Supramolecular Structure and Antimicrobial Activity of Ni(II) Complexes with s-Triazine/Hydrazine Type Ligand
Abstract
1. Introduction
2. Results and Discussion
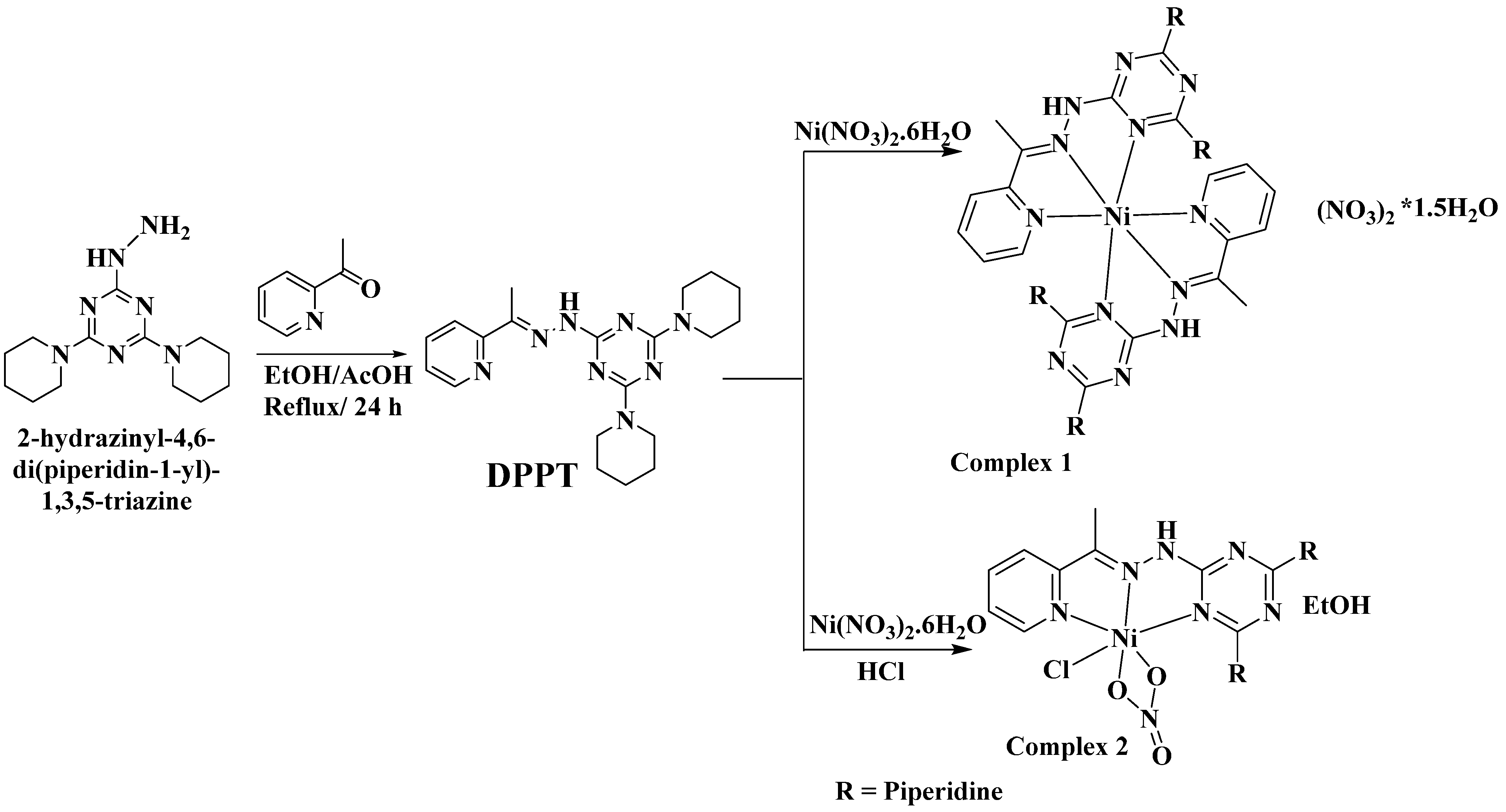
2.1. Synthesis and Characterizations
2.2. X-ray Structure
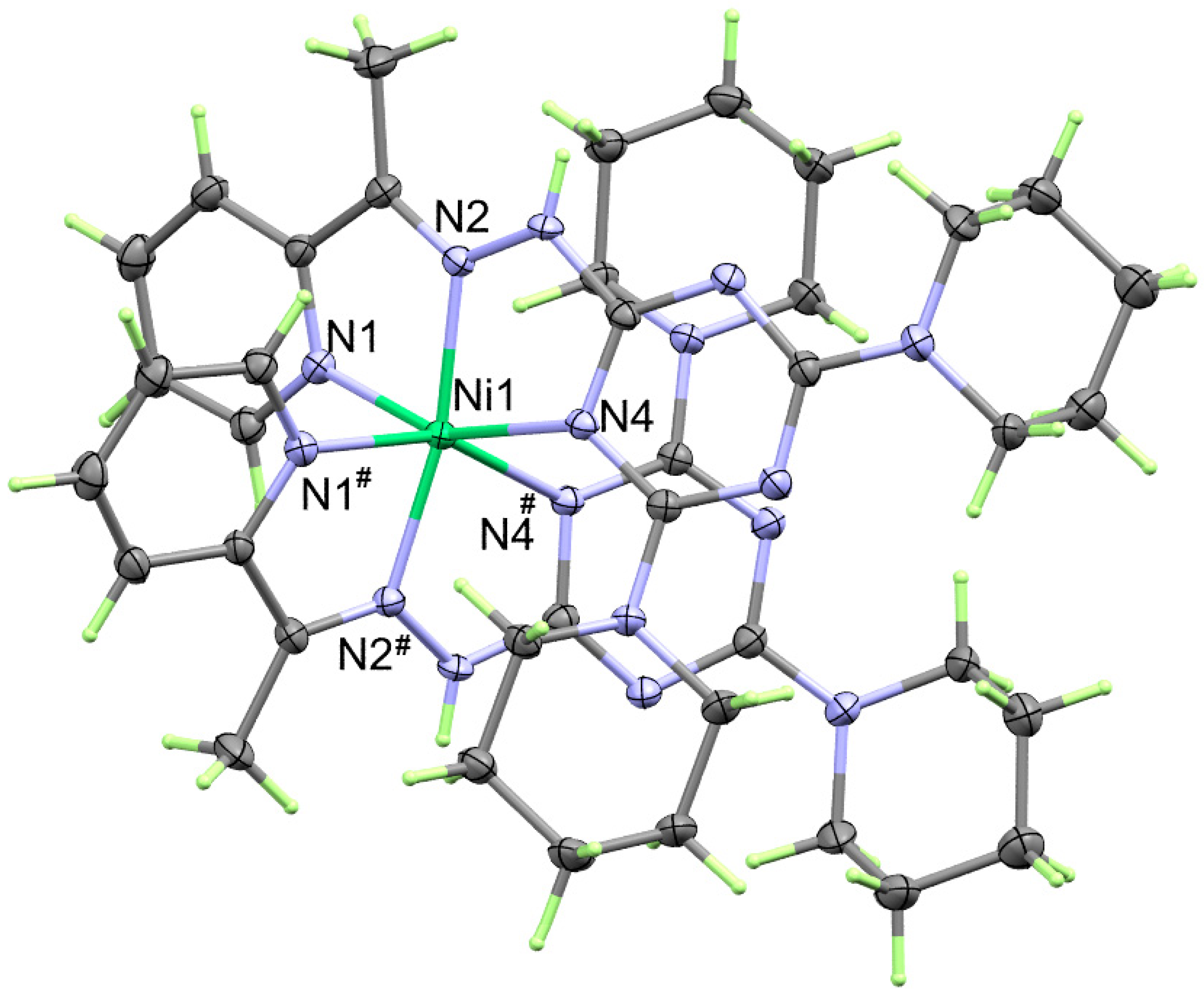
