Two-Dimensional Mixed-Ligand Metal–Organic Framework Constructed from Bridging Bidentate V-Shaped Ligands
Abstract
:1. Introduction
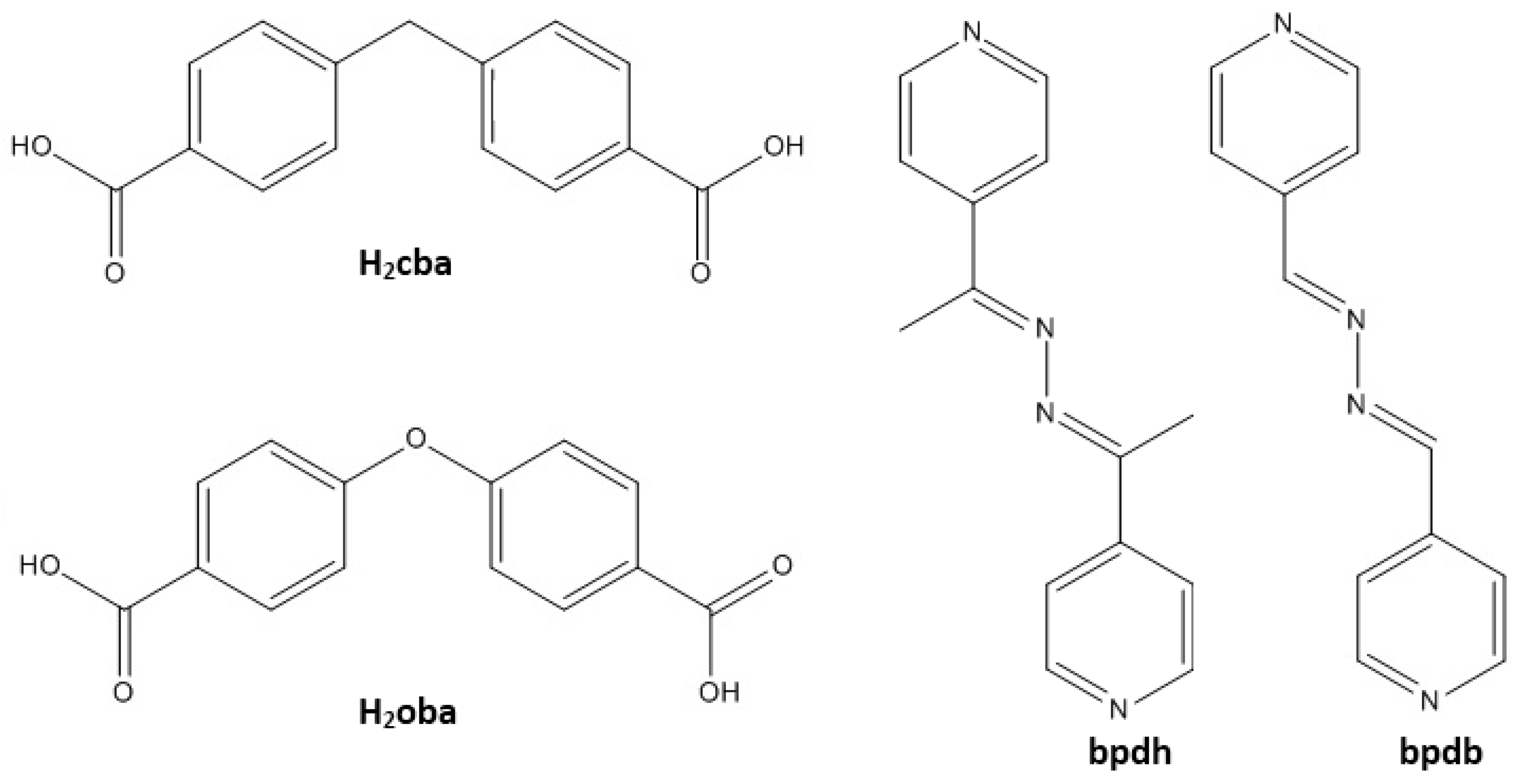
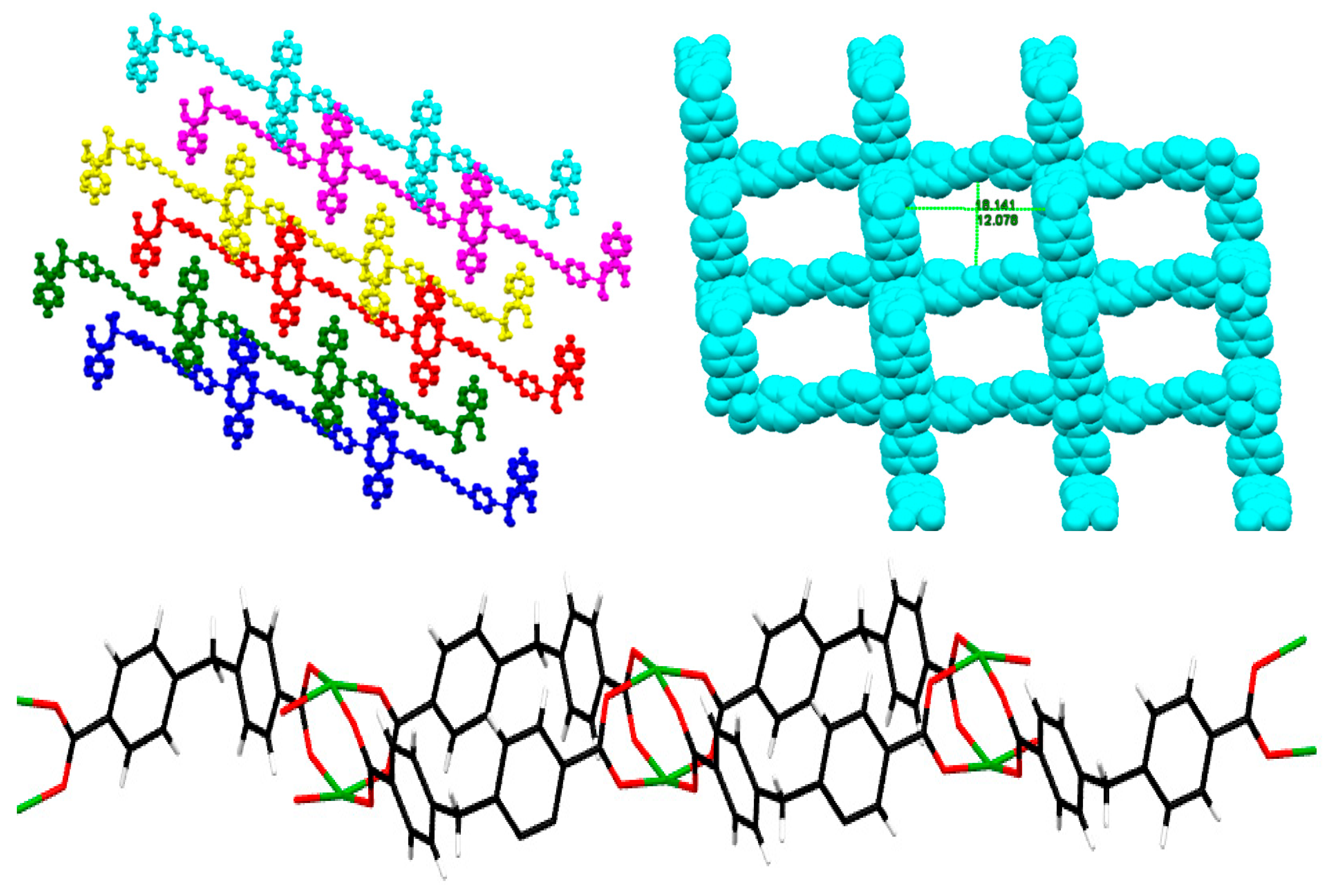
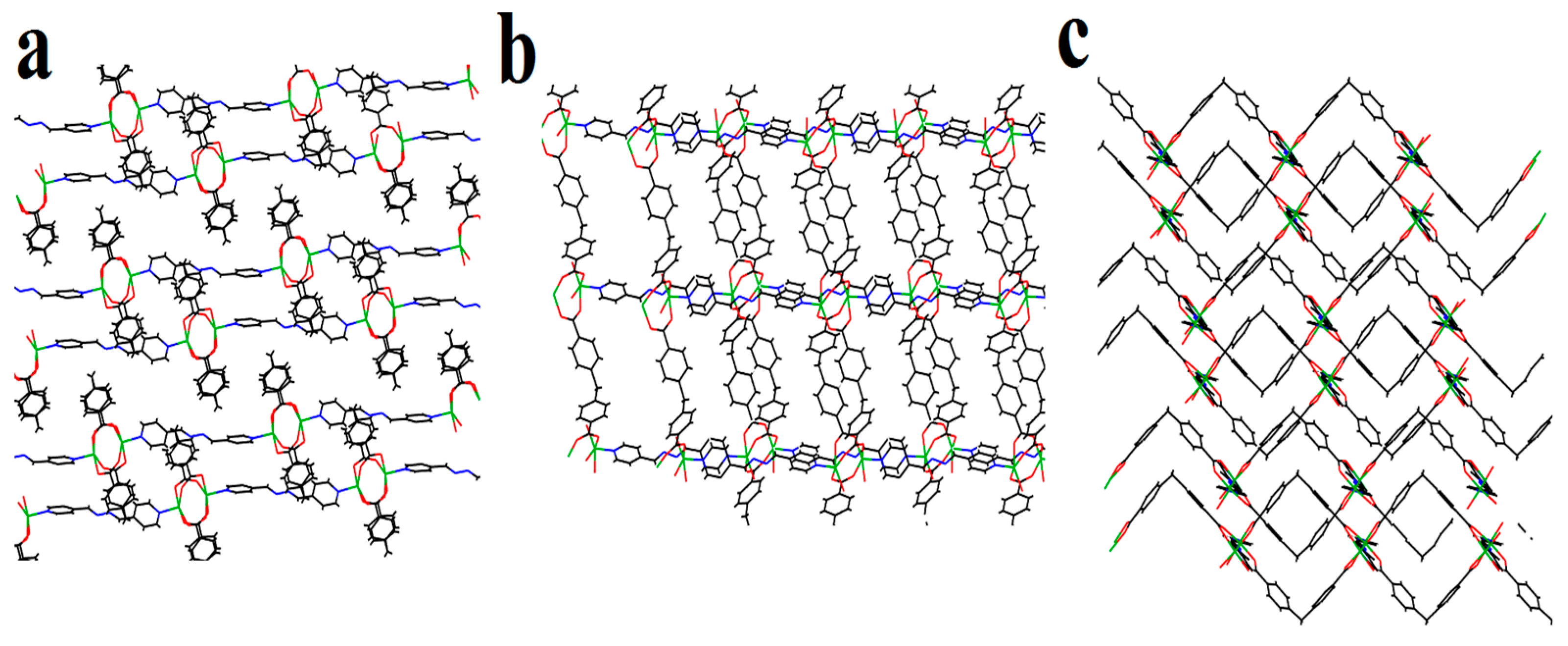
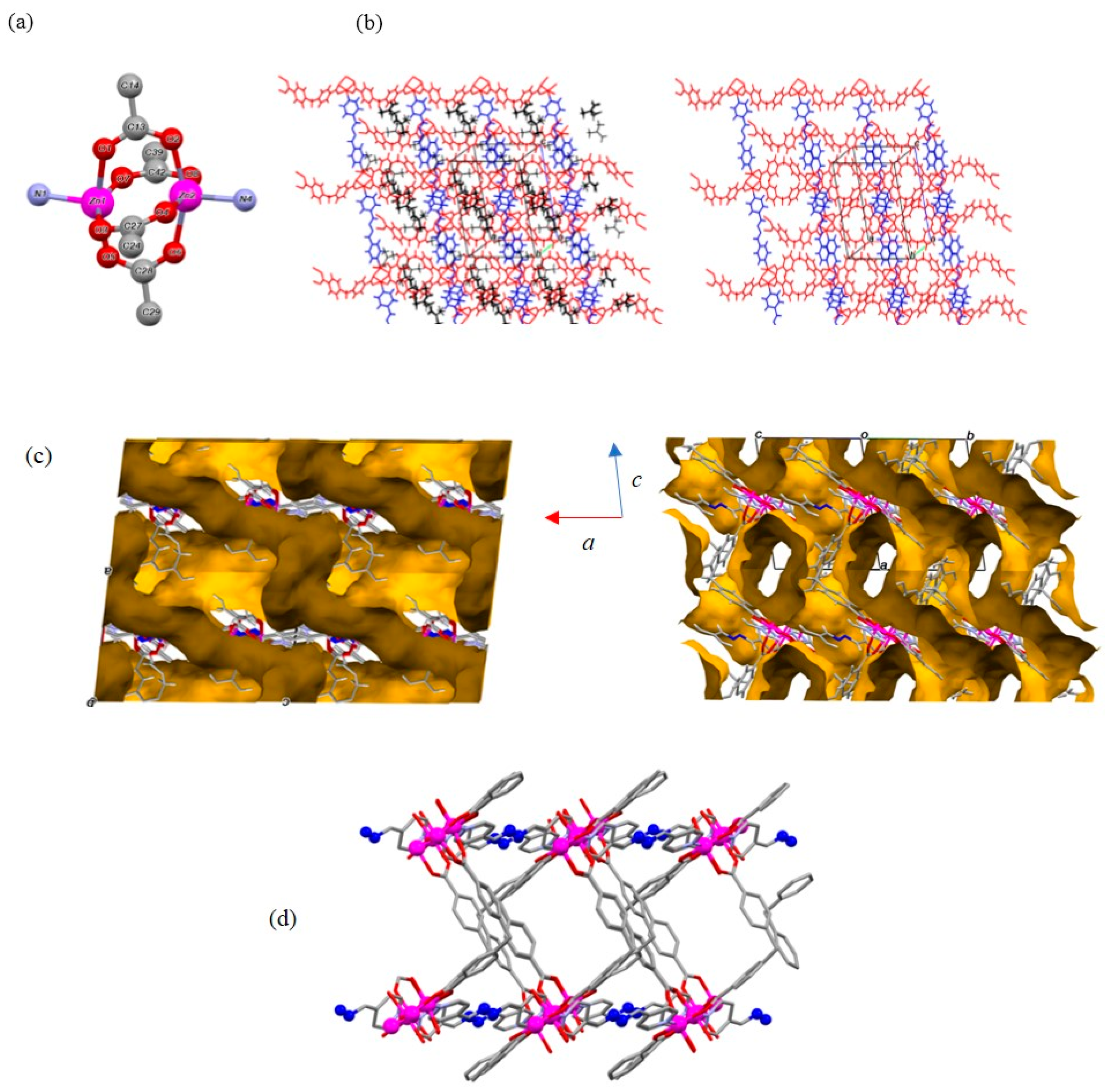
2. Results and Discussion
3. Experimental Section
3.1. Materials and Physical Measurements
3.2. Synthesis of the Ligand and MOF
3.3. Determination of the Crystal Structure of 2DTMU-1
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sanati, S.; Abazari, R.; Albero, J.; Morsali, A.; García, H.; Liang, Z.; Zou, R. Metal–organic framework derived bimetallic materials for electrochemical energy storage. Angew. Chem. Int. Ed. 2021, 60, 11048–11067. [Google Scholar] [CrossRef] [PubMed]
- Masoomi, M.Y.; Morsali, A.; Dhakshinamoorthy, A.; Garcia, H. Mixed-MOFs: Unique opportunities in metal–organic framework (MOF) functionality and design. Angew. Chem. 2019, 131, 15330–15347. [Google Scholar] [CrossRef]
- Liu, K.-G.; Sharifzadeh, Z.; Rouhani, F.; Ghorbanloo, M.; Morsali, A. Metal-organic framework composites as green/sustainable catalysts. Coord. Chem. Rev. 2021, 436, 213827. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Masoomi, M.Y.; Morsali, A. Morphology-dependent sensing performance of dihydro-tetrazine functionalized MOF toward Al (III). Ultrason. Sonochem. 2018, 41, 17–26. [Google Scholar] [CrossRef]
- Morsali, A.; Mahjoub, A. Coordination polymers of lead(II) with 4,4′-bipyridine: Syntheses and structures. Polyhedron 2004, 23, 2427–2436. [Google Scholar] [CrossRef]
