Structure–Function Relationship within Cu-Peptoid Electrocatalysts for Water Oxidation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization of Cu-peptoid Complexes in 0.1 M PBS pH 11
2.2. Electrocatalytic Activities of the Cu-Peptoid Complexes in PBS toward Water Oxidation
2.2.1. Electrochemical Properties
2.2.2. Kinetics Studies
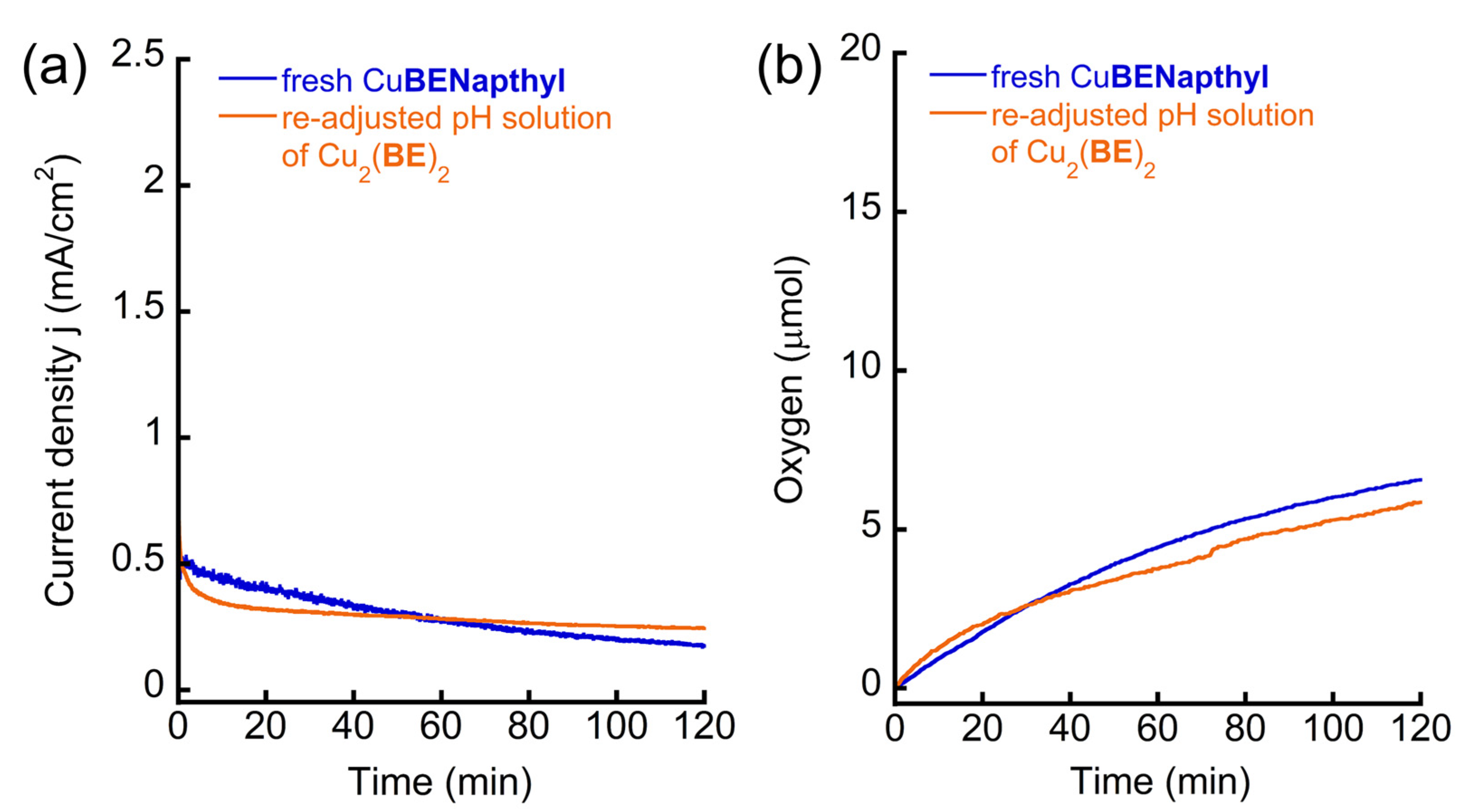
2.2.3. Electrocatalytic Water Oxidation and Homogeneity Studies
2.3. Structure–Function Relationship
3. Materials and Methods
3.1. Materials
3.2. Instrumentation
3.3. Synthesis of Cu-peptoids
3.4. Electrochemical Methods
3.5. Oxygen Evolution Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matheu, R.; Garrido-Barros, P.; Gil-Sepulcre, M.; Ertem, M.Z.; Sala, X.; Gimbert-Surinach, C.; Llobet, A. The development of molecular water oxidation catalysts. Nat. Rev. Chem. 2019, 3, 331–341. [Google Scholar] [CrossRef]
- Chatenet, M.; Pollet, B.G.; Dekel, D.R.; Dionigi, F.; Deseure, J.; Millet, P.; Braatz, R.D.; Bazant, M.Z.; Eikerling, M.; Staffell, I.; et al. Water electrolysis: From textbook knowledge to the latest scientific strategies and industrial developments. Chem. Soc. Rev. 2022, 51, 4583–4762. [Google Scholar] [CrossRef]
- Kondo, M.; Tatewaki, H.; Masaoka, S. Design of molecular water oxidation catalysts with earth-abundant metal ions. Chem. Soc. Rev. 2021, 50, 6790–6831. [Google Scholar] [CrossRef]
- Chen, Q.F.; Guo, Y.H.; Yu, Y.H.; Zhang, M.T. Bioinspired molecular clusters for water oxidation. Coord. Chem. Rev. 2021, 448, 214164. [Google Scholar] [CrossRef]
- Blakemore, J.D.; Crabtree, R.H.; Brudvig, G.W. Molecular Catalysts for Water Oxidation. Chem. Rev. 2015, 115, 12974–13005. [Google Scholar] [CrossRef]
- Ardo, S.; Rivas, D.F.; Modestino, M.A.; Greiving, V.S.; Abdi, F.F.; Alarcon Llado, E.; Artero, V.; Ayers, K.; Battaglia, C.; Becker, J.P.; et al. Pathways to electrochemical solar-hydrogen technologies. Energ. Env. Environ. Sci. 2018, 11, 2768–2783. [Google Scholar] [CrossRef] [Green Version]
- Kibsgaard, J.; Chorkendorff, I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy 2019, 4, 430–433. [Google Scholar] [CrossRef] [Green Version]
- Luque-Urrutia, J.A.; Ortiz-Garcia, T.; Sola, M.; Poater, A. Green Energy by Hydrogen Production from Water Splitting, Water Oxidation Catalysis and Acceptorless Dehydrogenative Coupling. Inorganics 2023, 11, 88. [Google Scholar] [CrossRef]
- Chavan, H.S.; Lee, C.H.; Inamdar, A.I.; Han, J.; Park, S.; Cho, S.; Shreshta, N.K.; Lee, S.U.; Hou, B.; Im, H.; et al. Designing and Tuning the Electronic Structure of Nickel-Vanadium Layered Double Hydroxides for Highly Efficient Oxygen Evolution Electrocatalysis. Acs Catal. 2022, 12, 3821–3831. [Google Scholar] [CrossRef]
- Inamdar, A.I.; Chavan, H.S.; Seok, J.H.; Lee, C.H.; Shin, G.; Park, S.; Yeon, S.; Cho, S.; Park, Y.; Shrestha, N.K.; et al. Optimal rule-of-thumb design of NiFeMo layered double hydroxide nanoflakes for highly efficient and durable overall water-splitting at large currents. J. Mater. Chem. A 2022, 10, 20497–20508. [Google Scholar] [CrossRef]
