 10−3 M; [CH3NO2] = 0.2 M and [metal complex] = 1 × 10−3 M.
After quenching for at least 10 min with an excess of solid PPh3, ca. 0.5 μL of sample was directly analyzed by GC using the following conditions: 100 °C (1 min), 100–160 °C (10 °C/min), 160 °C (1 min), 8 min total run time. The identification of the products (cyclohexanol, CyOH, and cyclohexanone, Cy’O) was made by comparison of their retention times with those of authentic samples.
3.3. Catalytic Studies in scCO2 or under MW Irradiation
Reactions in scCO2 were carried out in a home-built high-pressure setup described in detail elsewhere [23,31,45]. The experimental procedure was as follows: CO2 gas passed through a coil immersed in an ice bath to ensure that the fluid enters the high-pressure metering pump completely liquefied. The pump injected the fluid into the system; the pressure was controlled by a back-pressure regulator valve. Before entering the high-pressure reactor, the fluid passed through a pre-heater (a coil immersed in an oil bath) to ensure the fluid reaches the working temperature. The stainless-steel high-pressure reactor was charged with the catalyst, the selected volumes of solvent, GC internal standard, promoter (when applicable), substrate, the oxidant and a stirring bar, and then immersed in a thermostatic bath and heated to the working temperature; only then the system was filled with CO2 up to the working pressure and the stirring was turned on. The reaction mixture was stirred for 1 to 4 h at the selected temperature and pressure (ca. 120 bar). After reaction time completion, the reactor was immersed in an ice bath and the CO2 vented through an expansion valve previously heated to the reaction temperature (to avoid its clogging). The expanded CO2 passed through a cooled trap to avoid the loss of compounds by possible entrainment by the carbon dioxide flow (which was monitored by a flowmeter). A sample of the reaction mixture was collected, quenched with an excess of solid PPh3 for at least 10 min, and the GC protocol mentioned above was then followed.
Concerning the experiments under microwave irradiation, adequate 10 or 30 mL reaction tubes capable of being sealed were employed, and the protocol described in Section 3.2 was followed, though using lower reaction times (0.5 or 1 h) and higher temperatures (50, 70 or 80 °C).
4. Conclusions
The tetranuclear Cu(II) complex 1 acted as a catalyst towards the peroxidative oxidation of cyclohexane by aqueous H2O2 in acetonitrile medium, the best result being achieved with PCA as co-catalyst at 40 °C and 4 h reaction time (total yield of 26% and TON = 52.0). HNO3 generally proved to be the best promoter under the studied conditions (from r.t. to 50 °C and 1 to 6 h reaction time). When employing the tetranuclear Cu(II) complex 2 as catalyst, the best yield of oxygenates was afforded upon using PCA as acid promoter at 40 °C and during 4 h (24%, TON = 48.0).
The use of CH3CN/H2O as solvent mixture is relevant, namely to solubilize the substrate and the catalyst. The amount of water has a significant effect, and its increase led to a lowering of the total yield of oxygenates but with a slight increase in the cyclohexanone production.
The tetranuclear Cu(II) complexes 1 and 2 showed a higher catalytic activity than those of both the mononuclear Cu(II) complexes 3 and 4. For the latter catalysts, the best results were achieved with HNO3 at 50 °C and 4 h (total yields of 8 and 4%, respectively). However, these results are in the same order of magnitude as the ones obtained for the tetranuclear Cu(II) complexes if we consider the catalytic activity per metal center.
On the other hand, the trinuclear Co(II/III) complex 5 and the tetranuclear Zn(II) complex 6 did not fulfill a catalytic role since no oxygenated products were detected in the studied conditions (50 °C, 4 h, HNO3 or PCA as promoters).
Concerning the experiments employing supercritical carbon dioxide as reaction medium, generally there was a decrease in the catalytic activity of 1 towards the formation of the oxygenates with the exception of the experiment run at 50 °C for 6 h (17% total yield, TON = 34.0 and TOF = 5.7 h−1). Regarding the system cyclohexane/H2O2/PCA/2 at 50 °C and 4 h in scCO2, no oxygenated products were detected; however, the use of HNO3 together with the supercritical fluid resulted in a slight improvement of the catalytic performance (21%, TON = 42.0, TOF = 10.5 h−1).