- Fard-Jahromi, M.J.S.; Morsali, A. Sonochemical synthesis of nanoscale mixed-ligands lead (II) coordination polymers as precursors for preparation of Pb2(SO4)O and PbO nanoparticles; thermal, structural and X-ray powder diffraction studies. Ultrason. Sonochem. 2010, 17, 435–440. [Google Scholar] [CrossRef]
- Beobide, G.; Castillo, O.; Cepeda, J.; Luque, A.; Pérez-Yáñez, S.; Román, P.; Thomas-Gipson, J. Metal–carboxylato–nucleobase systems: From supramolecular assemblies to 3D porous materials. Coord. Chem. Rev. 2013, 257, 2716–2736. [Google Scholar] [CrossRef]
- Farrusseng, D. Metal-Organic Frameworks: Applications from Catalysis to Gas Storage; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- OYaghi, M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Thakuria, H.; Das, G. CuO micro plates from a 3D metallo-organic framework (MOF) of a binary copper (II) complex of N,N-bis(2-hydroxyethyl) glycine. Polyhedron 2007, 26, 149–153. [Google Scholar] [CrossRef]
- Bigdeli, F.; Morsali, A.; Retailleau, P. Syntheses and characterization of different zinc (II) oxide nano-structures from direct thermal decomposition of 1D coordination polymers. Polyhedron 2010, 29, 801–806. [Google Scholar] [CrossRef]
- Safarifard, V.; Rodríguez-Hermida, S.; Guillerm, V.; Imaz, I.; Bigdeli, M.; Tehrani, A.A.; Juanhuix, J.; Morsali, A.; Casco, M.E.; Silvestre-Albero, J. Influence of the amide groups in the CO2/N2 selectivity of a series of isoreticular, interpenetrated metal–organic frameworks. Cryst. Growth Des. 2016, 16, 6016–6023. [Google Scholar] [CrossRef]
- Sadeghzadeh, H.; Morsali, A. Hedge balls nano-structure of a mixed-ligand lead (II) coordination polymer; thermal, structural and X-ray powder diffraction studies. Cryst. Eng. Comm. 2010, 12, 370–372. [Google Scholar] [CrossRef]