- McEvoy, J.P.; Brudvig, G.W. Water-splitting chemistry of photosystem II. Chem. Rev. 2006, 106, 4455–4483. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.C.; Wang, Y.H. Homogeneous Water Oxidation Catalyzed by First-Row Transition Metal Complexes: Unveiling the Relationship between Turnover Frequency and Reaction Overpotential. Chemsuschem 2022, 15, e202102378. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, L.; Zhang, F.; Gao, L.L.; Geng, L.; Ge, J.B.; Tian, K.G.; Chai, H.; Niu, H.L.; Liu, Y.; et al. Doping with Rare Earth Elements and Loading Cocatalysts to Improve the Solar Water Splitting Performance of BiVO4. Inorganics 2023, 11, 203. [Google Scholar] [CrossRef]
- Li, J.; Triana, C.A.; Wan, W.; Saseendran, D.P.A.; Zhao, Y.; Balaghi, S.E.; Heidari, S.; Patzke, G.R. Molecular and heterogeneous water oxidation catalysts: Recent progress and joint perspectives. Chem. Soc. Rev. 2021, 50, 2444–2485. [Google Scholar] [CrossRef]
- Zhang, B.B.; Sun, L.C. Artificial photosynthesis: Opportunities and challenges of molecular catalysts. Chem. Soc. Rev. 2019, 48, 2216–2264. [Google Scholar] [CrossRef] [Green Version]
- Francke, R.; Schille, B.; Roemelt, M. Homogeneously Catalyzed Electroreduction of Carbon Dioxide-Methods, Mechanisms, and Catalysts. Chem. Rev. 2018, 118, 4631–4701. [Google Scholar] [CrossRef]
- Dau, H.; Limberg, C.; Reier, T.; Risch, M.; Roggan, S.; Strasser, P. The Mechanism of Water Oxidation: From Electrolysis via Homogeneous to Biological Catalysis. Chemcatchem 2010, 2, 724–761. [Google Scholar] [CrossRef]
- Chalkley, M.J.; Garrido-Barros, P.; Peters, J.C. A molecular mediator for reductive concerted proton-electron transfers via electrocatalysis. Science 2020, 369, 850–854. [Google Scholar] [CrossRef]
- Garrido-Barros, P.; Derosa, J.; Chalkley, M.J.; Peters, J.C. Tandem electrocatalytic N-2 fixation via proton-coupled electron transfer. Nature 2022, 609, 71–76. [Google Scholar] [CrossRef]
- Nitopi, S.; Bertheussen, E.; Scott, S.B.; Liu, X.Y.; Engstfeld, A.K.; Horch, S.; Seger, B.; Stephens, I.E.L.; Chan, K.; Hahn, C.; et al. Progress and Perspectives of Electrochemical CO2 Reduction on Copper in Aqueous Electrolyte. Chem. Rev. 2019, 119, 7610–7672. [Google Scholar] [CrossRef] [Green Version]
- Gil-Sepulcre, M.; Garrido-Barros, P.; Oldengott, J.; Funes-Ardoiz, I.; Bofill, R.; Sala, X.; Benet-Buchholz, J.; Llobet, A. Consecutive Ligand-Based Electron Transfer in New Molecular Copper-Based Water Oxidation Catalysts. Angew. Chem. Int. Ed. 2021, 60, 18639–18644. [Google Scholar] [CrossRef] [PubMed]
- Matheu, R.; Ertem, M.Z.; Gimbert-Surinach, C.; Benet-Buchholz, J.; Sala, X.; Llobet, A. Hydrogen Bonding Rescues Overpotential in Seven-Coordinated Ru Water Oxidation Catalysts. Acs Catal. 2017, 7, 6525–6532. [Google Scholar] [CrossRef]
- Wasylenko, D.J.; Ganesamoorthy, C.; Henderson, M.A.; Koivisto, B.D.; Osthoff, H.D.; Berlinguette, C.P. Electronic modification of the [Ru(II)(tpy)(bpy)(OH(2))](2+) scaffold: Effects on catalytic water oxidation. J. Am. Chem. Soc. 2010, 132, 16094–16106. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.Q.; Zhang, B.B.; Sun, L.C.; Ahlquist, M.S.G. Hydrophobic/Hydrophilic Directionality Affects the Mechanism of Ru-Catalyzed Water Oxidation Reaction. Acs Catal. 2020, 10, 13364–13370. [Google Scholar] [CrossRef]
- Yi, J.J.; Zhan, S.Q.; Chen, L.; Tian, Q.; Wang, N.; Li, J.; Xu, W.H.; Zhang, B.B.; Ahlquist, M.S.G. Electrostatic Interactions Accelerating Water Oxidation Catalysis via Intercatalyst O-O Coupling. J. Am. Chem. Soc. 2021, 143, 2484–2490. [Google Scholar] [CrossRef]