Using microwave irradiation as alternative heating source, total yields of oxygenates up to 14% (with 1; TOF = 56.0 h−1) and 13% (with 2; TOF = 52.0 h−1) were achieved, at a higher temperature of 70 °C but in the shorter time of 0.5 h. Nevertheless, these yields are below the best ones recorded for the experiments held in conventional conditions when using nitric acid as co-catalyst. Therefore, a higher temperature under MW irradiation or a longer reaction time under conventional heating are essential to achieve better results.
The highest total yields of cyclohexanol and cyclohexanone when using the tetranuclear Cu(II) complexes 1 and 2 as catalysts and HNO3 as co-catalyst are comparable to those achieved for the best copper(II) catalysts.
Data on the application of 3d metal complexes containing hydrazone-derived ligands as potential catalysts in the peroxidative oxidation of cyclohexane by aqueous H2O2 are scarce and therefore this work is a significant contribution to this area. Moreover, it explored non-conventional conditions such as the employment of supercritical fluids (scCO2) and the use of MW irradiation, which falls in the scope of Green Chemistry and its quest for more sustainable and “greener” chemical processes.
Author Contributions
Conceptualization, N.R.C. and B.P.N.; investigation, N.R.C. and B.P.N.; writing—original draft, N.R.C.; writing—review and editing, N.R.C., B.P.N., A.V.G., A.M.F.P., M.F.C.G.d.S., K.T.M. and A.J.L.P. All authors have read and agreed to the published version of the manuscript.
Funding
Centro de Química Estrutural (CQE) acknowledges the financial support of Fundação para a Ciência e Tecnologia (FCT) (projects UIDB/00100/2020, UIDP/00100/2020 and LA/P/0056/2020). N.R.C. acknowledges the financial support from Research fellowship (for PhD student) BL2/2020_IST-ID of CQE (project 1801P.00974.1.01.01, UIDB/00100/2020) and Programa Doutoral FCT “Catalysis and Sustainability” CATSUS (PD/00248/2012). B.P.N. expresses her gratitude to Instituto Superior Técnico and FCT for the Scientific Employment contract IST-ID/14/2018 under Decree-Law no. 57/2016, of August 29. A.V.G. and K.T.M. thank FCT and Instituto Superior Técnico (DL 57/2016, L 57/2017 and CEEC Institutional 2018 Programs, Contracts no: IST-ID/110/2018 and IST-ID/85/2018), as well as Baku State University (Azerbaijan). This publication has been supported by the RUDN University Strategic Academic Leadership Program (recipient A.J.L.P, catalysis).
Data Availability Statement
The data presented in this study are available in [current manuscript].
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pombeiro, A.J.L.; Guedes da Silva, M.F.C. Alkane Functionalization, 1st ed.; John Wiley & Sons Ltd: Chichester, UK, 2019. [Google Scholar]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J.L. Multicopper complexes and coordination polymers for mild oxidative functionalization of alkanes. Coord. Chem. Rev. 2012, 256, 2741–2759. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M.; Kuznetsov, M.L.; Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Alkanes to carboxylic acids in aqueous medium: Metal-free and metal-promoted highly efficient and mild conversions. Chem. Commun. 2009, 17, 2353–2355. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, M.V.; Kirillov, A.M.; Pombeiro, A.J.L. Mild, Single-Pot Hydrocarboxylation of Gaseous Alkanes to Carboxylic Acids in Metal-Free and Copper-Promoted Aqueous Systems. Chem. Eur. J. 2010, 16, 9485–9493. [Google Scholar] [CrossRef]
