Electrode with a Carbon Nanotube Array for a Proton Exchange Membrane Fuel Cell
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Making an Electrode
3.2. Growing a CNTA
3.3. Growing a Catalytic Layer
3.4. Electrode Studies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Okonkwo, P.C.; Emori, W.; Uzoma, P.C.; Mansir, I.B.; Radwan, A.B.; Ige, O.O.; Abdullah, A.M. A Review of Bipolar Plates Materials and Graphene Coating Degradation Mechanism in Proton Exchange Membrane Fuel Cell. Int. J. Energy Res. 2022, 46, 3766–3781. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ben Belgacem, I.; Emori, W.; Uzoma, P.C. Nafion Degradation Mechanisms in Proton Exchange Membrane Fuel Cell (PEMFC) System: A Review. Int. J. Hydrogen Energy 2021, 46, 27956–27973. [Google Scholar] [CrossRef]
- Prokop, M.; Drakselova, M.; Bouzek, K. Review of the Experimental Study and Prediction of Pt-Based Catalyst Degradation during PEM Fuel Cell Operation. Curr. Opin. Electrochem. 2020, 20, 20–27. [Google Scholar] [CrossRef]
- Speder, J.; Zana, A.; Spanos, I.; Kirkensgaard, J.J.K.; Mortensen, K.; Hanzlik, M.; Arenz, M. Comparative Degradation Study of Carbon Supported Proton Exchange Membrane Fuel Cell Electrocatalysts—The Influence of the Platinum to Carbon Ratio on the Degradation Rate. J. Power Source 2014, 261, 14–22. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Ige, O.O.; Barhoumi, E.M.; Uzoma, P.C.; Emori, W.; Benamor, A.; Abdullah, A.M. Platinum Degradation Mechanisms in Proton Exchange Membrane Fuel Cell (PEMFC) System: A Review. Int. J. Hydrogen Energy 2021, 46, 15850–15865. [Google Scholar] [CrossRef]