- Lin, H.-Y.; Mu, B.; Wang, X.-L.; Tian, A.-X. Three copper (II) complexes connected through tetradentate carboxylate linkers and bidentate N-heterocyclic ligands: From 3-D MOF to 1-D chain. J. Organomet. Chem. 2012, 702, 36–44. [Google Scholar] [CrossRef]
- Hashemi, L.; Morsali, A. Microwave assisted synthesis of a new lead (II) porous three-dimensional coordination polymer: Study of nanostructured size effect on high iodide adsorption affinity. Cryst. Eng. Comm. 2012, 14, 779–781. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Morsali, A.; Junk, P.C. Ultrasound assisted synthesis of a Zn (II) metal–organic framework with nano-plate morphology using non-linear dicarboxylate and linear N-donor ligands. RSC Adv. 2014, 4, 47894–47898. [Google Scholar] [CrossRef]
- Ranjbar, Z.R.; Morsali, A. Sonochemical syntheses of a new nano-sized porous lead (II) coordination polymer as precursor for preparation of lead (II) oxide nanoparticles. J. Mol. Struct. 2009, 936, 206–212. [Google Scholar] [CrossRef]
- Teo, P.; Hor, T.A. Spacer directed metallo-supramolecular assemblies of pyridine carboxylates. Coord. Chem. Rev. 2011, 255, 273–289. [Google Scholar] [CrossRef]
- Razavi, S.A.A.; Morsali, A.; Piroozzadeh, M. A Dihydrotetrazine-Functionalized Metal–Organic Framework as a Highly Selective Luminescent Host–Guest Sensor for Detection of 2,4,6-Trinitrophenol. Inorg. Chem. 2022, 61, 7820–7834. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef]
- Rouhani, F.; Morsali, A. Fast and Selective Heavy Metal Removal by a Novel Metal-Organic Framework Designed with In-Situ Ligand Building Block Fabrication Bearing Free Nitrogen. Chem. Eur. J. 2018, 24, 5529–5537. [Google Scholar] [CrossRef]