- Dang, L.L.; Feng, H.J.; Lin, Y.J.; Jin, G.X. Self-Assembly of Molecular Figure-Eight Knots Induced by Quadruple Stacking Interactions. J. Am. Chem. Soc. 2020, 142, 18946–18954. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, N.; Li, X.; Zhang, Z.; Zhao, J.; Ren, W.; Ding, S.; Xu, G.; Li, J.; Apfel, U.P.; et al. Homolytic versus Heterolytic Hydrogen Evolution Reaction Steered by a Steric Effect. Angew. Chem. Int. Ed. Engl. 2020, 59, 8941–8946. [Google Scholar] [CrossRef]
- Liu, T.Q.; Zhan, S.Q.; Shen, N.N.; Wang, L.Q.; Szabo, Z.; Yang, H.; Ahlquist, M.S.G.; Sun, L.C. Bioinspired Active Site with a Coordination-Adaptive Organosulfonate Ligand for Catalytic Water Oxidation at Neutral pH. J. Am. Chem. Soc. 2023, 145, 11818–11828. [Google Scholar] [CrossRef]
- Liu, T.Q.; Li, G.; Shen, N.N.; Wang, L.Q.; Timmer, B.J.J.; Zhou, S.Y.; Zhang, B.B.; Kravchenko, A.; Xu, B.; Ahlquist, M.S.G.; et al. Isolation and Identification of Pseudo Seven-Coordinate Ru(III) Intermediate Completing the Catalytic Cycle of Ru-bda Type of Water Oxidation Catalysts. Ccs Chem. 2022, 4, 2481–2490. [Google Scholar] [CrossRef]
- Barnett, S.M.; Goldberg, K.I.; Mayer, J.M. A soluble copper-bipyridine water-oxidation electrocatalyst. Nat. Chem. 2012, 4, 498–502. [Google Scholar] [CrossRef]
- Su, X.J.; Gao, M.; Jiao, L.; Liao, R.Z.; Siegbahn, P.E.; Cheng, J.P.; Zhang, M.T. Electrocatalytic water oxidation by a dinuclear copper complex in a neutral aqueous solution. Angew. Chem. Int. Ed. Engl. 2015, 54, 4909–4914. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.Q.; Su, X.J.; Zhang, M.T. Electrocatalytic Water Oxidation by an Unsymmetrical Di-Copper Complex. Inorg. Chem. 2018, 57, 10481–10484. [Google Scholar] [CrossRef] [PubMed]
- Su, X.J.; Zheng, C.; Hu, Q.Q.; Du, H.Y.; Liao, R.Z.; Zhang, M.T. Bimetallic cooperative effect on O-O bond formation: Copper polypyridyl complexes as water oxidation catalyst. Dalton Trans. 2018, 47, 8670–8675. [Google Scholar] [CrossRef]
- Chen, Q.F.; Cheng, Z.Y.; Liao, R.Z.; Zhang, M.T. Bioinspired Trinuclear Copper Catalyst for Water Oxidation with a Turnover Frequency up to 20000 s(-1). J. Am. Chem. Soc. 2021, 143, 19761–19768. [Google Scholar] [CrossRef]
- Fisher, K.J.; Materna, K.L.; Mercado, B.Q.; Crabtree, R.H.; Brudvig, G.W. Electrocatalytic Water Oxidation by a Copper(II) Complex of an Oxidation-Resistant Ligand. Acs Catal. 2017, 7, 3384–3387. [Google Scholar] [CrossRef]
- Li, T.T.; Zheng, Y.Q. Electrocatalytic water oxidation using a chair-like tetranuclear copper(II) complex in a neutral aqueous solution. Dalton T 2016, 45, 12685–12690. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, C.; Liu, S.B.; Wang, J.L.; Lin, W.B. A Biomimetic Copper Water Oxidation Catalyst with Low Overpotential. J. Am. Chem. Soc. 2014, 136, 273–281. [Google Scholar] [CrossRef]
- Ruan, G.; Fridman, N.; Maayan, G. Borate Buffer as a Key Player in Cu-Based Homogeneous Electrocatalytic Water Oxidation. Chem-Eur. J. 2022, 28, e202202407. [Google Scholar] [CrossRef]
- Lin, J.Q.; Wang, N.N.; Chen, X.; Yang, X.L.; Hong, L.; Ruan, Z.J.; Ye, H.; Chen, Y.M.; Liang, X.M. Electrocatalytic water oxidation by copper(II) complexes with a pentadentate amine-pyridine ligand. Sustain. Energ. Fuels 2022, 6, 1312–1318. [Google Scholar] [CrossRef]
- Jian, J.X.; Liao, J.X.; Zhou, M.H.; Yao, M.M.; Chen, Y.J.; Liang, X.W.; Liu, C.P.; Tong, Q.X. Enhanced Photoelectrochemical Water Splitting of Black Silicon Photoanode with pH-Dependent Copper-Bipyridine Catalysts. Chem-Eur. J. 2022, 28, e202201520. [Google Scholar] [CrossRef]