2.2.1. X-ray Structure of 1
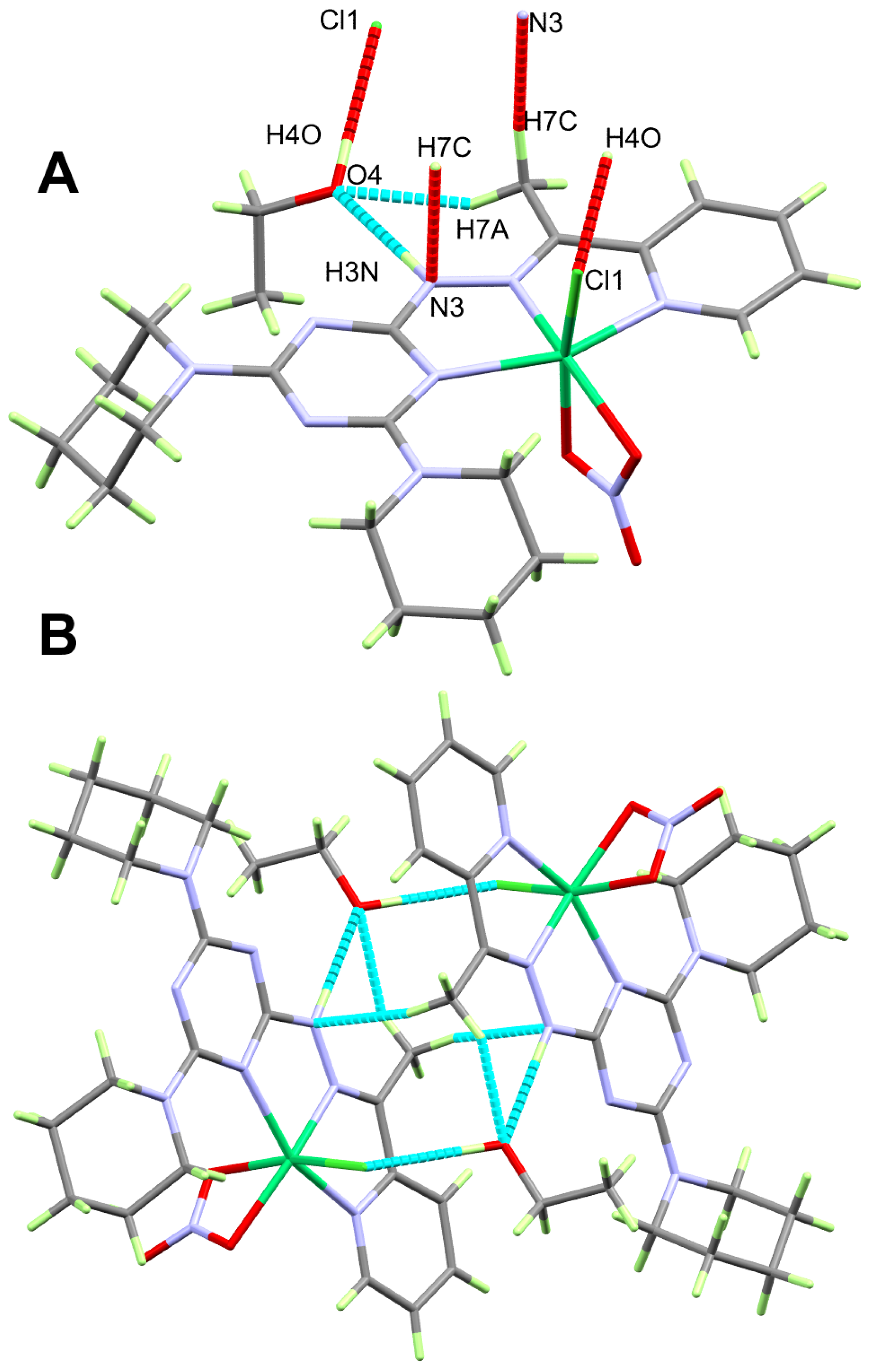
2.2.2. X-ray Structure of 2
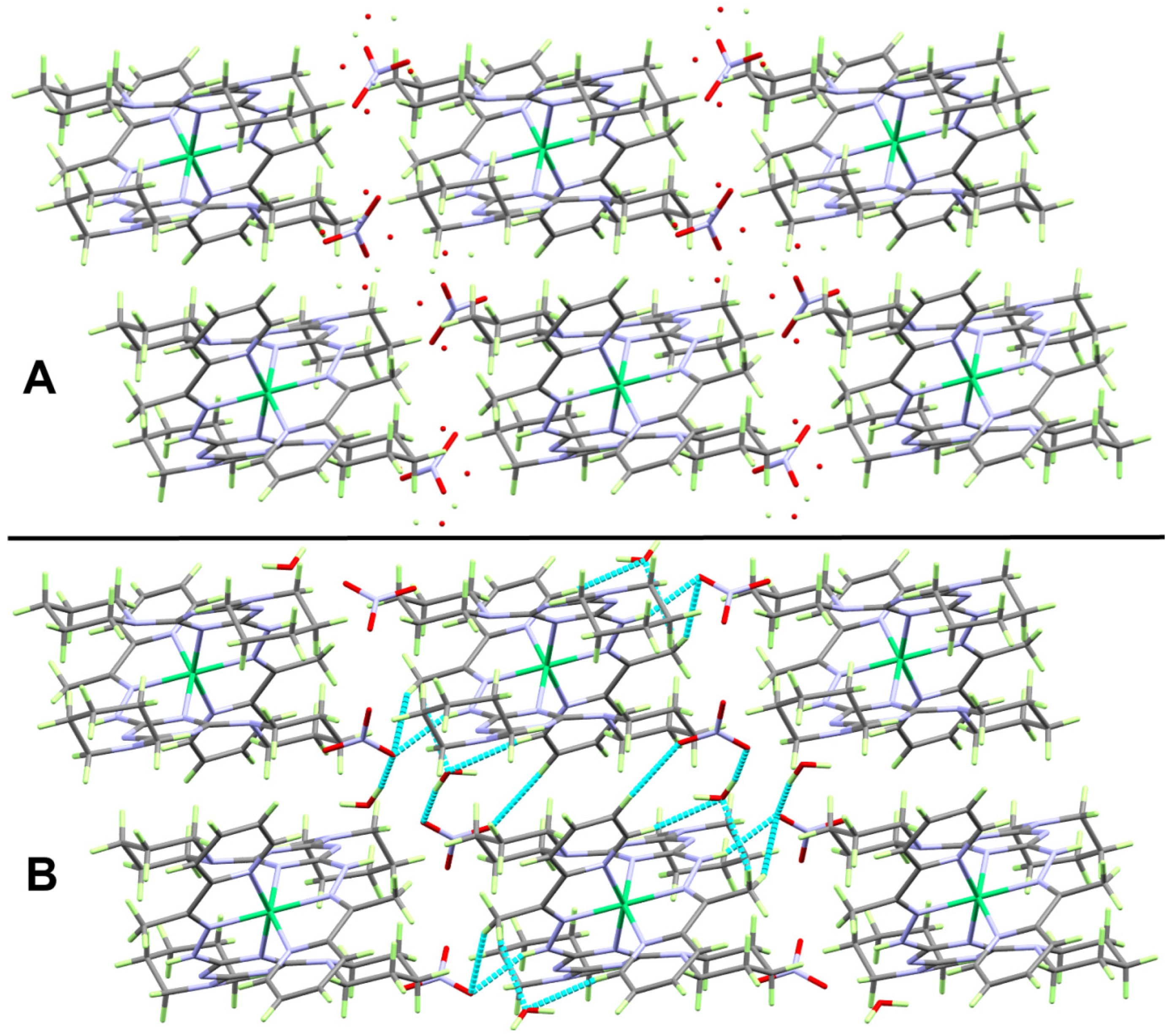
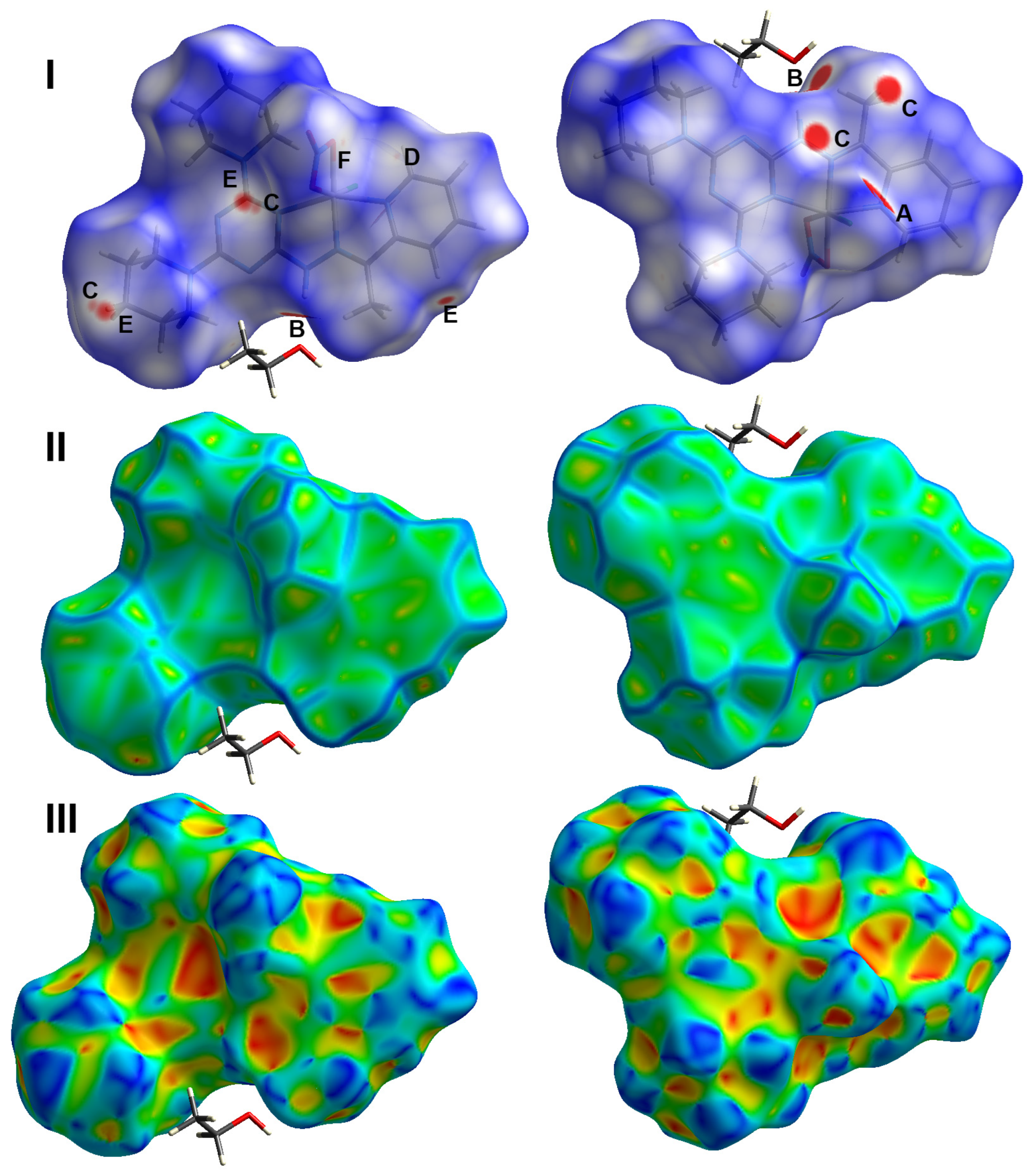
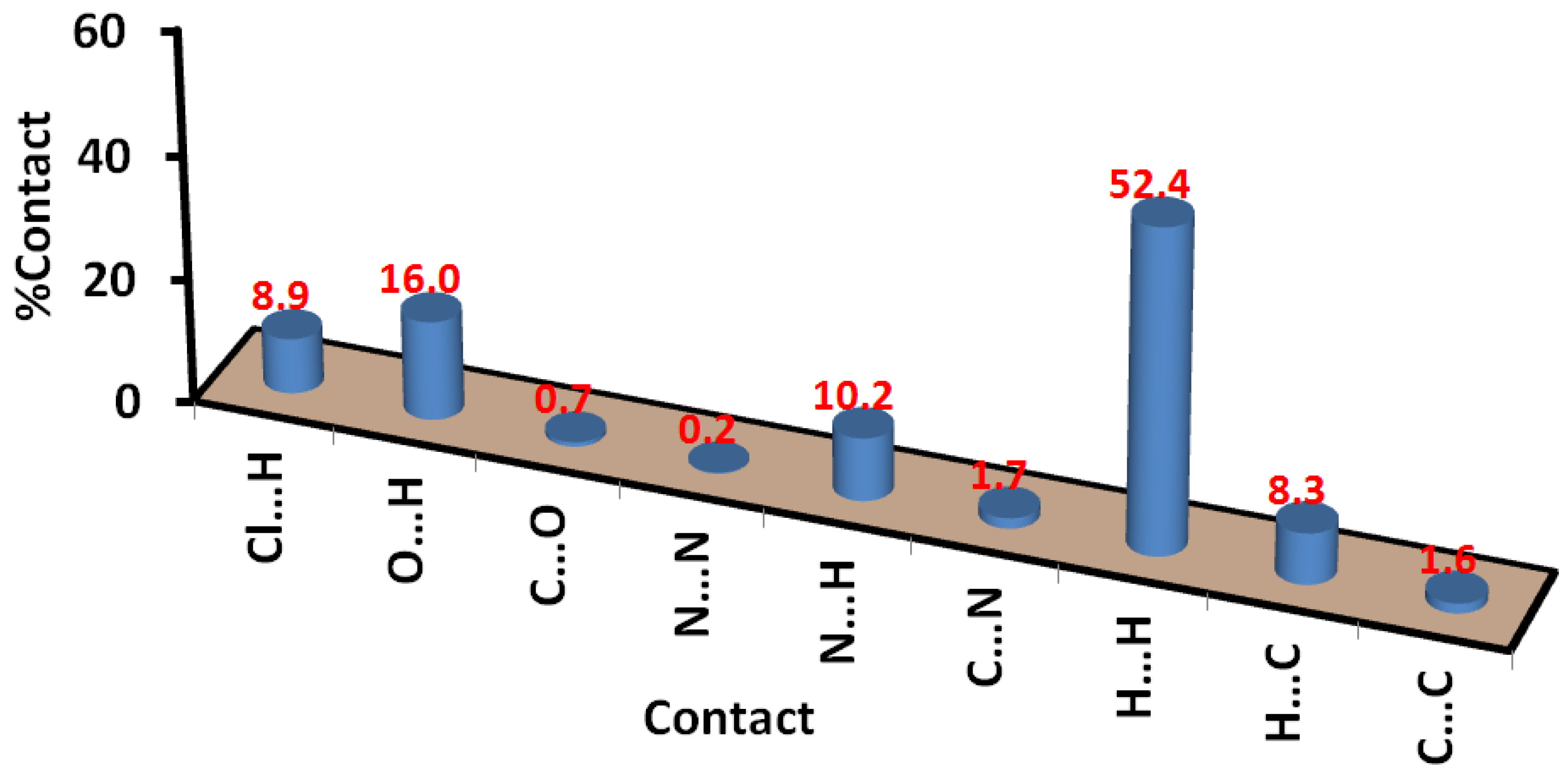
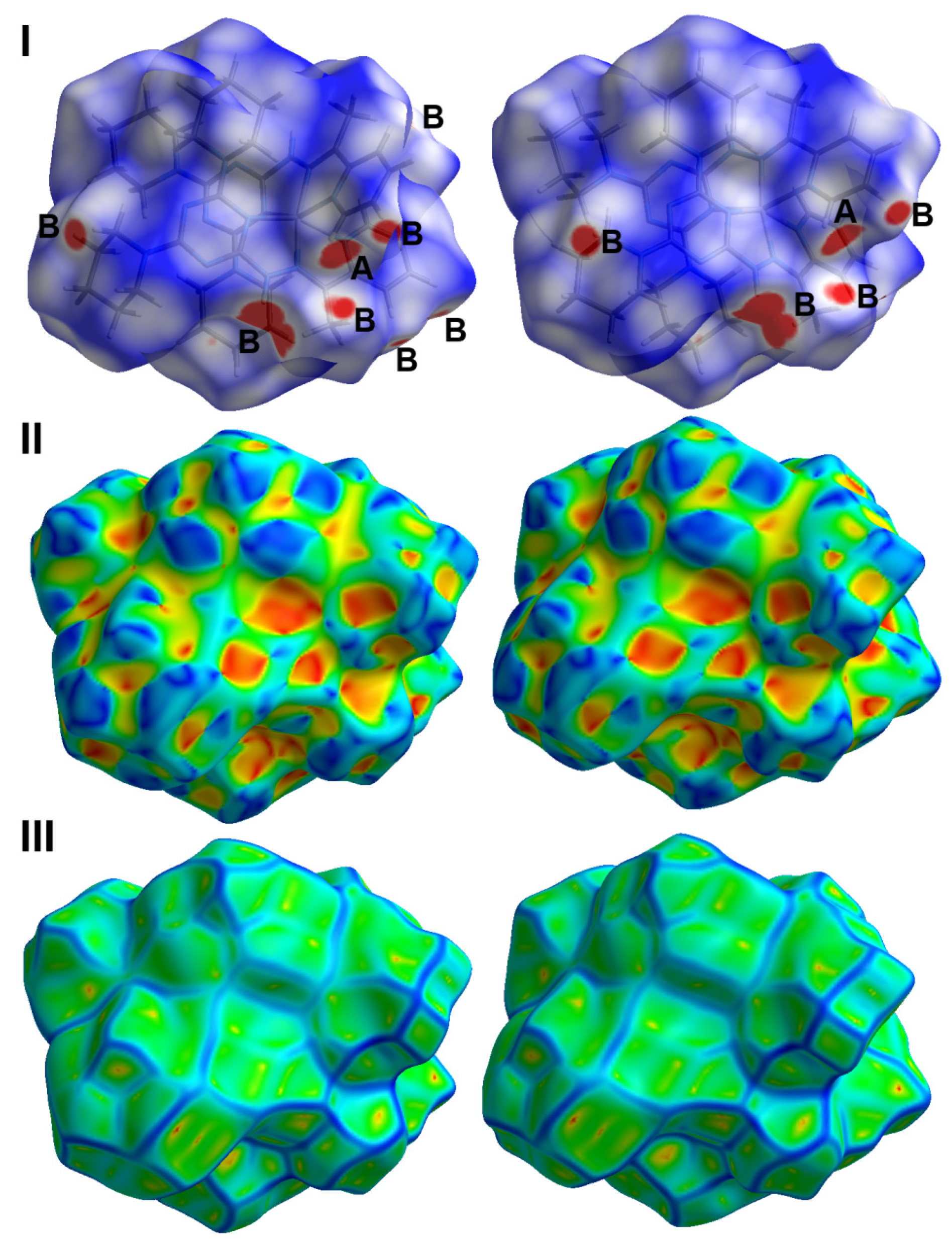
2.3. Hirshfeld Analysis of Molecular Packing
2.4. Antimicrobial Evaluations
3. Materials and Methods
3.1. Physical Measurements
3.2. Synthesis of DPPT Ligand [36,37]
3.3. Synthesis of Ni(II) Complexes
3.3.1. Synthesis of [Ni(DPPT)2](NO3)2*1.5H2O (1)
3.3.2. Synthesis of [Ni(DPPT)(NO3)Cl].EtOH (2)
3.4. Crystal Structure Determination
3.5. Hirshfeld Analysis
3.6. Antimicrobial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raithby, G.L.; De Leon, J.; Karra-Aly, A.; Osman, A.A.; Boyd, C.L.; Saini, G.K.; Sehra, J.S.; Ikenyei, U.C. An Innovative Approach to Improve Canada’s Infectious Disease Pandemic Rapid Response in Marginalized Communities. Glob. Health Annu. Rev. 2020, 1, 3. [Google Scholar]
- World Health Organization. Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report: 2021; World Health Organization: 2021. Available online: https://www.who.int/publications/i/item/9789240027336 (accessed on 22 May 2023).