- Hu, M.-L.; Razavi, S.A.A.; Piroozzadeh, M.; Morsali, A. Sensing organic analytes by metal–organic frameworks: A new way of considering the topic. Inorg. Chem. Front. 2020, 7, 1598–1632. [Google Scholar] [CrossRef]
- Bigdeli, F.; Abedi, S.; Hosseini-Monfared, H.; Morsali, A. An investigation of the catalytic activity in a series of isoreticular Zn (II)-based metal-organic frameworks. Inorg. Chem. Commun. 2016, 72, 122–127. [Google Scholar] [CrossRef]
- Motakef-Kazemi, N.; Shojaosadati, S.A.; Morsali, A. In situ synthesis of a drug-loaded MOF at room temperature. Microporous Mesoporous Mater. 2014, 186, 73–79. [Google Scholar] [CrossRef]
- Akhbari, K.; Morsali, A. Modulating methane storage in anionic nano-porous MOF materials via post-synthetic cation exchange process. Dalton Trans. 2013, 42, 4786–4789. [Google Scholar] [CrossRef] [PubMed]
- Afshariazar, F.; Morsali, A. A dual-response regenerable luminescent 2D-MOF for nitroaromatic sensing via target-modulation of active interaction sites. J. Mater. Chem. C 2021, 9, 12849–12858. [Google Scholar] [CrossRef]
- Parsa, F.; Ghorbanloo, M.; Morsali, A.; Wang, J.; Junk, P.C.; Retailleau, P. Azobenzene based 2D-MOF for high selective quinone fluorescence sensing performance. Inorg. Chim. Acta 2020, 510, 119699. [Google Scholar] [CrossRef]
- Chakraborty, G.; Park, I.-H.; Medishetty, R.; Vittal, J.J. Two-dimensional metal-organic framework materials: Synthesis, structures, properties and applications. Chem. Rev. 2021, 121, 3751–3891. [Google Scholar] [CrossRef]