- Mao, Q.Y.; Pang, Y.J.; Li, X.C.; Chen, G.J.; Tan, H.W. Theoretical Study of the Mechanisms of Two Copper Water Oxidation Electrocatalysts with Bipyridine Ligands. Acs Catal. 2019, 9, 8798–8809. [Google Scholar] [CrossRef]
- Akbari, M.S.A.; Nandy, S.; Chae, K.H.; Bikas, R.; Kozakiewicz-Piekarz, A.; Najafpour, M.M. Water Oxidation by a Copper(II) Complex with 6,6?-Dihydroxy-2,2?-Bipyridine Ligand: Challenges and an Alternative Mechanism. Langmuir 2023, 39, 5542–5553. [Google Scholar] [CrossRef]
- Gerlach, D.L.; Bhagan, S.; Cruce, A.A.; Burks, D.B.; Nieto, I.; Truong, H.T.; Kelley, S.P.; Herbst-Gervasoni, C.J.; Jernigan, K.L.; Bowman, M.K.; et al. Studies of the Pathways Open to Copper Water Oxidation Catalysts Containing Proximal Hydroxy Groups During Basic Electrocatalysis. Inorg. Chem. 2014, 53, 12689–12698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koepke, S.J.; Light, K.M.; VanNatta, P.E.; Wiley, K.M.; Kieber-Emmons, M.T. Electrocatalytic Water Oxidation by a Homogeneous Copper Catalyst Disfavors Single-Site Mechanisms. J. Am. Chem. Soc. 2017, 139, 8586–8600. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.Y.; Wang, M.; Zhang, P.L.; Jiang, J.; Sun, L.C. Electrocatalytic water oxidation by copper(II) complexes containing a tetra- or pentadentate amine-pyridine ligand. Chem. Commun. 2017, 53, 4374–4377. [Google Scholar] [CrossRef]
- Shen, J.Y.; Wang, M.; Gao, J.S.; Han, H.X.; Liu, H.; Sun, L.C. Improvement of Electrochemical Water Oxidation by Fine-Tuning the Structure of Tetradentate N-4 Ligands of Molecular Copper Catalysts. Chemsuschem 2017, 10, 4581–4588. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Wu, X.J.; Sun, L.C. Copper-based homogeneous and heterogeneous catalysts for electrochemical water oxidation. Nanoscale 2020, 12, 4187–4218. [Google Scholar] [CrossRef]
- Lukacs, D.; Szyrwiel, L.; Pap, J.S. Copper Containing Molecular Systems in Electrocatalytic Water Oxidation-Trends and Perspectives. Catalysts 2019, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.T.; Chen, Z.; Kang, P.; Meyer, T.J. Electrocatalytic water oxidation with a copper(II) polypeptide complex. J. Am. Chem. Soc. 2013, 135, 2048–2051. [Google Scholar] [CrossRef] [PubMed]
- Pap, J.S.; Szyrwiel, L.; Sranko, D.; Kerner, Z.; Setner, B.; Szewczuk, Z.; Malinka, W. Electrocatalytic water oxidation by Cu(II) complexes with branched peptides. Chem. Commun. 2015, 51, 6322–6324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szyrwiel, L.; Lukacs, D.; Ishikawa, T.; Brasun, J.; Szczukowski, L.; Szewczuk, Z.; Setner, B.; Pap, J.S. Electrocatalytic water oxidation influenced by the ratio between Cu(2+)and a multiply branched peptide ligand. Catal. Commun. 2019, 122, 5–9. [Google Scholar] [CrossRef]
- Lukacs, D.; Nemeth, M.; Szyrwiel, L.; Illes, L.; Pecz, B.; Shen, S.H.; Pap, J.S. Behavior of a Cu-Peptide complex under water oxidation conditions—Molecular electrocatalyst or precursor to nanostructured CuO films? Sol. Energ. Mat. Sol. C 2019, 201, 110079. [Google Scholar] [CrossRef]
- dos Santos, L.; Climent, V.; Blanford, C.F.; Armstrong, F.A. Mechanistic studies of the ‘blue’ Cu enzyme, bilirubin oxidase, as a highly efficient electrocatalyst for the oxygen reduction reaction. Phys. Chem. Chem. Phys. 2010, 12, 13962–13974. [Google Scholar] [CrossRef]
