Influence of Ga Substitution on the Local Structure and Luminescent Properties of Eu-Doped CaYAlO4 Phosphors
Abstract
:1. Introduction
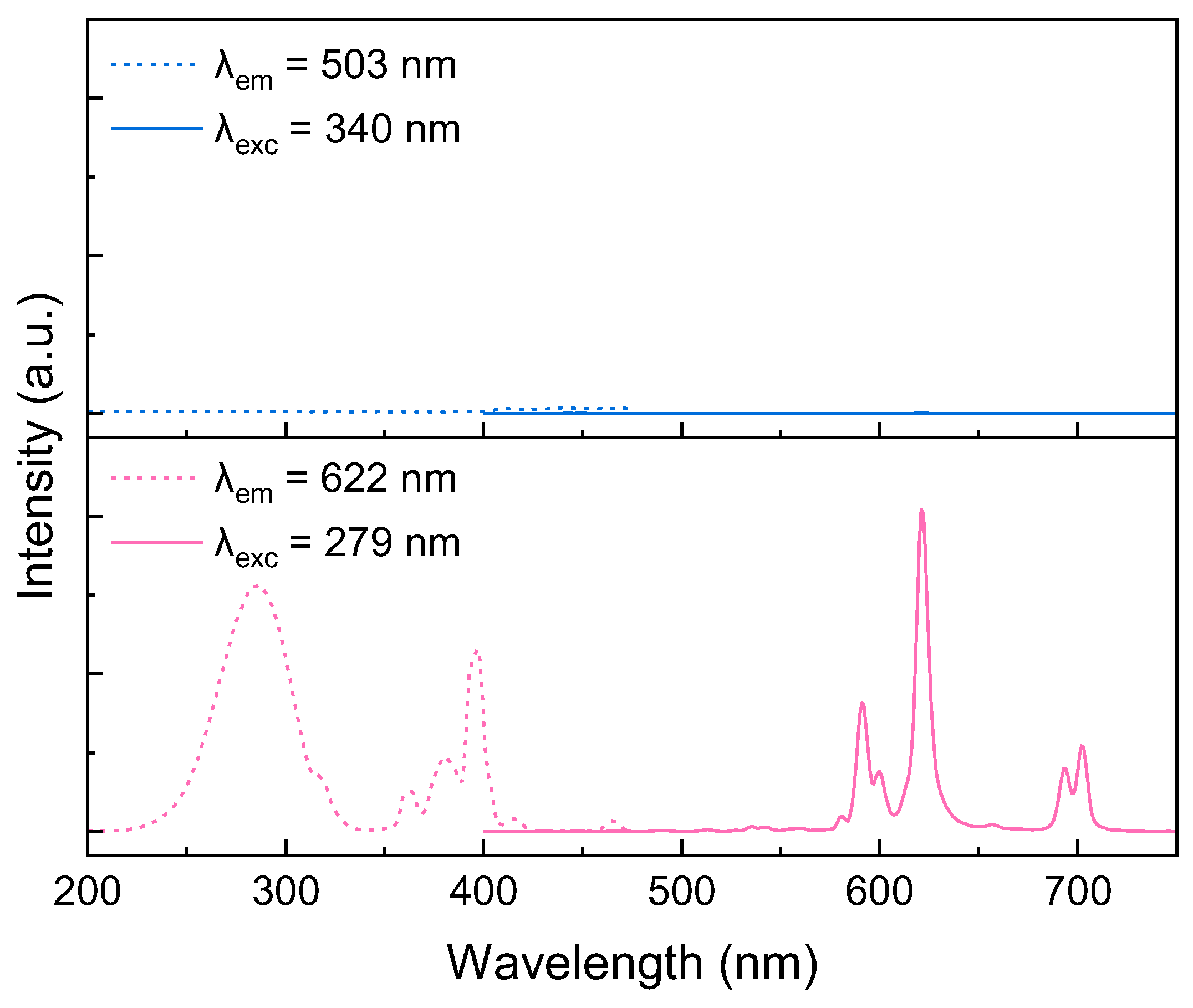
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ma, Z.; Ji, X.; Wang, M.; Chen, X.; Wu, D.; Li, X.; Shan, C.; Shi, Z. Emerging new-generation white light-emitting diodes based on luminescent lead-free halide perovskites and perovskite derivatives. Nano Sel. 2022, 3, 280–297. [Google Scholar] [CrossRef]
- Menéndez-Velázquez, A.; Morales, D.; García-Delgado, A.B. Sunlike White Light-Emitting Diodes Based on Rare-Earth-Free Luminescent Materials. Materials 2022, 15, 1680. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, H.; Cao, W.; Cui, Y.; Qian, G. Luminescent Metal–Organic Frameworks for White LEDs. Adv. Opt. Mater. 2021, 9, 2001817. [Google Scholar] [CrossRef]
- Bispo-Jr, A.G.; Saraiva, L.F.; Lima, S.A.M.; Pires, A.M.; Davolos, M.R. Recent prospects on phosphor-converted LEDs for lighting, displays, phototherapy, and indoor farming. J. Lumin. 2021, 237, 118167. [Google Scholar] [CrossRef]
- Nair, G.B.; Swart, H.C.; Dhoble, S.J. A review on the advancements in phosphor-converted light emitting diodes (pc-LEDs): Phosphor synthesis, device fabrication and characterization. Prog. Mater. Sci. 2020, 109, 100622. [Google Scholar] [CrossRef]
- Shi, W.; Chen, J.; Kong, J.; Ma, Z.; Gao, J.; Guo, J.; Hu, Z.; Lv, Q.; Deng, B.; Chen, W.; et al. A novel highly thermal-stable red-emitting CaGdSbWO8: Eu3+ phosphor with scheelite structure for high CRI w-LEDs, security ink, and latent fingerprint. J. Alloys Compd. 2022, 914, 165134. [Google Scholar] [CrossRef]
- Singh, K.; Rajendran, M.; Devi, R.; Vaidyanathan, S. Narrow-Band Red-Emitting Phosphors with High Color Purity, Trifling Thermal and Concentration Quenching for Hybrid White LEDs and Li3Y3BaSr(MoO4)8:Sm3+, Eu3+-Based Deep-Red LEDs for Plant Growth Applications. Inorg. Chem. 2022, 61, 2768–2782. [Google Scholar] [CrossRef]