- Abdollahi, N.; Masoomi, M.Y.; Morsali, A.; Junk, P.C.; Wang, J. Sonochemical synthesis and structural characterization of a new Zn (II) nanoplate metal–organic framework with removal efficiency of Sudan red and Congo red. Ultrason. Sonochem. 2018, 45, 50–56. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Bagheri, M.; Morsali, A. Porosity and dye adsorption enhancement by ultrasonic synthesized Cd (II) based metal-organic framework. Ultrason. Sonochem. 2017, 37, 244–250. [Google Scholar] [CrossRef]
- Zhang, X.; Xamena, F.L.I.; Corma, A. Gold (III)–metal organic framework bridges the gap between homogeneous and heterogeneous gold catalysts. J. Catal. 2009, 265, 155–160. [Google Scholar] [CrossRef]
- Wang, M.; Dong, R.; Feng, X. Two-dimensional conjugated metal–organic frameworks (2D c-MOFs): Chemistry and function for MOFtronics. Chem. Soc. Rev. 2021, 50, 2764–2793. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Campbell, M.G.; Dincă, M. Electrically conductive porous metal–organic frameworks. Angew. Chem. Int. Ed. 2016, 55, 3566–3579. [Google Scholar] [CrossRef]
- Afshariazar, F.; Morsali, A. Target-Architecture Engineering of a Novel Two-Dimensional Metal–Organic Framework for High Catalytic Performance. Cryst. Growth Des. 2019, 19, 4239–4245. [Google Scholar] [CrossRef]
- Su, J.; He, W.; Li, X.-M.; Sun, L.; Wang, H.-Y.; Lan, Y.-Q.; Ding, M.; Zuo, J.-L. High electrical conductivity in a 2D MOF with intrinsic superprotonic conduction and interfacial pseudo-capacitance. Matter 2020, 2, 711–722. [Google Scholar] [CrossRef]
- Li, J.; Song, S.; Meng, J.; Tan, L.; Liu, X.; Zheng, Y.; Li, Z.; Yeung, K.W.K.; Cui, Z.; Liang, Y. 2D MOF periodontitis photodynamic ion therapy. J. Am. Chem. Soc. 2021, 143, 15427–15439. [Google Scholar] [CrossRef]