- Goldfeder, M.; Kanteev, M.; Isaschar-Ovdat, S.; Adir, N.; Fishman, A. Determination of tyrosinase substrate-binding modes reveals mechanistic differences between type-3 copper proteins. Nat. Commun. 2014, 5, 4505. [Google Scholar] [CrossRef] [Green Version]
- Fujieda, N.; Umakoshi, K.; Ochi, Y.; Nishikawa, Y.; Yanagisawa, S.; Kubo, M.; Kurisu, G.; Itoh, S. Copper-Oxygen Dynamics in the Tyrosinase Mechanism. Angew. Chem. Int. Ed. 2020, 59, 13385–13390. [Google Scholar] [CrossRef]
- Kim, M.K.; Martell, A.E. Copper(2) Complexes of Triglycine and Tetraglycine. J. Am. Chem. Soc. 1966, 88, 914–918. [Google Scholar] [CrossRef]
- Sanna, D.; Micera, G.; Kallay, C.; Rigo, V.; Sovago, I. Copper(II) complexes of N-terminal protected tri- and tetrapeptides containing histidine residues. Dalton Trans. 2004, 2702–2707. [Google Scholar] [CrossRef] [PubMed]
- Szyrwiel, L.; Szczukowski, L.; Pap, J.S.; Setner, B.; Szewczuk, Z.; Malinka, W. The Cu2+ Binding Properties of Branched Peptides Based on L-2,3-Diaminopropionic Acid. Inorg. Chem. 2014, 53, 7951–7959. [Google Scholar] [CrossRef]
- Simon, R.J.; Kania, R.S.; Zuckermann, R.N.; Huebner, V.D.; Jewell, D.A.; Banville, S.; Ng, S.; Wang, L.; Rosenberg, S.; Marlowe, C.K.; et al. Peptoids: A modular approach to drug discovery. Proc. Natl. Acad. Sci. USA 1992, 89, 9367–9371. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N.; Kerr, J.M.; Kent, S.B.H.; Moos, W.H. Efficient Method for the Preparation of Peptoids [Oligo(N-Substituted Glycines)] by Submonomer Solid-Phase Synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647. [Google Scholar] [CrossRef]
- Baskin, M.; Maayan, G. Water-Soluble Chiral Metallopeptoids. Biopolymers 2015, 104, 577–584. [Google Scholar] [CrossRef]
- Baskin, M.; Maayan, G. A rationally designed metal-binding helical peptoid for selective recognition processes. Chem. Sci. 2016, 7, 2809–2820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskin, M.; Panz, L.; Maayan, G. Versatile ruthenium complexes based on 2,2′-bipyridine modified peptoids. Chem. Commun. 2016, 52, 10350–10353. [Google Scholar] [CrossRef] [Green Version]
- Tigger-Zaborov, H.; Maayan, G. Nanoparticles assemblies on demand: Controlled aggregation of Ag(0) mediated by modified peptoid sequences. J. Colloid. Interf. Sci. 2017, 508, 56–64. [Google Scholar] [CrossRef]
- Baskin, M.; Maayan, G. Chiral Cu(ii), Co(ii) and Ni(ii) complexes based on 2,2′-bipyridine modified peptoids. Dalton Trans. 2018, 47, 10767–10774. [Google Scholar] [CrossRef] [PubMed]
- Baskin, M.; Zhu, H.; Qu, Z.W.; Chill, J.H.; Grimme, S.; Maayan, G. Folding of unstructured peptoids and formation of hetero-bimetallic peptoid complexes upon side-chain-to-metal coordination. Chem. Sci. 2019, 10, 620–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amato, A.; Ghosh, P.; Costabile, C.; Della Sala, G.; Izzo, I.; Maayan, G.; De Riccardis, F. Peptoid-based siderophore mimics as dinuclear Fe3+ chelators. Dalton Trans. 2020, 49, 6020–6029. [Google Scholar] [CrossRef]
- Ghosh, P.; Maayan, G. A Water-Soluble Peptoid that Can Extract Cu2+ from Metallothionein via Selective Recognition. Chem-Eur. J. 2021, 27, 1383–1389. [Google Scholar] [CrossRef]
- Behar, A.E.; Sabater, L.; Baskin, M.; Hureau, C.; Maayan, G. A Water-Soluble Peptoid Chelator that Can Remove Cu2+ from Amyloid-beta Peptides and Stop the Formation of Reactive Oxygen Species Associated with Alzheimer’s Disease. Angew. Chem. Int. Ed. 2021, 60, 24588–24597. [Google Scholar] [CrossRef]