- Gupta, C.; Maheshwari, P.H.; Sasikala, S.; Mathur, R.B. Processing of Pristine Carbon Nanotube Supported Platinum as Catalyst for PEM Fuel Cell. Mater. Renew. Sustain. Energy 2014, 3, 36. [Google Scholar] [CrossRef]
- Sandström, R.; Ekspong, J.; Annamalai, A.; Sharifi, T.; Klechikov, A.; Wågberg, T. Fabrication of Microporous Layer-Free Hierarchical Gas Diffusion Electrode as a Low Pt-Loading PEMFC Cathode by Direct Growth of Helical Carbon Nanofibers. RSC Adv. 2018, 8, 41566–41574. [Google Scholar] [CrossRef]
- Kozlova, M.; Butrim, S.; Solovyev, M.; Pushkarev, A.; Pushkareva, I.; Kalinichenko, V.; Akelkina, S.; Grigoriev, S. Structural and Electrochemical Characteristics of Platinum Nanoparticles Supported on Various Carbon Carriers. C 2022, 8, 14. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Graphene Oxide: A Promising Membrane Material for Fuel Cells. Renew. Sustain. Energy Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- Voznyakovskii, A.; Vozniakovskii, A.; Kidalov, S. New Way of Synthesis of Few-Layer Graphene Nanosheets by the Self Propagating High-Temperature Synthesis Method from Biopolymers. Nanomaterials 2022, 12, 657. [Google Scholar] [CrossRef]
- Fu, X.; Hassan, F.M.; Zamani, P.; Jiang, G.; Higgins, D.C.; Choi, J.Y.; Wang, X.; Xu, P.; Liu, Y.; Chen, Z. Engineered Architecture of Nitrogenous Graphene Encapsulating Porous Carbon with Nano-Channel Reactors Enhancing the PEM Fuel Cell Performance. Nano Energy 2017, 42, 249–256. [Google Scholar] [CrossRef]