- Martins, N.M.R.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Green oxidation of cyclohexane catalyzed by recyclable magnetic transition-metal silica coated nanoparticles. Catal. Commun. 2019, 125, 15–20. [Google Scholar] [CrossRef]
- Da Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Amavadin, a Vanadium Natural Complex: Its Role and Applications. Coord. Chem. Rev. 2013, 257, 2388–2400. [Google Scholar] [CrossRef]
- Da Silva, J.A.L.; Guedes da Silva, M.F.C.; Sutradhar, M.; Pombeiro, A.J.L. Amavadin and Related Complexes as Oxidation Catalysts. In Vanadium Catalysis, 1st ed.; Sutradhar, M., Pombeiro, A.J.L., da Silva, J.A.L., Eds.; Royal Society of Chemistry: London, UK, 2021; Chapter 2; pp. 12–34. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Fernandes, T.A.; André, V.; Kirillov, A.M. Mild C–H functionalization of alkanes catalyzed by bioinspired copper(II) cores. Org. Biomol. Chem. 2019, 17, 7706–7714. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M.; Pombeiro, A.J.L. Metal-Free and Copper-Promoted Single-Pot Hydrocarboxylation of Cycloalkanes to Carboxylic Acids in Aqueous Medium. Adv. Synth. Catal. 2009, 351, 2936–2948. [Google Scholar] [CrossRef]
- Nesterov, D.S.; Nesterova, O.V.; Pombeiro, A.J.L. Homo- and heterometallic polynuclear transition metal catalysts for alkane C–H bonds oxidative functionalization: Recent advances. Coord. Chem. Rev. 2018, 355, 199–222. [Google Scholar] [CrossRef]
- Gawlig, C.; Schindler, S.; Becker, S. One-Pot Conversion of Cyclohexane to Adipic Acid Using a μ4-Oxido-Copper Cluster as Catalyst Together with Hydrogen Peroxide. Eur. J. Inorg. Chem. 2020, 3, 248–252. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalyzed hydrocarbon oxygenations in solutions: The dramatic role of additives: A review. J. Mol. Catal. A Chem. 2002, 189, 39–66. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Metal-catalysed hydrocarbon oxidations. Comptes Rendus Chim. 2013, 6, 163–178. [Google Scholar] [CrossRef]
- Shul’pin, G.B. Hydrocarbon Oxygenations with Peroxides Catalyzed by Metal Compounds. Mini-Rev. Org. Chem. 2009, 6, 95–104. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kirillova, M.V.; Pombeiro, A.J.L. Homogeneous Multicopper Catalysts for Oxidation and Hydrocarboxylation of Alkanes. In Advances in Inorganic Chemistry – Homogeneous Catalysis; van Eldik, R., Hubbard, C.D., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 65, Chapter 1; pp. 1–31. [Google Scholar] [CrossRef]
- Han, X.; Poliakoff, M. Continuous reactions in supercritical carbon dioxide: Problems, solutions and possible ways forward. Chem. Soc. Rev. 2012, 41, 1428–1436. [Google Scholar] [CrossRef]
- Scott Oakes, R.; Clifford, A.A.; Rayner, C.M. The use of supercritical fluids in synthetic organic chemistry. J. Chem. Soc. Perkin Trans. 2001, 1, 917–941. [Google Scholar] [CrossRef]
- Sahle-Demessie, E.; Gonzalez, M.A.; Enriquez, J.; Zhao, Q. Selective Oxidation in Supercritical Carbon Dioxide Using Clean Oxidants. Ind. Eng. Chem. Res. 2000, 39, 4858–4864. [Google Scholar] [CrossRef]
- Sutradhar, M.; Ribeiro, A.P.C.; Guedes da Silva, M.F.C.; Palavra, A.M.F.; Pombeiro, A.J.L. Application of molybdenum complexes for the oxidation of cyclohexane in acetonitrile, ionic liquid and supercritical CO2 media, a comparative study. Mol. Catal. 2020, 482, 100356. [Google Scholar] [CrossRef]
- Dapurkar, S.E.; Kawanami, H.; Yokoyama, T.; Ikushima, Y. Catalytic Oxidation of Oleic Acid in Supercritical Carbon Dioxide Media with Molecular Oxygen. Top. Catal. 2009, 52, 707–713. [Google Scholar] [CrossRef]