- Ahmad, N.; Younus, H.A.; Chughtai, A.H.; Van Hecke, K.; Khattak, Z.A.; Gaoke, Z.; Danish, M.; Verpoort, F. Synthesis of 2D MOF having potential for efficient dye adsorption and catalytic applications. Catal. Sci. Technol. 2018, 8, 4010–4017. [Google Scholar] [CrossRef]
- Ghasempour, H.; Wang, K.-Y.; Powell, J.A.; ZareKarizi, F.; Lv, X.-L.; Morsali, A.; Zhou, H.-C. Metal–organic frameworks based on multicarboxylate linkers. Coord. Chem. Rev. 2021, 426, 213542. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Masoomi, M.Y.; Stylianou, K.C.; Morsali, A.; Retailleau, P.; Maspoch, D. Selective CO2 capture in metal–organic frameworks with azine-functionalized pores generated by mechanosynthesis. Cryst. Growth Des. 2014, 14, 2092–2096. [Google Scholar] [CrossRef]
- OD, R. CrysAlis PRO; Rigaku Oxford Diffraction: Oxfordshire, UK, 2015. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Ciurtin, D.M.; Dong, Y.-B.; Smith, M.D.; Barclay, T.; Loye, H.-C.Z. Two versatile N,N′-bipyridine-type ligands for preparing organic− inorganic coordination polymers: New cobalt-and nickel-containing framework materials. Inorg. Chem. 2001, 40, 2825–2834. [Google Scholar] [CrossRef]
- Spek, A.L. checkCIF validation ALERTS: What they mean and how to respond. Acta Crystallogr. Sect. E Crystallogr. Commun. 2020, 76, 1–11. [Google Scholar] [CrossRef]



| Zn(1)-O(5) | 2.040(2) | O(5)-Zn(1)-N(1) | 99.68(9) |
| Zn(1)-N(1) | 2.041(2) | O(5)-Zn(1)-O(3) #1 | 87.60(9) |
| Zn(1)-O(3) #1 | 2.047(2) | N(1)-Zn(1)-O(3) #1 | 110.72(10) |
| Zn(1)-O(1) #2 | 2.052(2) | O(5)-Zn(1)-O(1) #2 | 163.74(8) |
| Zn(1)-O(7) #3 | 2.056(2) | N(1)-Zn(1)-O(1) #2 | 96.35(9) |
| Zn(1)-Zn(2) #2 | 2.9338(5) | O(3) #1-Zn(1)-O(1) #2 | 89.28(9) |
| Zn(2)-O(2) | 2.033(2) | O(5)-Zn(1)-O(7) #3 | 88.22(9) |
| Zn(2)-N(4) | 2.042(2) | N(1)-Zn(1)-O(7) #3 | 94.92(10) |