- Wang, F.; Chen, L.; Li, H.; Duan, G.; He, S.; Zhang, L.; Zhang, G.; Zhou, Z.; Jiang, S. N-Doped Honeycomb-like Porous Carbon towards High-Performance Supercapacitor. Chin. Chem. Lett. 2020, 31, 1986–1990. [Google Scholar] [CrossRef]
- Sharma, S.; Pollet, B.G. Support Materials for PEMFC and DMFC Electrocatalysts—A Review. J. Power Source 2012, 208, 96–119. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Graphene in Electrocatalyst and Proton Conductiong Membrane in Fuel Cell Applications: An Overview. Renew. Sustain. Energy Rev. 2017, 69, 862–870. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Wei, J.-M.; Lin, J.-S.; Chen, W.-Y. Pulse Electrodeposition of Pt Nanocatalysts on Graphene-Based Electrodes for Proton Exchange Membrane Fuel Cells. Catal. Commun. 2011, 16, 220–224. [Google Scholar] [CrossRef]
- Remy, E.; Thomas, Y.R.J.; Guetaz, L.; Fouda-Onana, F.; Jacques, P.A.; Heitzmann, M. Optimization and Tunability of 2d Graphene and 1d Carbon Nanotube Electrocatalysts Structure for Pem Fuel Cells. Catalysts 2018, 8, 377. [Google Scholar] [CrossRef]
- Mariani, M.; Latorrata, S.; Patrignani, S.; Gallo Stampino, P.; Dotelli, G. Characterization of Novel Graphene-Based Microporous Layers for Polymer Electrolyte Membrane Fuel Cells Operating under Low Humidity and High Temperature. Int. J. Hydrogen Energy 2020, 45, 7046–7058. [Google Scholar] [CrossRef]
- Ageev, O.A.; Blinov, Y.F.; Il’ina, M.V.; Il’in, O.I.; Smirnov, V.A.; Tsukanova, O.G. Study of Adhesion of Vertically Aligned Carbon Nanotubes to a Substrate by Atomic-Force Microscopy. Phys. Solid State 2016, 58, 309–314. [Google Scholar] [CrossRef]