- Jiang, L.H.; Hu, C.T.; De Riccardis, F.; Kirshenbaum, K. Elaborate Supramolecular Architectures Formed by Co-Assembly of Metal Species and Peptoid Macrocycles. Cryst. Growth Des. 2021, 21, 3889–3901. [Google Scholar] [CrossRef]
- D’Amato, A.; Schettini, R.; Pierri, G.; Izzo, I.; Grisi, F.; Tedesco, C.; De Riccardis, F.; Costabile, C. Synthesis and characterization of new Na+ complexes of N-benzyl cyclic peptoids and their role in the ring opening polymerization of l-lactide. New J. Chem. 2021, 45, 5410–5420. [Google Scholar] [CrossRef]
- Maayan, G.; Ward, M.D.; Kirshenbaum, K. Folded biomimetic oligomers for enantioselective catalysis. Proc. Natl. Acad. Sci. USA 2009, 106, 13679–13684. [Google Scholar] [CrossRef]
- Prathap, K.J.; Maayan, G. Metallopeptoids as efficient biomimetic catalysts. Chem. Commun. 2015, 51, 11096–11099. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Ghosh, P.; Maayan, G. A Copper-Peptoid as a Highly Stable, Efficient, and Reusable Homogeneous Water Oxidation Electrocatalyst. Acs Catal. 2018, 8, 10631–10640. [Google Scholar] [CrossRef]
- Darapaneni, C.M.; Ghosh, P.; Ghosh, T.; Maayan, G. Unique beta-Turn Peptoid Structures and Their Application as Asymmetric Catalysts. Chem-Eur. J. 2020, 26, 9573–9579. [Google Scholar] [CrossRef]
- Ruan, G.; Engelberg, L.; Ghosh, P.; Maayan, G. A unique Co(III)-peptoid as a fast electrocatalyst for homogeneous water oxidation with low overpotential. Chem. Commun. 2021, 57, 939–942. [Google Scholar] [CrossRef]
- Ruan, G.; Ghosh, P.; Fridman, N.; Maayan, G. A Di-Copper-Peptoid in a Noninnocent Borate Buffer as a Fast Electrocatalyst for Homogeneous Water Oxidation with Low Overpotential. J. Am. Chem. Soc. 2021, 143, 10614–10623. [Google Scholar] [CrossRef] [PubMed]
- Culf, A.S.; Ouellette, R.J. Solid-Phase Synthesis of N-Substituted Glycine Oligomers (alpha-Peptoids) and Derivatives. Molecules 2010, 15, 5282–5335. [Google Scholar] [CrossRef]
- Zborovsky, L.; Smolyakova, A.; Baskin, M.; Maayan, G. A Pure Polyproline Type I-like Peptoid Helix by Metal Coordination. Chem-Eur. J. 2018, 24, 1159–1167. [Google Scholar] [CrossRef]
- Ghosh, P.; Torner, J.; Arora, P.S.; Maayan, G. Dual Control of Peptide Conformation with Light and Metal Coordination. Chem-Eur. J. 2021, 27, 8956–8959. [Google Scholar] [CrossRef]
- Ghosh, T.; Fridman, N.; Kosa, M.; Maayan, G. Self-Assembled Cyclic Structures from Copper(II) Peptoids. Angew. Chem. Int. Ed. 2018, 57, 7703–7708. [Google Scholar] [CrossRef]
- Ghosh, P.; Ruan, G.; Fridman, N.; Maayan, G. Amide bond hydrolysis of peptoids. Chem. Commun. 2022, 58, 9922–9925. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Y.; Xu, H.Z. Synthesis, Structures and Properties of Cu(II) and Mn(II) Complexes with 1,10-Phenanthroline-2-carboxylic acid and 2,2′-Bipyridine Ligands. Molecules 2010, 15, 8349–8359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constable, E.C.; Housecroft, C.E. Packing Motifs in [M(bpy)(2)X-2] Coordination Compounds (bpy=2,2′-bipyridine; X = F, Cl, Br, I). Crystals 2023, 13, 505. [Google Scholar] [CrossRef]
- Mishra, B.K.; Sathyamurthy, N. pi-pi interaction in pyridine. J. Phys. Chem. A 2005, 109, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Hohenstein, E.G.; Sherrill, C.D. Effects of Heteroatoms on Aromatic pi-pi Interactions: Benzene-Pyridine and Pyridine Dimer. J. Phys. Chem. A 2009, 113, 878–886. [Google Scholar] [CrossRef]