- Paninho, A.B.; Ventura, A.L.R.; Branco, L.C.; Pombeiro, A.J.L.; Guedes da Silva, M.F.C.; Nunes da Ponte, M.; Mahmudov, K.T.; Nunes, A.V.M. CO2 + ionic liquid biphasic system for reaction/product separation in the synthesis of cyclic carbonates. J. Supercrit. Fluids 2018, 132, 71–75. [Google Scholar] [CrossRef]
- Tsang, S.C.; Zhu, J.; Yu, K.M.K. Selective catalytic oxidation of alkylaromatic molecules by nanosize water droplets containing Co2+ species in supercritical carbon dioxide fluid. J. Exp. Nanosci. 2006, 1, 435–456. [Google Scholar] [CrossRef]
- Fernandes, R.R.; Lasri, J.; Guedes da Silva, M.F.C.; Palavra, A.M.F.; da Silva, J.A.L.; Fraústo da Silva, J.J.R.; Pombeiro, A.J.L. Oxadiazoline and Ketoimine Palladium(II) Complexes as Highly Efficient Catalysts for Suzuki–Miyaura Cross-Coupling Reactions in Supercritical Carbon Dioxide. Adv. Synth. Catal. 2011, 353, 1153–1160. [Google Scholar] [CrossRef]
- Laskar, R.; Pal, T.; Bhattacharya, T.; Maiti, S.; Akita, M.; Maiti, D. Sustainable C–H functionalization under ballmilling, microwave-irradiation and aqueous media. Green Chem. 2022, 24, 2296. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Pombeiro, A.J.L. Coordination chemistry of arylhydrazones of methylene active compounds. Coord. Chem. Rev. 2013, 257, 1244–1281. [Google Scholar] [CrossRef]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Figiel, P.J.; Karabach, Y.Y.; Pombeiro, A.J.L. New copper(II) dimer with 3-(2-hydroxy-4-nitrophenylhydrazo)pentane-2,4-dione and its catalytic activity in cyclohexane and benzyl alcohol oxidations. J. Mol. Catal. A Chem. 2010, 318, 44–50. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Kouznetsov, M.L.; Mahmudov, K.T.; Pombeiro, A.J.L. Synthesis, structure and electrochemical behaviour of Na, MgII, MnII, ZnII, CdII and NiII complexes of 3-(2-carboxyphenylhydrazone)pentane-2,4-dione. Polyhedron 2013, 50, 374–382. [Google Scholar] [CrossRef]
- Gurbanov, A.V.; Maharramov, A.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Trinuclear and polymeric cobalt(II or II/III) complexes with an arylhydrazone of acetoacetanilide and their application in cyanosilylation of aldehydes. Inorg. Chim. Acta 2017, 466, 632–637. [Google Scholar] [CrossRef]
- Kopylovich, M.N.; Mizar, A.; Guedes da Silva, M.F.C.; Mac Leod, T.C.O.; Mahmudov, K.T.; Pombeiro, A.J.L. Template Syntheses of Copper(II) Complexes from Arylhydrazones of Malononitrile and their Catalytic Activity towards Alcohol Oxidations and the Nitroaldol Reaction: Hydrogen Bond-Assisted Ligand Liberation and E/Z Isomerisation. Chem. Eur. J. 2013, 19, 588–600. [Google Scholar] [CrossRef]
- Ribeiro, A.P.C.; Martins, L.M.D.R.S.; Alegria, E.C.B.A.; Matias, I.A.S.; Duarte, T.A.G.; Pombeiro, A.J.L. Catalytic Performance of Fe(II)-Scorpionate Complexes towards Cyclohexane Oxidation in Organic, Ionic Liquid and/or Supercritical CO2 Media: A Comparative Study. Catalysts 2017, 7, 230. [Google Scholar] [CrossRef]
- Reis Conceição, N.; Nobre, B.P.; Karmakar, A.; Palavra, A.M.F.; Mahmudov, K.T.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Knoevenagel condensation reaction in supercritical carbon dioxide medium using a Zn(II) coordination polymer as catalyst. Inorg. Chim. Acta 2022, 538, 120981. [Google Scholar] [CrossRef]
- Löw, S.; Becker, J.; Würtele, C.; Miska, A.; Kleeberg, C.; Behrens, U.; Walter, O.; Schindler, S. Reactions of Copper(II) Chloride in Solution: Facile Formation of Tetranuclear Copper Clusters and Other Complexes That Are Relevant in Catalytic Redox Processes. Chem. Eur. J. 2013, 19, 5342–5351. [Google Scholar] [CrossRef]