- Luo, C.; Xie, H.; Wang, Q.; Luo, G.; Liu, C. A Review of the Application and Performance of Carbon Nanotubes in Fuel Cells. J. Nanomater. 2015, 2015, 560392. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, H.S.; Yun, E.T.; Ham, S.Y.; Park, J.H.; Ahn, C.H.; Lee, S.H.; Park, H.D. Vertically Aligned Carbon Nanotube Membranes: Water Purification and Beyond. Membranes 2020, 10, 273. [Google Scholar] [CrossRef]
- Zanin, H.; May, P.W.; Harniman, R.L.; Risbridger, T.; Corat, E.J.; Fermin, D.J. High Surface Area Diamond-like Carbon Electrodes Grown on Vertically Aligned Carbon Nanotubes. Carbon N. Y. 2015, 82, 288–296. [Google Scholar] [CrossRef]
- Cho, W.; Schulz, M.; Shanov, V. Growth and Characterization of Vertically Aligned Centimeter Long CNT Arrays. Carbon N. Y. 2014, 72, 264–273. [Google Scholar] [CrossRef]
- Kudinova, E.S.; Vorobyeva, E.A.; Ivanova, N.A.; Tishkin, V.V.; Alekseeva, O.K. A Magnetron Sputtering Method for the Application of the Ni Catalyst for the Synthesis Process of Carbon Nanotube Arrays. Nanotechnol. Russ. 2020, 15, 715–722. [Google Scholar] [CrossRef]
- Meng, Q.H.; Hao, C.; Yan, B.; Yang, B.; Liu, J.; Shen, P.K.; Tian, Z.Q. High-Performance Proton Exchange Membrane Fuel Cell with Ultra-Low Loading Pt on Vertically Aligned Carbon Nanotubes as Integrated Catalyst Layer. J. Energy Chem. 2022, 71, 497–506. [Google Scholar] [CrossRef]
- Murata, S.; Imanishi, M.; Hasegawa, S.; Namba, R. Vertically Aligned Carbon Nanotube Electrodes for High Current Density Operating Proton Exchange Membrane Fuel Cells. J. Power Source 2014, 253, 90–97. [Google Scholar] [CrossRef]