- Guo, Z.; Sadler, P.J. Medicinal inorganic chemistry. Adv. Inorg. Chem. 1999, 49, 183–306. [Google Scholar]
- Barrios, A.M.; Cohen, S.M.; Lim, M.H. Medicinal inorganic chemistry: A web themed issue. Chem. Commun. 2013, 49, 5910–5911. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- El-Sherif, A.A. Synthesis, spectroscopic characterization and biological activity on newly synthesized copper (II) and nickel (II) complexes incorporating bidentate oxygen–nitrogen hydrazone ligands. Inorg. Chim. Acta. 2009, 362, 4991–5000. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, Y.; Puri, P.; Kumar, M.; Sharma, C. Cobalt, nickel, copper and zinc complexes with 1, 3-diphenyl-1H-pyrazole-4-carboxaldehyde Schiff bases: Antimicrobial, spectroscopic, thermal and fluorescence studies. Eur. J. Med. Chem. 2012, 52, 313–321. [Google Scholar] [CrossRef]
- Tharmaraj, P.; Kodimunthiri, D.; Sheela, C.D.; Shanmuga Priya, C.S. Synthesis, spectral characterization, and antimicrobial activity of copper (II), cobalt (II), and nickel (II) complexes of 3-formylchromoniminopropylsilatrane. J. Coord. Chem. 2009, 62, 2220–2228. [Google Scholar] [CrossRef]
- Raman, N.; Mutijuraj, V.; Rovichandran, S.; Kulandaisamy, A. Electrochemical Behavior of Cu (II), Co (II), Ni (II) and Zn (II) complexes derived from acetylacetone and p-anisidine and their antimicrobial activity. Proc. Indian Acad. Sci. 2003, 115, 161–167. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Farooq, M.A. Synthesis, characterization, ligational and biological properties of some acylhydrazine derived furanyl and thienyl Schiff bases with Co(II), Cu(II), Ni(II), and Zn(II) metal ions. Synth. React. Inorg. Met. Org. Chem. 2001, 31, 1853–1871. [Google Scholar] [CrossRef]
- Jouad, E.M.; Larcher, G.; Allain, M.; Riou, A.; Bouet, G.M.; Khan, M.A.; Do Thanh, X. Synthesis, structure and biological activity of nickel (II) complexes of 5-methyl 2-furfural thiosemicarbazone. J. Inorg. Biochem. 2001, 86, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Dixon, N.E.; Gazzola, C.; Blakeley, R.L.; Zerner, B. Jack bean urease (EC 3.5. 1.5). Metalloenzyme. Simple biological role for nickel. J. Am. Chem. Soc. 1975, 97, 4131–4133. [Google Scholar] [CrossRef]
- Raj, P.; Singh, A.; Singh, A.; Singh, N. Syntheses and photophysical properties of Schiff base Ni (II) complexes: Application for sustainable antibacterial activity and cytotoxicity. ACS Sustain. Chem. Eng. 2017, 5, 6070–6080. [Google Scholar] [CrossRef]
- Kasuga, N.C.; Sekino, K.; Koumo, C.; Shimada, N.; Ishikawa, M.; Nomiya, K. Synthesis, structural characterization and antimicrobial activities of 4-and 6-coordinate nickel (II) complexes with three thiosemicarbazones and semicarbazone ligands. J. Inorg. Biochem. 2001, 84, 55–65. [Google Scholar] [CrossRef]
- Kurtaran, R.; Yıldırım, L.T.; Azaz, A.D.; Namli, H.; Atakol, O. Synthesis, characterization, crystal structure and biological activity of a novel heterotetranuclear complex: [NiLPb (SCN) 2 (DMF)(H2O)] 2, bis-{[μ-N, N′-bis (salicylidene)-1, 3-propanediaminato-aqua-nickel(II)](thiocyanato)(μ-thiocyanato)(μ-N,N′ dimethylformamide) lead (II)}. J. Inorg. Biochem. 2005, 99, 1937–1944. [Google Scholar]
- Kasuga, N.C.; Ohashi, A.; Koumo, C.; Uesugi, J.; Oda, M.; Nomiya, K. Synthesis, Structural Characterization, and Biological Activity of Two Different Nickel (II) Complexes Derived from N′-[1-(2-pyridyl) ethylidene] morpholine-4-carbothiohydrazide. Chem. Lett. 1997, 26, 609–610. [Google Scholar] [CrossRef]
- Oladipo, S.D.; Omondi, B.; Mocktar, C. Synthesis and structural studies of nickel (II)-and copper (II)-N, N′-diarylformamidine dithiocarbamate complexes as antimicrobial and antioxidant agents. Polyhedron 2019, 170, 712–722. [Google Scholar] [CrossRef]
- Alexiou, M.; Tsivikas, I.; Dendrinou-Samara, C.; Pantazaki, A.A.; Trikalitis, P.; Lalioti, N.; Kyriakidis, D.A.; Kessissoglou, D.P. High nuclearity nickel compounds with three, four or five metal atoms showing antibacterial activity. J. Inorg. Biochem. 2003, 93, 256–264. [Google Scholar] [CrossRef]
- Skyrianou, K.C.; Efthimiadou, E.K.; Psycharis, V.; Terzis, A.; Kessissoglou, D.P.; Psomas, G. Nickel–quinolones interaction. Part 1–Nickel (II) complexes with the antibacterial drug sparfloxacin: Structure and biological properties. J. Inorg. Biochem. 2009, 103, 1617–1625. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A review on recent advances in nitrogen-containing molecules and their biological applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Gordon, E.M.; Barrett, R.W.; Dower, W.J.; Fodor, S.P.A.; Gallop, M.A. Applications of combinatorial technologies to drug discovery, combinatorial organic synthesis, library screening strategies, and future directions. J. Med. Chem. 1994, 37, 1385–1401. [Google Scholar] [CrossRef]
- Zhang, B.; Studer, A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef]