- Yu, F.S.; Li, F.; Hu, J.X.; Bai, L.C.; Zhu, Y.; Sun, L.C. Electrocatalytic water oxidation by a macrocyclic Cu(II) complex in neutral phosphate buffer. Chem. Commun. 2016, 52, 10377–10380. [Google Scholar] [CrossRef]
- Xiang, R.J.; Wang, H.Y.; Xin, Z.J.; Li, C.B.; Lu, Y.X.; Gao, X.W.; Sun, H.M.; Cao, R. A Water-Soluble Copper-Polypyridine Complex as a Homogeneous Catalyst for both Photo-Induced and Electrocatalytic O-2 Evolution. Chem-Eur. J. 2016, 22, 1602–1607. [Google Scholar] [CrossRef]
- Kafentzi, M.C.; Papadakis, R.; Gennarini, F.; Kochem, A.; Iranzo, O.; Le Mest, Y.; Le Poul, N.; Tron, T.; Faure, B.; Simaan, A.J.; et al. Electrochemical Water Oxidation and Stereoselective Oxygen Atom Transfer Mediated by a Copper Complex. Chem-Eur. J. 2018, 24, 5213–5224. [Google Scholar] [CrossRef] [Green Version]
- Shahadat, H.M.; Younus, H.A.; Ahmad, N.; Zhang, S.G.; Zhuiykov, S.; Verpoort, F. Macrocyclic cyanocobalamin (vitamin B-12) as a homogeneous electrocatalyst for water oxidation under neutral conditions. Chem. Commun. 2020, 56, 1968–1971. [Google Scholar] [CrossRef]
- Costentin, C.; Drouet, S.; Robert, M.; Savéant, J.-M. Correction to Turnover Numbers, Turnover Frequencies, and Overpotential in Molecular Catalysis of Electrochemical Reactions. Cyclic Voltammetry and Preparative-Scale Electrolysis. J. Am. Chem. Soc. 2012, 134, 19949–19950. [Google Scholar] [CrossRef]
- Martin, D.J.; Mercado, B.Q.; Mayer, J.M. Combining scaling relationships overcomes rate versus overpotential trade-offs in O-2 molecular electrocatalysis. Sci. Adv. 2020, 6, eaaz3318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artero, V.; Saveant, J.M. Toward the rational benchmarking of homogeneous H-2-evolving catalysts. Energ. Env. Environ. Sci. 2014, 7, 3808–3814. [Google Scholar] [CrossRef] [Green Version]
- Garrido-Barros, P.; Funes-Ardoiz, I.; Drouet, S.; Benet-Buchholz, J.; Maseras, F.; Llobet, A. Redox Non-innocent Ligand Controls Water Oxidation Overpotential in a New Family of Mononuclear Cu-Based Efficient Catalysts. J. Am. Chem. Soc. 2015, 137, 6758–6761. [Google Scholar] [CrossRef] [PubMed]
- Matheu, R.; Neudeck, S.; Meyer, F.; Sala, X.; Llobet, A. Foot of the Wave Analysis for Mechanistic Elucidation and Benchmarking Applications in Molecular Water Oxidation Catalysis. Chemsuschem 2016, 9, 3361–3369. [Google Scholar] [CrossRef]
- Gentil, S.; Molloy, J.K.; Carriere, M.; Hobballah, A.; Dutta, A.; Cosnier, S.; Shaw, W.J.; Gellon, G.; Belle, C.; Artero, V.; et al. A Nanotube-Supported Dicopper Complex Enhances Pt-free Molecular H-2/Air Fuel Cells. Joule 2019, 3, 2020–2029. [Google Scholar] [CrossRef]
- Zhang, X.F.; Li, Y.Y.; Jiang, J.; Zhang, R.; Liao, R.Z.; Wang, M. A Dinuclear Copper Complex Featuring a Flexible Linker as Water Oxidation Catalyst with an Activity Far Superior to Its Mononuclear Counterpart. Inorg. Chem. 2020, 59, 5424–5432. [Google Scholar] [CrossRef]
- Geer, A.M.; Musgrave, C.; Webber, C.; Nielsen, R.J.; McKeown, B.A.; Liu, C.; Schleker, P.P.M.; Jakes, P.; Jia, X.F.; Dickie, D.A.; et al. Electrocatalytic Water Oxidation by a Trinuclear Copper(II) Complex. Acs Catal. 2021, 11, 7223–7240. [Google Scholar] [CrossRef]
- Zhong, D.C.; Gong, Y.N.; Zhang, C.; Lu, T.B. Dinuclear metal synergistic catalysis for energy conversion. Chem. Soc. Rev. 2023, 52, 3170–3214. [Google Scholar] [CrossRef]