- Meng, X.; Deng, X.; Zhou, L.; Hu, B.; Tan, W.; Zhou, W.; Liu, M.; Shao, Z. A Highly Ordered Hydrophilic–Hydrophobic Janus Bi-Functional Layer with Ultralow Pt Loading and Fast Gas/Water Transport for Fuel Cells. Energy Environ. Mater. 2021, 4, 126–133. [Google Scholar] [CrossRef]
- Lu, C.; Shi, F.; Jin, J.; Peng, X. Study on the Properties of Vertical Carbon Nanotube Films Grown on Stainless Steel Bipolar Plates. Materials 2019, 16, 899. [Google Scholar] [CrossRef]
- Fontana, M.; Ramos, R.; Morin, A.; Dijon, J. Direct Growth of Carbon Nanotubes Forests on Carbon Fibers to Replace Microporous Layers in Proton Exchange Membrane Fuel Cells. Carbon N. Y. 2021, 172, 762–771. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Y.; Zhang, H.; Su, B.; Yang, Y. Optimization of CNTs Growth on TiB2-Based Composite Powders by CVD with Fe as Catalyst. Ceram. Int. 2020, 46, 3837–3843. [Google Scholar] [CrossRef]
- Mensharapov, R.M.; Ivanova, N.A.; Zasypkina, A.A.; Spasov, D.D.; Sinyakov, M.V.; Grigoriev, S.A.; Fateev, V.N. Model Study of CNT-Based PEMFCs’ Electrocatalytic Layers. Catalysts 2022, 12, 1227. [Google Scholar] [CrossRef]
- Ivanova, N.A.; Alekseeva, O.K.; Fateev, V.N.; Shapir, B.L.; Spasov, D.; Nikitin, S.M.; Presnyakov, M.Y.; Kolobylina, N.N.; Soloviev, M.A.; Mikhalev, A.I.; et al. Activity and Durability of Electrocatalytic Layers with Low Platinum Loading Prepared by Magnetron Sputtering onto Gas Diffusion Electrodes. Int. J. Hydrogen Energy 2019, 44, 29529–29536. [Google Scholar] [CrossRef]