| Zn(2)-O(6) #4 | 2.048(2) | O(3) #1-Zn(1)-O(7) #3 | 154.36(8) |
| Zn(2)-O(8) #5 | 2.050(2) | O(1) #2-Zn(1)-O(7) #3 | 87.71(9) |
| Zn(2)-O(4) #6 | 2.098(2) | O(5)-Zn(1)-Zn(2) #2 | 86.65(6) |
| N(1)-Zn(1)-Zn(2) #2 | 162.94(8) | ||
| O(3) #1-Zn(1)-Zn(2) #2 | 85.21(6) | ||
| O(1) #2-Zn(1)-Zn(2) #2 | 77.19(6) | ||
| O(7) #3-Zn(1)-Zn(2) #2 | 69.30(6) | ||
| O(2)-Zn(2)-N(4) | 102.58(9) | ||
| O(2)-Zn(2)-O(6) #4 | 155.79(8) | ||
| N(4)-Zn(2)-O(6) #4 | 100.92(9) | ||
| O(2)-Zn(2)-O(8) #5 | 88.07(9) | ||
| N(4)-Zn(2)-O(8) #5 | 103.56(9) | ||
| O(6) #4-Zn(2)-O(8) #5 | 92.06(9) | ||
| O(2)-Zn(2)-O(4) #6 | 87.52(9) | ||
| N(4)-Zn(2)-O(4) #6 | 91.10(9) | ||
| O(6) #4-Zn(2)-O(4) #6 | 86.28(9) | ||
| O(8) #5-Zn(2)-O(4) #6 | 165.29(8) | ||
| O(2)-Zn(2)-Zn(1) #4 | 82.66(6) | ||
| N(4)-Zn(2)-Zn(1) #4 | 164.91(8) | ||
| O(6) #4-Zn(2)-Zn(1) #4 | 73.13(6) | ||
| O(8) #5-Zn(2)-Zn(1) #4 | 90.66(6) | ||
| O(4) #6-Zn(2)-Zn(1) #4 | 74.87(6) |
| Identification Code | 2DTMU-1 [Zn2(cba)2(bpdb)]·(DMF)y |
|---|---|
| Empirical formula | C42 H30 N4 O8 Zn2, 4 (C3 H7 N O) |
| Formula weight | 1141.82 |
| Temperature | 100(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Triclinic |
| Space group | P-1 |
| Unit cell dimensions | a = 12.6848(9) Å, α = 78.658(5)° |
| b = 12.9028(8) Å, β = 80.243(5)° | |
| c = 18.1796(9) Å, γ = 72.620(6)° | |
| Volume | 2764.6(3) Å3 |
| Z | 2 |
| Density (calculated) | 1.382 Mg/m3 |
| Absorption coefficient | 0.942 mm−1 |
| F(000) | 1188 |
| Crystal size | 0.12 mm × 0.11 mm × 0.07 mm |
| θ range for data collection | 2.550 to 25.349° |
| Index ranges | −15 ≤ h ≤ 15, −15 ≤ k ≤ 14, −21 ≤ l ≤ 21 |
| Reflections collected | 40891 |
| Independent reflections | 10063 [R(int) = 0.1061] |