- Kaeffer, N.; Morozan, A.; Fize, J.; Martinez, E.; Guetaz, L.; Artero, V. The Dark Side of Molecular Catalysis: Diimine-Dioxime Cobalt Complexes Are Not the Actual Hydrogen Evolution Electrocatalyst in Acidic Aqueous Solutions. Acs Catal. 2016, 6, 3727–3737. [Google Scholar] [CrossRef]
- Wickramasinghe, L.D.; Zhou, R.W.; Zong, R.F.; Vo, P.; Gagnon, K.J.; Thummel, R.P. Iron Complexes of Square Planar Tetradentate Polypyridyl-Type Ligands as Catalysts for Water Oxidation. J. Am. Chem. Soc. 2015, 137, 13260–13263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.F.; Xiao, Y.; Liao, R.-Z.; Zhang, M.T. Trinuclear Nickel Catalyst for Water Oxidation: Intramolecular Proton-Coupled Electron Transfer Triggered Trimetallic Cooperative O–O Bond Formation. CCS Chem. 2023, 5, 245–256. [Google Scholar] [CrossRef]
- Cao, R.; Saracini, C.; Ginsbach, J.W.; Kieber-Emmons, M.T.; Siegler, M.A.; Solomon, E.I.; Fukuzumi, S.; Karlin, K.D. Peroxo and Superoxo Moieties Bound to Copper Ion: Electron-Transfer Equilibrium with a Small Reorganization Energy. J. Am. Chem. Soc. 2016, 138, 7055–7066. [Google Scholar] [CrossRef] [Green Version]
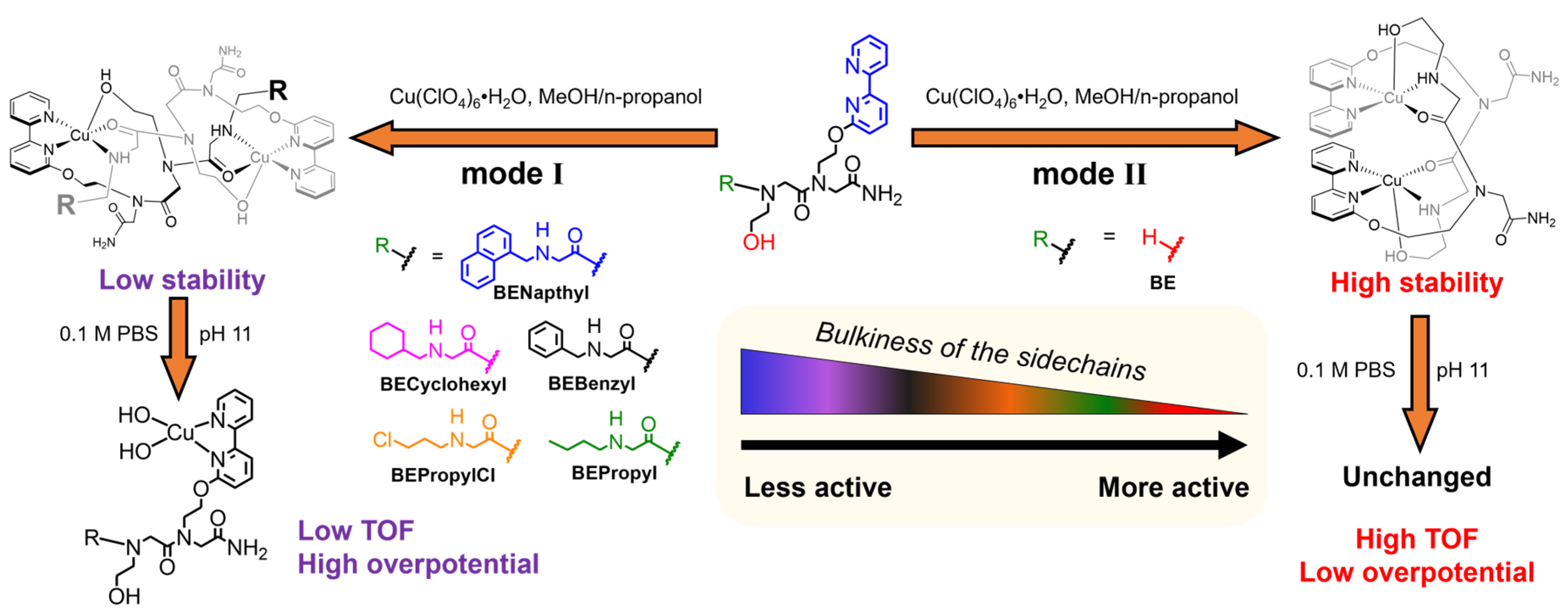

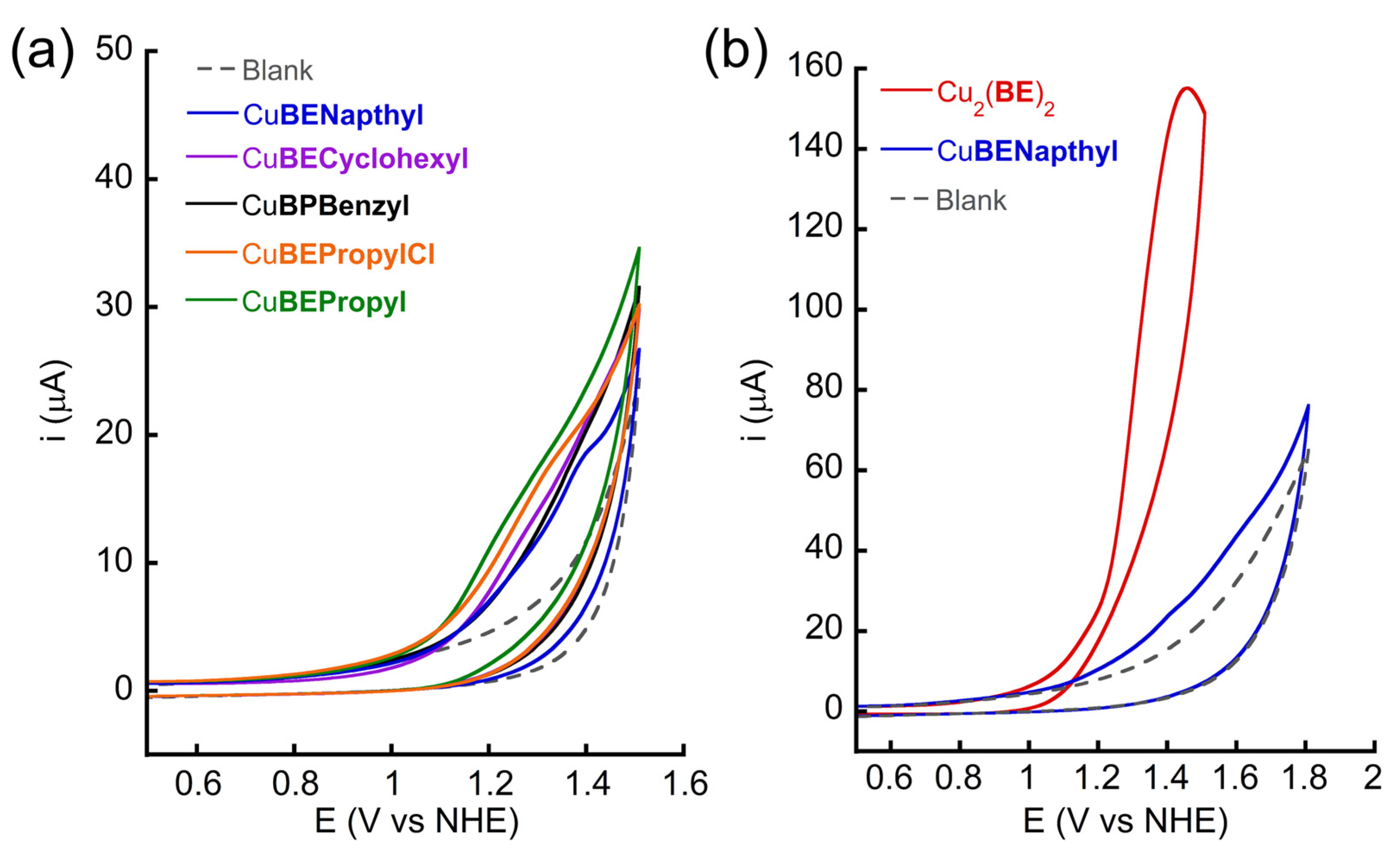
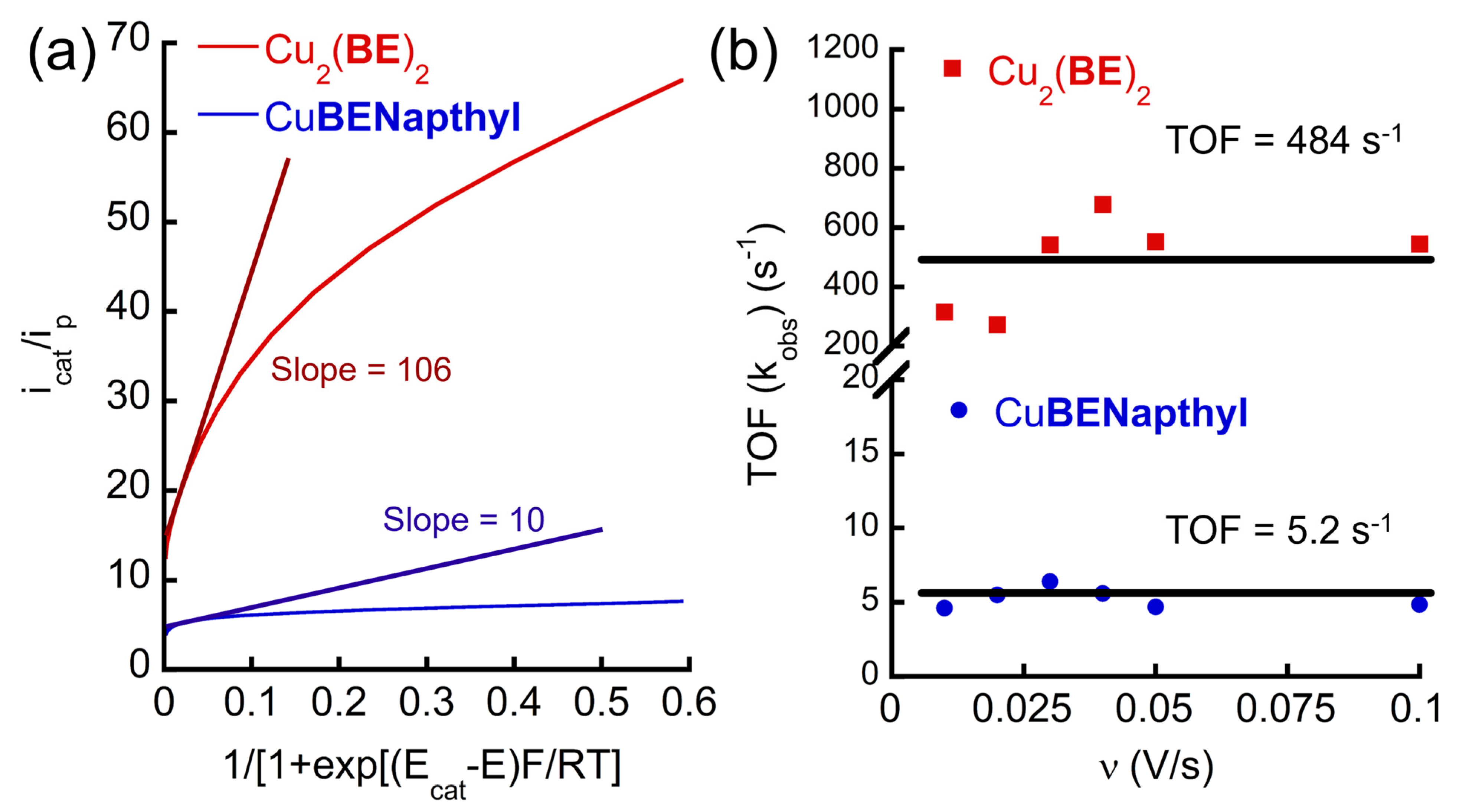

| Complex a | Solid State or in Pure Water | Cu···Cu Distance in Dinuclear Form | In 0.1 M PBS (pH 11) | TOF (s−1) | Onset Potential (V vs. NHE) |
|---|---|---|---|---|---|
| CuBENapthyl | Dinuclear (mode I) | 6.787 Å | Mononuclear | 5.2 | +1.26 |
| CuBECyclohexyl | Dinuclear (mode I) | 6.757 Å | Mononuclear | 5.3 | +1.26 |
| CuBEBenzyl | Dinuclear (mode I) | 6.805 Å | Mononuclear | 5.8 | +1.24 |
| CuBEPropylCl | Dinuclear (mode I) | 6.912 Å | Mononuclear | 6.1 | +1.20 |
| CuBEPropyl | Dinuclear (mode I) | 6.921 Å | Mononuclear | 7.7 | +1.18 |
| Cu2(BE)2 | Dinuclear (mode II) | 4.270 Å | Dinuclear (mode II) | 484 (43) b | +1.08 |
| Complex a | Nuclearity | Onset Potential (V vs. NHE) a | TOF (s−1) | Reference |
|---|---|---|---|---|
| Cu2(BE)2 | Dinuclear | +1.08 | 484 (43) b | This work |
| CuBENapthyl | Mononuclear | +1.26 | 5.2 | |
| TNC-Cu | Trinuclear | ~+1.6 * | 20,000 | [34] b |
| HappCu2 | Dinuclear | ~+1.65 * | 1375 | |
| F-N2O2Cu | Mononuclear | ~+1.85 * | 131.6 | |
| cat 1 | Dinuclear | ~+1.25 * | 144 | [97] c |
| cat 2 | Mononuclear | ~+1.35 * | 4.86 | |
| Fe(dpa) | Dinuclear | ~+1.2 * | 2.2 | [101] d |
| Fe(ppq) | Mononuclear | ~+1.4 * | 0.23 | |
| TNC-Ni3 | Trinuclear | +1.24 | 0.54 | [102] e |
| TNC-Ni2 | Dinuclear | ~+1.44 * | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, G.; Fridman, N.; Maayan, G. Structure–Function Relationship within Cu-Peptoid Electrocatalysts for Water Oxidation. Inorganics 2023, 11, 312. https://doi.org/10.3390/inorganics11070312
Ruan G, Fridman N, Maayan G. Structure–Function Relationship within Cu-Peptoid Electrocatalysts for Water Oxidation. Inorganics. 2023; 11(7):312. https://doi.org/10.3390/inorganics11070312
Chicago/Turabian StyleRuan, Guilin, Natalia Fridman, and Galia Maayan. 2023. "Structure–Function Relationship within Cu-Peptoid Electrocatalysts for Water Oxidation" Inorganics 11, no. 7: 312. https://doi.org/10.3390/inorganics11070312
APA StyleRuan, G., Fridman, N., & Maayan, G. (2023). Structure–Function Relationship within Cu-Peptoid Electrocatalysts for Water Oxidation. Inorganics, 11(7), 312. https://doi.org/10.3390/inorganics11070312