- Chechenin, N.G.; Chernykh, P.N.; Vorobyeva, E.A.; Timofeev, O.S. Synthesis and Electroconductivity of Epoxy/Aligned CNTs Composites. Proc. Appl. Surf. Sci. 2013, 275, 217–221. [Google Scholar] [CrossRef]
- Lin, J.; Yang, Y.; Zhang, H.; Yang, Y.; Hu, S. Effects of Synthesis Temperature and Fe Catalyst Amount on the Performance of in Situ CNTs/TiB2 Composites. J. Alloys Compd. 2018, 745, 817–824. [Google Scholar] [CrossRef]
- Ivanova, N.A.; Spasov, D.D.; Zasypkina, A.A.; Alekseeva, O.K.; Kukueva, E.V.; Vorobyeva, E.A.; Kudinova, E.S.; Chumakov, R.G.; Millet, P.; Grigoriev, S.A. Comparison of the Performance and Durability of PEM Fuel Cells with Different Pt-Activated Microporous Layers. Int. J. Hydrogen Energy 2021, 46, 18093–18106. [Google Scholar] [CrossRef]
- Ivanova, N.A.; Spasov, D.D.; Grigoriev, S.A.; Kamyshinsky, R.A.; Peters, G.S.; Mensharapov, R.M.; Seregina, E.A.; Millet, P.; Fateev, V.N. On the Influence of Methanol Addition on the Performances of PEM Fuel Cells Operated at Subzero Temperatures. Int. J. Hydrogen Energy 2021, 46, 18116–18127. [Google Scholar] [CrossRef]

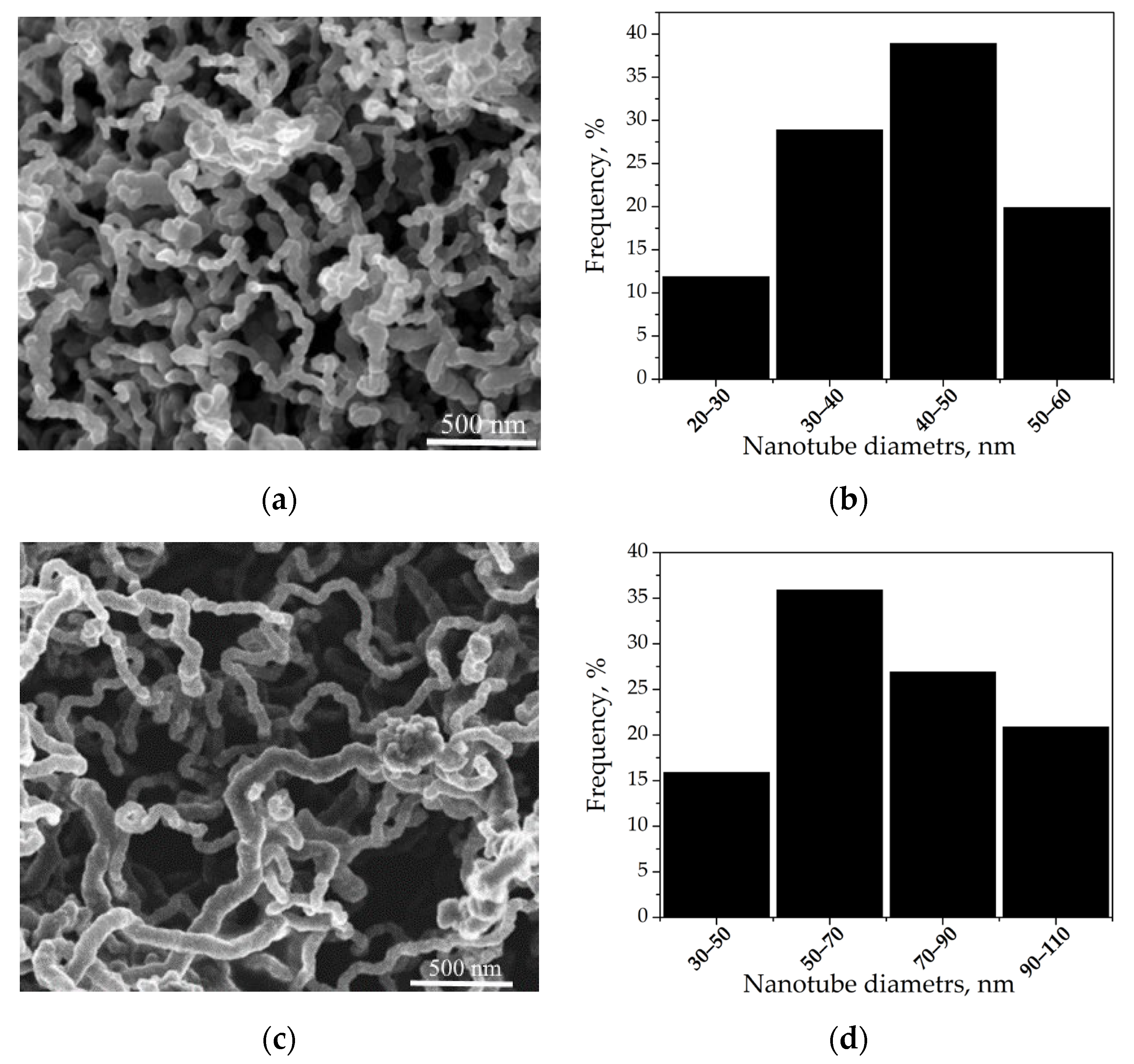
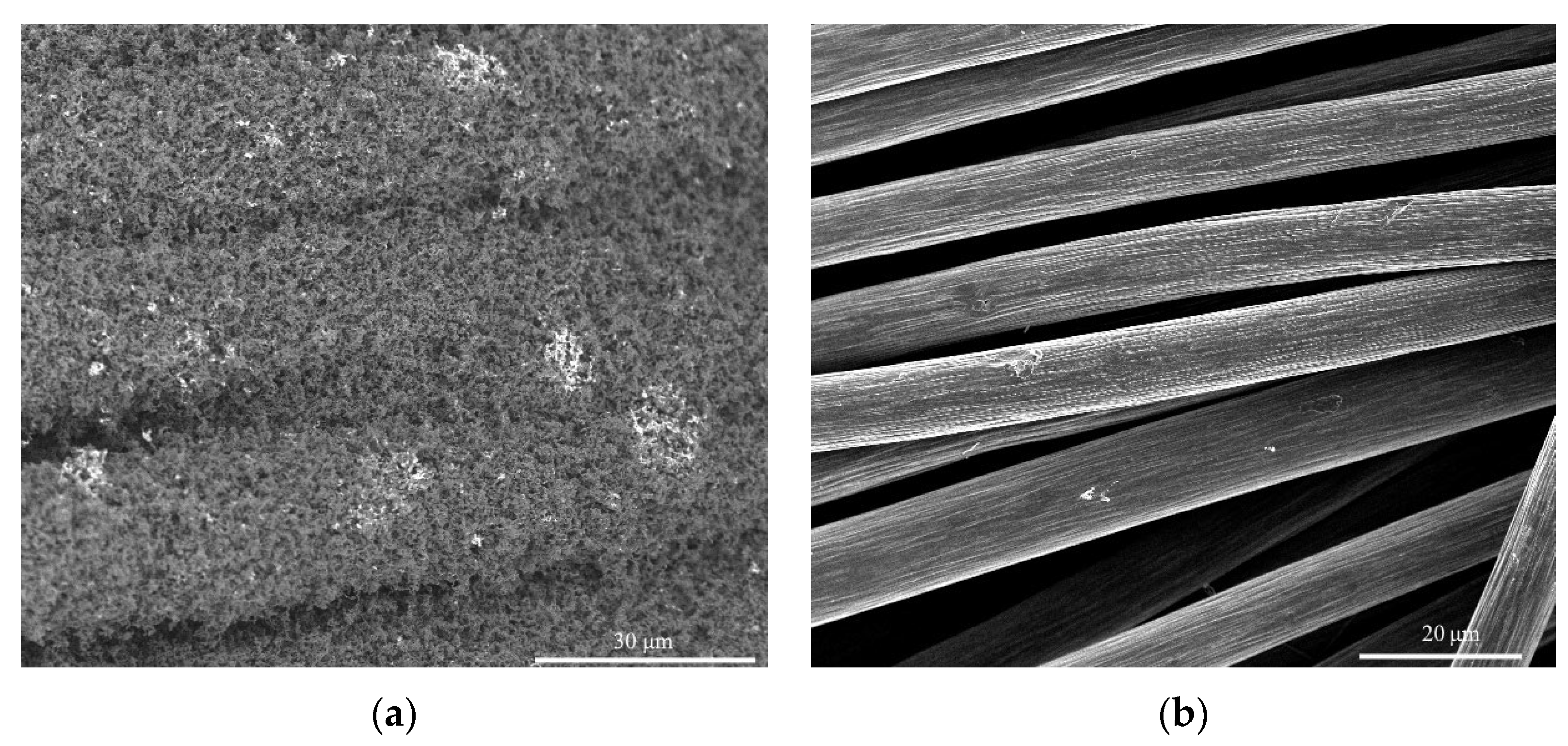



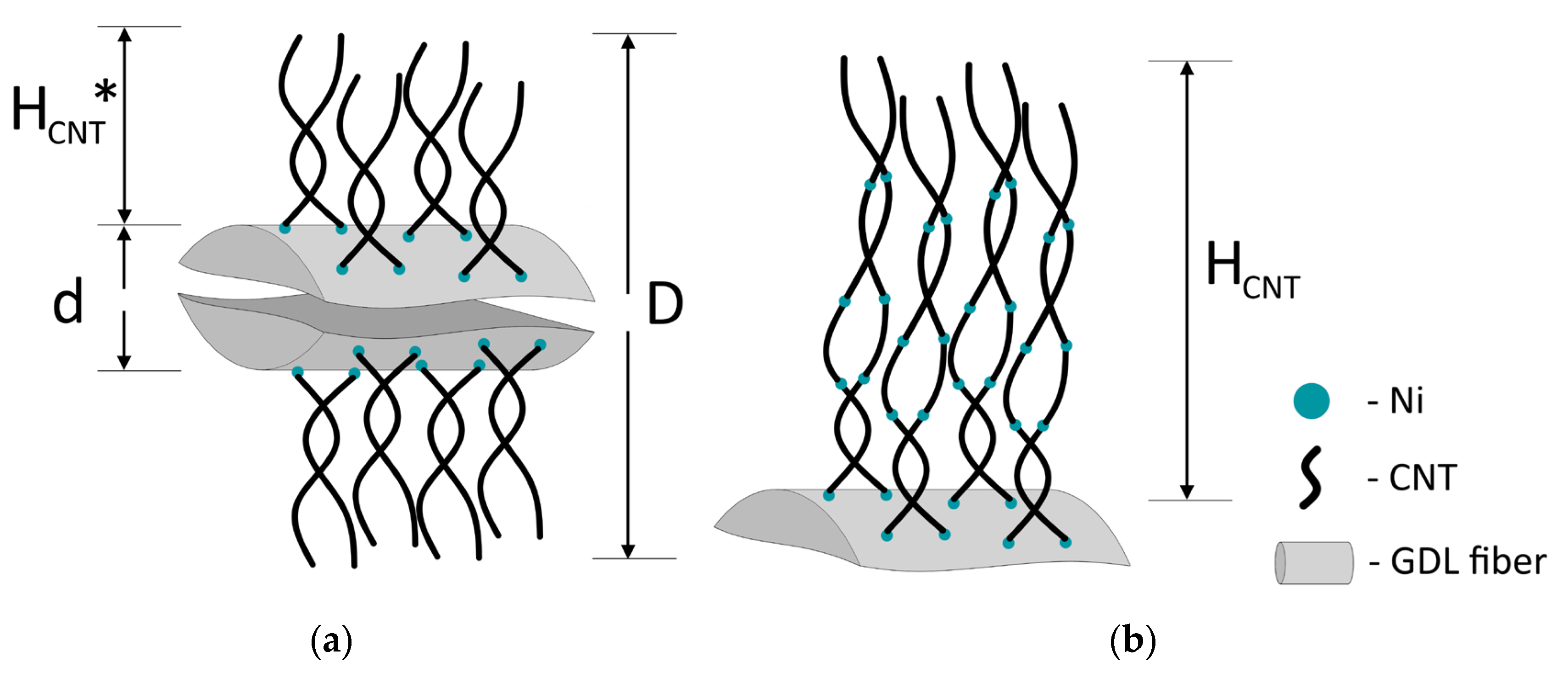
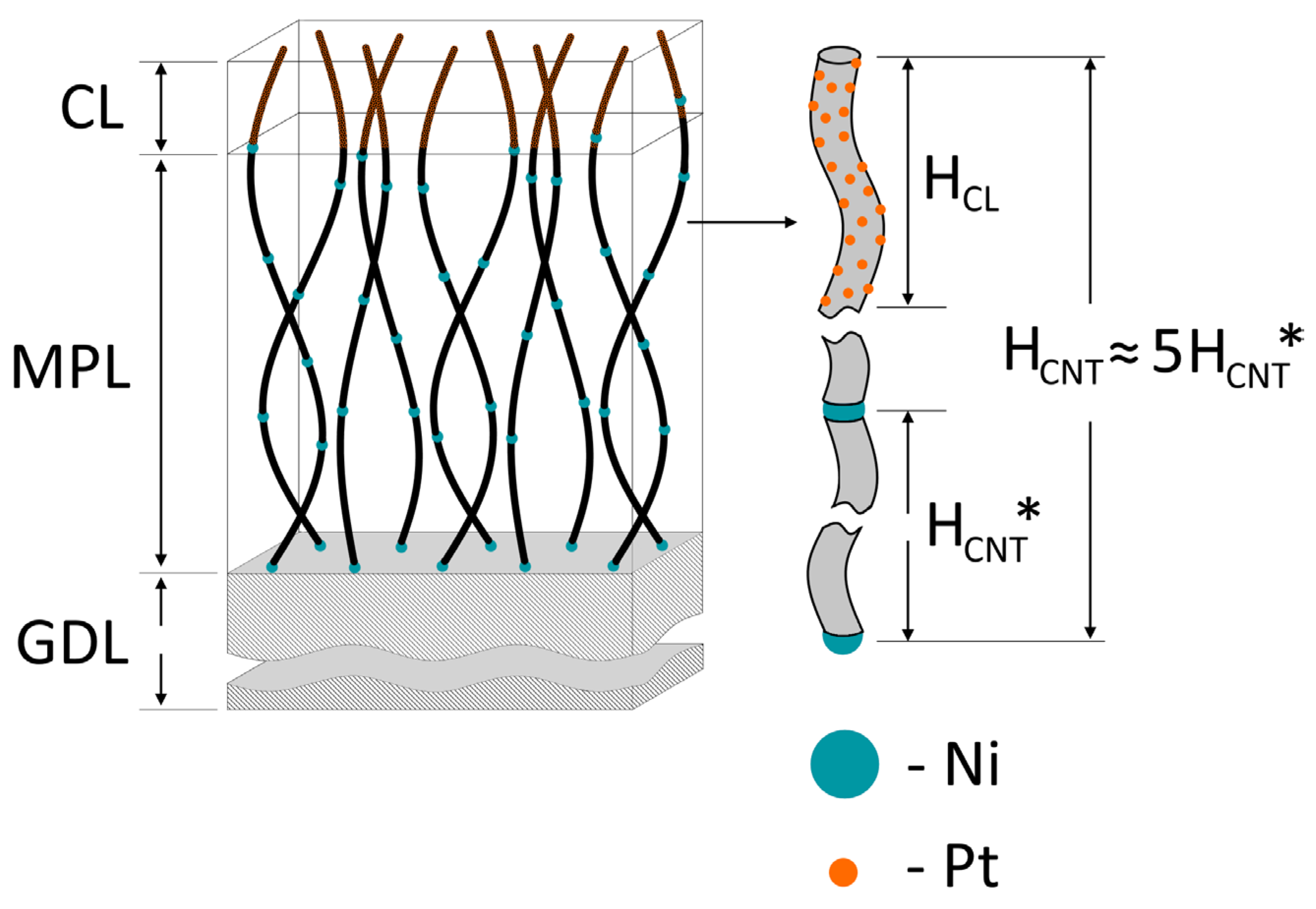
| Sample | Initial GDL | Diameter (Average) of Ni Particles, nm | Diameter (Average) of Nanotubes, nm | D, μm | d, μm | HCNT*, μm (One-Step Growth) | HCNT, μm |
|---|---|---|---|---|---|---|---|
| CNTA 45 | ELAT LT 1400 W without a microporous layer | 40 ± 5 | 45 ± 5 | 17 ± 1 | 9 ± 1 | 4 ± 1 | - |
| CNTA 50 | 50 ± 5 | 50 ± 5 | 20 ± 1 | 5 ± 1 | 27 ± 2 | ||