- Walsh, C.T. Nature loves nitrogen heterocycles. Tetrahedron Lett. 2015, 56, 3075–3081. [Google Scholar] [CrossRef]
- Angelusiu, M.V.; Barbuceanu, S.F.; Draghici, C.; Almajan, G.L. New Cu (II), Co (II), Ni (II) complexes with aroyl-hydrazone based ligand. Synthesis, spectroscopic characterization and in vitro antibacterial evaluation. Eur. J. Med. Chem. 2010, 45, 2055–2062. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, Z.Y.; Qi, G.F.; Qin, D.D. Crystal structures, DNA-binding studies and antioxidant activities of the Ln(III) complexes with 7-methoxychromone-3-carbaldehyde-isonicotinoyl hydrazone. BioMetals 2009, 22, 927–940. [Google Scholar] [CrossRef]
- Aslan, H.G.; Özcan, S.; Karacan, N. Synthesis, characterization and antimicrobial activity of salicylaldehyde benzenesulfonylhydrazone (Hsalbsmh) and its Nickel (II), Palladium (II), Platinum (II), Copper (II), Cobalt (II) complexes. Inorg. Chem. Commun. 2011, 14, 1550–1553. [Google Scholar] [CrossRef]
- Naskar, S.; Naskar, S.; Butcher, R.J.; Chattopadhyay, S.K. Synthesis, X-ray crystal structures and spectroscopic properties of two Ni (II) complexes of pyridoxal Schiff’s bases with diamines: Importance of steric factor in stabilization of water helices in the lattices of metal complex. Inorg. Chim. Acta. 2010, 363, 404–411. [Google Scholar] [CrossRef]
- Xu, Z.H.; Zhang, X.W.; Zhang, W.Q.; Gao, Y.H.; Zeng, Z.Z. Synthesis, characterization, DNA interaction and antibacterial activities of two tetranuclear cobalt (II) and nickel (II) complexes with salicylaldehyde 2-phenylquinoline-4-carboylhydrazone. Inorg. Chem. Commun. 2011, 14, 1569–1573. [Google Scholar] [CrossRef]
- Kratz, F.; Beyer, U.; Roth, T.; Tarasova, N.; Collery, P.; Lechenault, F.; Cazabat, A.; Schumacher, P.; Unger, C.; Falken, U. Transferrin conjugates of doxorubicin: Synthesis, characterization, cellular uptake, and in vitro efficacy. J. Pharm. Sci. 1998, 87, 338–346. [Google Scholar] [CrossRef]
- Soliman, S.M.; El-Faham, A.; Albering, J.H. Synthesis, X-ray crystal structure and DFT studies of two octahedral cobalt(II) complexes with N,N,N-tridentate triazine-type ligand. J. Coord. Chem. 2017, 70, 2261–2279. [Google Scholar] [CrossRef]
- Zerkowski, J.A.; Seto, C.T.; Whitesides, G.M. Solid-state structures of rosette and crinkled tape motifs derived from the cyanuric acid melamine lattice. J. Am. Chem. Soc. 1992, 114, 5473–5475. [Google Scholar] [CrossRef]
- Demeshko, S.; Dechert, S.; Meyer, F. Anion-π Interactions in a Carousel Copper(II)-Triazine Complex. J. Am. Chem. Soc. 2004, 126, 4508–4509. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Stadler, A.-M.; Brelot, L.; Lehn, J.-M. Coordinative, conformational and motional behaviour of triazine-based ligand strands on binding of Pb(II) cations. Tetrahedron 2008, 64, 8402–8410. [Google Scholar] [CrossRef]
- Vilaivan, T.; Saesaengseerung, N.; Jarprung, D.; Kamchonwongpaisan, S.; Sirawaraporn, W.; Yuthavong, Y. Synthesis of Solution-Phase Combinatorial Library of 4,6-Diamino-1,2-dihydro-1,3,5-triazine and Identification of New Leads Against A16V+S108T Mutant Dihydrofolate Reductase of Plasmodium falciparum. Bioorgan. Med. Chem. 2002, 11, 217–224. [Google Scholar] [CrossRef]
- Osman, S.M.; Khattab, S.; Aly, E.-S.A.; Kenawy, E.-R.; El-Faham, A. 1,3,5-Triazine-based polymer: Synthesis, characterization and application for immobilization of silver nanoparticles. J. Polym. Res. 2017, 24, 231. [Google Scholar] [CrossRef]
- Fathalla, E.M.; Abu-Youssef, M.A.M.; Sharaf, M.M.; El-Faham, A.; Barakat, A.; Badr, A.M.A.; Soliman, S.M.; Slawin, A.M.Z.; Woollins, J.D. Synthesis, Characterizations, Antitumor and Antimicrobial Evaluations of Novel Mn(II) and Cu(II) Complexes with NNN-tridentate s-Triazine-Schiff base Ligand. Inorg. Chim. Acta 2023, 555, 121586. [Google Scholar] [CrossRef]
- Fathalla, E.M.; Abu-Youssef, M.A.M.; Sharaf, M.M.; El-Faham, A.; Barakat, A.; Haukka, M.; Soliman, S.M. Synthesis, X-ray Structure of Two Hexa-Coordinated Ni(II) Complexes with s-Triazine Hydrazine Schiff Base Ligand. Inorganics 2023, 11, 222. [Google Scholar] [CrossRef]
- Sherif, O.E.; Abdel-Kader, N.S. DFT calculations, spectroscopic studies, thermal analysis and biological activity of supramolecular Schiff base complexes. Arab. J. Chem. 2018, 11, 700–713. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-ray Diffraction Data Collected in Oscillation Mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar]
- Sheldrick, G.M. Shelxt–integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SADABS—Bruker Nonius Scaling and Absorption Correction; Bruker AXS, Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Lu, P.-L.; Liu, Y.-C.; Toh, H.-S.; Lee, Y.-L.; Liu, Y.-M.; Ho, C.-M.; Huang, C.-C.; Liu, C.-E.; Ko, W.-C.; Wang, J.-H. Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009–2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents 2012, 40, S37–S43. [Google Scholar] [CrossRef] [PubMed]