- Becker, S.; Dürr, M.; Miska, A.; Becker, J.; Gawlig, C.; Behrens, U.; Ivanović-Burmazović, I.; Schindler, S. Copper Chloride Catalysis: Do μ4-Oxido Copper Clusters Play a Significant Role? Inorg. Chem. 2016, 55, 3759–3766. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Kopylovich, M.N.; Kirillova, M.V.; Karabach, E.Y.; Haukka, M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Mild Peroxidative Oxidation of Cyclohexane Catalyzed by Mono-, Di-, Tri-, Tetra- and Polynuclear Copper Triethanolamine Complexes. Adv. Synth. Catal. 2006, 348, 159. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Santos, C.I.M.; André, V.; Dias, S.S.P.; Kirillova, M.V.; Kirillov, A.M. New aqua-soluble dicopper(II) aminoalcoholate cores for mild and water-assisted catalytic oxidation of alkanes. Catal. Sci. Technol. 2016, 6, 4584–4593. [Google Scholar] [CrossRef]
- Roy, P.; Manassero, M. Tetranuclear copper(II)–Schiff-base complexes as active catalysts for oxidation of cyclohexane and toluene. Dalton Trans. 2010, 39, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Mukherjee, S.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. A cyclic tetranuclear cuboid type copper(II) complex doubly supported by cyclohexane-1,4-dicarboxylate: Molecular and supramolecular structure and cyclohexane oxidation activity. RSC Adv. 2014, 4, 48449–48457. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Dey, S.; Roy, P. Synthesis, characterization and catalytic properties of dinuclear complexes of copper(II) and nickel(II): Oxidation of cyclohexane, toluene and cyclopentane. Inorg. Chim. Acta 2019, 490, 93–103. [Google Scholar] [CrossRef]
- Sutradhar, M.; Alegria, E.C.B.A.; Guedes da Silva, M.F.C.; Martins, L.M.D.R.S.; Pombeiro, A.J.L. Aroylhydrazone Cu(II) Complexes in keto Form: Structural Characterization and Catalytic Activity towards Cyclohexane Oxidation. Molecules 2016, 21, 425. [Google Scholar] [CrossRef]
- Detoni, C.; Carvalho, N.M.F.; Aranda, D.A.G.; Louis, B.; Antunes, O.A.C. Cyclohexane and toluene oxidation catalyzed by 1,10-phenantroline Cu(II) complexes. Appl. Catal. A Gen. 2009, 365, 281–286. [Google Scholar] [CrossRef]
- Bilyachenko, A.N.; Khrustalev, V.N.; Gutsul, E.I.; Zueva, A.Y.; Korlyukov, A.A.; Shul’pina, L.S.; Ikonnikov, N.S.; Dorovatovskii, P.V.; Gelman, D.; Shubina, E.S.; et al. Hybrid Silsesquioxane/Benzoate Cu7-Complexes: Synthesis, Unique Cage Structure, and Catalytic Activity. Molecules 2022, 27, 8505. [Google Scholar] [CrossRef] [PubMed]
- Hazra, S.; Rocha, B.G.M.; Guedes da Silva, M.F.C.; Karmakar, A.; Pombeiro, A.J.L. Syntheses, Structures, and Catalytic Hydrocarbon Oxidation Properties of N-Heterocycle-Sulfonated Schiff Base Copper(II) Complexes. Inorganics 2019, 7, 17. [Google Scholar] [CrossRef]
- Fırıncı, E. Copper(II) complexes with barbiturate derivatives: Synthesis, characterization and catalytic applications. Polyhedron 2019, 164, 132–137. [Google Scholar] [CrossRef]
- Galassi, R.; Simon, O.C.; Burini, A.; Tosi, G.; Conti, C.; Graiff, C.; Martins, N.M.R.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Copper(I) and copper(II) metallacycles as catalysts for microwave assisted selective oxidation of cyclohexane. Polyhedron 2017, 134, 143–152. [Google Scholar] [CrossRef]
- Fernandes, R.J.R. Bioinspired Iron and Copper Catalyzed Oxidations and Reactions in Supercritical CO2 Media. Ph.D. Thesis, Instituto Superior Técnico, Universidade de Lisboa, Lisbon, Portugal, 2011. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).