| CNTA 75 | 70 ± 5 | 75 ± 5 | 22 ± 1 | 6 ± 1 | - | ||
| CNTA 120 | 110 ± 10 | 120 ± 10 | 25 ± 1 | 8 ± 1 | - |
| Sample | Longitudinal Resistivity (Ohm·µm) | Thickness (µm) | Longitudinal Resistivity of the MPL (Ohm·µm) |
|---|---|---|---|
| ELAT LT 1400 W | 330 | 400 | 100 |
| ELAT LT 1400 W without MPL | 230 | 370 | - |
| ELAT LT 1400 W + CNTA 50 | 300 | 400 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zasypkina, A.A.; Ivanova, N.A.; Spasov, D.D.; Mensharapov, R.M.; Alekseeva, O.K.; Vorobyeva, E.A.; Kukueva, E.V.; Fateev, V.N. Electrode with a Carbon Nanotube Array for a Proton Exchange Membrane Fuel Cell. Inorganics 2023, 11, 219. https://doi.org/10.3390/inorganics11050219
Zasypkina AA, Ivanova NA, Spasov DD, Mensharapov RM, Alekseeva OK, Vorobyeva EA, Kukueva EV, Fateev VN. Electrode with a Carbon Nanotube Array for a Proton Exchange Membrane Fuel Cell. Inorganics. 2023; 11(5):219. https://doi.org/10.3390/inorganics11050219
Chicago/Turabian StyleZasypkina, Adelina A., Nataliya A. Ivanova, Dmitry D. Spasov, Ruslan M. Mensharapov, Olga K. Alekseeva, Ekaterina A. Vorobyeva, Elena V. Kukueva, and Vladimir N. Fateev. 2023. "Electrode with a Carbon Nanotube Array for a Proton Exchange Membrane Fuel Cell" Inorganics 11, no. 5: 219. https://doi.org/10.3390/inorganics11050219
APA StyleZasypkina, A. A., Ivanova, N. A., Spasov, D. D., Mensharapov, R. M., Alekseeva, O. K., Vorobyeva, E. A., Kukueva, E. V., & Fateev, V. N. (2023). Electrode with a Carbon Nanotube Array for a Proton Exchange Membrane Fuel Cell. Inorganics, 11(5), 219. https://doi.org/10.3390/inorganics11050219