| Bond | Distance | Bond | Distance |
| Ni1-N2 | 2.015(2) | Ni1-N4 | 2.139(2) |
| Ni1-N1 | 2.087(2) | ||
| Bonds | Angle | Bonds | Angle |
| N2 #1-Ni1-N2 | 168.65(13) | N1-Ni1-N4 | 154.63(9) |
| N2 #1-Ni1-N1 | 93.91(9) | N1 #1-Ni1-N4 | 100.12(9) |
| N2-Ni1-N1 | 77.67(9) | N4 #1-Ni1-N4 | 85.46(12) |
| N1-Ni1-N1 #1 | 85.44(13) | N2-Ni1-N4 #1 | 111.45(9) |
| N2-Ni1-N4 | 77.27(9) |
| D-H…A | D-H | H…A | D…A | D-H…A |
|---|---|---|---|---|
| C7-H7A…O3 #1 | 0.98 | 2.4 | 3.292(6) | 150.6 |
| N3-H3…O3 #1 | 0.84(3) | 2.05(3) | 2.848(5) | 158(3) |
| C1-H1…O4 #2 | 0.95 | 2.43 | 3.261(6) | 146.2 |
| C2-H2…O1 #2 | 0.95 | 2.45 | 3.388(6) | 171.4 |
| C7-H7B…O4 #3 | 0.98 | 2.41 | 3.109(7) | 127.9 |
| O4-H4A…O3 | 0.85 | 1.86 | 2.665(8) | 158.7 |
| Bond | Distance | Bond | Distance |
| Ni1-N2 | 1.9832(14) | Ni1-O1 | 2.0982(14) |
| Ni1-N1 | 2.0765(13) | Ni1-O2 | 2.1352(14) |
| Ni1-N7 | 2.2249(13) | Ni1-Cl1 | 2.3407(5) |
| Bonds | Angle | Bonds | Angle |
| N2-Ni1-N1 | 78.69(5) | O1-Ni1-N7 | 90.53(5) |
| N2-Ni1-O1 | 96.13(6) | O2-Ni1-N7 | 112.05(5) |
| N1-Ni1-O1 | 92.47(5) | N2-Ni1-Cl1 | 100.40(4) |
| N2-Ni1-O2 | 153.98(6) | N1-Ni1-Cl1 | 91.64(4) |
| N1-Ni1-O2 | 89.29(5) | O1-Ni1-Cl1 | 163.44(4) |
| O1-Ni1-O2 | 61.09(6) | O2-Ni1-Cl1 | 102.95(4) |
| N2-Ni1-N7 | 78.23(5) | N7-Ni1-Cl1 | 91.96(4) |
| N1-Ni1-N7 | 156.91(5) |
| D-H…A | D-H | H…A | D…A | D-H…A |
|---|---|---|---|---|
| N3-H3N…O4 | 0.82(2) | 2.05(2) | 2.870(2) | 178(2) |
| C7-H7A…O4 | 0.98 | 2.33 | 3.166(3) | 143.2 |
| C7-H7C…N3 #1 | 0.98 | 2.47 | 3.412(3) | 161.1 |
| O4-H4O…Cl1 #1 | 0.90(3) | 2.20(3) | 3.1048(17) | 174(3) |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| Cl1…H4O | 2.125 | H4…H4 | 2.044 |
| O4…H7A | 2.244 | H21B…H10A | 2.16 |
| O4…H3N | 1.861 | C15…H12B | 2.65 |
| N3…H7C | 2.373 | C16…O3 | 3.204 |
| N7…H12B | 2.584 |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| H1…H4B | 1.677 | O4…H1 | 2.317 |
| O1B…H14B | 2.213 | O3…H4A | 1.732 |
| O2B…H3 | 1.928 | O3B…H4B | 1.711 |
| O2B…H7A | 2.208 | O1…H3A | 2.507 |
| O3…H7A | 2.315 | O1B…H3A | 2.456 |
| O3…H3 | 1.900 | O1B…H4 | 2.560 |
| O2…H17B | 2.540 | O2…H4 | 2.418 |
| O4…H7B | 2.347 | O1B…H2 | 2.338 |
| Microbes | DPPT | 1 | 2 | Control |
|---|---|---|---|---|
| Fungi | ||||
| A. fumigatus | NA b (ND) c | 20 (312) | 18 (312) | 17 (156) d |
| C. albicans | NA b (ND) c | 21 (312) | 19 (312) | 20 (312) d |
| Gram-positive | ||||
| S. aureus | NAb (ND) c | 7 (5000) | 8 (2500) | 24 (9.7) e |
| B. subtilis | 10 (1250) | 19 (312) | 22 (78) | 26 (4.8) e |
| Gram-negative | ||||
| E.coli | NA b (ND) c | NAb (ND) c | NAb (ND) c | 30 (4.8) e |
| P.vulgaris | NA b (ND) c | NAb (ND) c | NAb (ND) c | 25 (4.8) e |
| 1 | 2 | |
|---|---|---|
| CCDC no. | 2264028 | 2264029 |
| empirical formula | C80H118N36Ni2O15 | C22H34ClN9NiO4 |
| fw | 1941.52 | 582.74 |
| temp (K) | 170(2) | 170(2) |
| λ (Å) | 0.71073 | 0.71073 Å |
| cryst syst | Monoclinic | Triclinic |
| space group | C2/c | P 1 |
| a (Å) | 22.5323(7) | 8.8007(2) |
| b (Å) | 13.1439(2) | 12.2506(2) |
| c (Å) | 15.9387(5) | 12.5275(2) |
| α (deg) | 81.2940(10) | |
| β (deg) | 106.8490(10) | 82.3740(10) |
| γ (deg) | 74.8340(10) | |
| V (Å3) | 4517.8(2) | 1282.43(4) |
| Z | 2 | 2 |
| ρcalc (Mg/m3) | 1.427 | 1.509 |
| μ(Mo Kα) (mm−1) | 0.501 | 0.909 |
| No. reflns. | 25970 | 27302 |
| Completeness to theta = 25.242° | 99.8% | 99.5% |
| Unique reflns. | 4271 | 7451 |
| GOOF (F2) | 1.087 | 1.070 |
| Rint | 0.0502 | 0.0284 |
| R1a (I ≥ 2σ) | 0.0535 | 0.0382 |
| wR2b (I ≥ 2σ) | 0.1096 | 0.0807 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathalla, E.M.; Abu-Youssef, M.A.M.; Sharaf, M.M.; El-Faham, A.; Barakat, A.; Haukka, M.; Soliman, S.M. Supramolecular Structure and Antimicrobial Activity of Ni(II) Complexes with s-Triazine/Hydrazine Type Ligand. Inorganics 2023, 11, 253. https://doi.org/10.3390/inorganics11060253
Fathalla EM, Abu-Youssef MAM, Sharaf MM, El-Faham A, Barakat A, Haukka M, Soliman SM. Supramolecular Structure and Antimicrobial Activity of Ni(II) Complexes with s-Triazine/Hydrazine Type Ligand. Inorganics. 2023; 11(6):253. https://doi.org/10.3390/inorganics11060253
Chicago/Turabian StyleFathalla, Eman M., Morsy A. M. Abu-Youssef, Mona M. Sharaf, Ayman El-Faham, Assem Barakat, Matti Haukka, and Saied M. Soliman. 2023. "Supramolecular Structure and Antimicrobial Activity of Ni(II) Complexes with s-Triazine/Hydrazine Type Ligand" Inorganics 11, no. 6: 253. https://doi.org/10.3390/inorganics11060253
APA StyleFathalla, E. M., Abu-Youssef, M. A. M., Sharaf, M. M., El-Faham, A., Barakat, A., Haukka, M., & Soliman, S. M. (2023). Supramolecular Structure and Antimicrobial Activity of Ni(II) Complexes with s-Triazine/Hydrazine Type Ligand. Inorganics, 11(6), 253. https://doi.org/10.3390/inorganics11060253