| Completeness to θ = 25.242 Å | 99.2% |
| Absorption correction | Sphere r 0.1 Å T 0.872 |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 10,060/401/764 |
| Goodness-of-fit for F2 | 0.966 |
| Final R indices [I > 2σ(I)] | R1 = 0.0452, wR2 = 0.0896 |
| R indices (all data) | R1 = 0.0801, wR2 = 0.1003 |
| Largest diff. peak and hole | 0.444 and −0.472 e. Å−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, W.-W.; Dehghani Firuzabadi, F.; Hanifehpour, Y.; Zeng, X.; Feng, Y.-J.; Liu, K.-G.; Joo, S.W.; Morsali, A.; Retailleau, P. Two-Dimensional Mixed-Ligand Metal–Organic Framework Constructed from Bridging Bidentate V-Shaped Ligands. Inorganics 2023, 11, 184. https://doi.org/10.3390/inorganics11050184
Zhong W-W, Dehghani Firuzabadi F, Hanifehpour Y, Zeng X, Feng Y-J, Liu K-G, Joo SW, Morsali A, Retailleau P. Two-Dimensional Mixed-Ligand Metal–Organic Framework Constructed from Bridging Bidentate V-Shaped Ligands. Inorganics. 2023; 11(5):184. https://doi.org/10.3390/inorganics11050184
Chicago/Turabian StyleZhong, Wen-Wu, Fahimeh Dehghani Firuzabadi, Younes Hanifehpour, Xue Zeng, Yuan-Jiao Feng, Kuan-Guan Liu, Sang Woo Joo, Ali Morsali, and Pascal Retailleau. 2023. "Two-Dimensional Mixed-Ligand Metal–Organic Framework Constructed from Bridging Bidentate V-Shaped Ligands" Inorganics 11, no. 5: 184. https://doi.org/10.3390/inorganics11050184
APA StyleZhong, W.-W., Dehghani Firuzabadi, F., Hanifehpour, Y., Zeng, X., Feng, Y.-J., Liu, K.-G., Joo, S. W., Morsali, A., & Retailleau, P. (2023). Two-Dimensional Mixed-Ligand Metal–Organic Framework Constructed from Bridging Bidentate V-Shaped Ligands. Inorganics, 11(5), 184. https://doi.org/10.3390/inorganics11050184