- Zhao, M.; Liao, H.; Molokeev, M.S.; Zhou, Y.; Zhang, Q.; Liu, Q.; Xia, Z. Emerging ultra-narrow-band cyan-emitting phosphor for white LEDs with enhanced color rendition. Light Sci. Appl. 2019, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Qiang, Y.; Liu, Y.; Chen, J.; Liu, S.; Zhang, L.; Kang, H.; Xu, F.; Xiao, Z.; You, W.; Han, L.; et al. BaY1.95Al2Ga2SiO12:0.05Ce3+: A novel green-emitting phosphor with extra-high quantum yield, small thermal quenching and excellent water resistance for high-color-rendering white LEDs. J. Lumin. 2020, 224, 117293. [Google Scholar] [CrossRef]
- Shao, B.; Huo, J.; You, H. Prevailing Strategies to Tune Emission Color of Lanthanide-Activated Phosphors for WLED Applications. Adv. Opt. Mater. 2019, 7, 1900319. [Google Scholar] [CrossRef]
- Wang, C.; Lv, Q.; Ma, J.; Li, Y.; Shao, B.; Zhao, X.; Zhu, G. A novel single-phased white light emitting phosphor with single Eu2+ doped whitlockite structure. Adv. Powder Technol. 2022, 33, 103394. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, J.; Yang, N.; Liang, Q.; Xu, Y.; Fu, S.; Yan, J.; Zhou, J.; Shi, J.; Wu, M. A novel multi-center activated single-component white light-emitting phosphor for deep UV chip-based high color-rendering WLEDs. Chem. Eng. J. 2020, 390, 124601. [Google Scholar] [CrossRef]
- Panigrahi, K.; Nag, A. Challenges and Strategies to Design Phosphors for Future White Light Emitting Diodes. J. Phys. Chem. C 2022, 126, 8553–8564. [Google Scholar] [CrossRef]
- Zhou, L.; Hong, J.; Li, X.; Shi, J.; Tanner, P.A.; Wong, K.L.; Wu, M. Bright Green Emitting CaYAlO4:Tb3+, Ce3+ Phosphor: Energy Transfer and 3D-Printing Artwork. Adv. Opt. Mater. 2020, 8, 202000523. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, M.; Liang, C.; Zhu, H.; Zhong, Y.; Yang, N.; Zhou, Z.; Xia, M. Tuning the luminescence properties of Mn4+-activated CaYAlO4 phosphor by co-doping cations for indoor plant cultivation. J. Am. Ceram. Soc. 2020, 103, 4373–4383. [Google Scholar] [CrossRef]
- Matsushima, Y.; Ishizawa, N.; Kodama, N. Synchrotron X-ray and molecular dynamics studies of CaYAlO4: The role of heterovalent solutes in K2NiF4-type solid solutions. Phys. C Supercond. 2000, 338, 166–169. [Google Scholar] [CrossRef]
- Ueda, A.; Higuchi, M.; Yamada, D.; Namiki, S.; Ogawa, T.; Wada, S.; Tadanaga, K. Float zone growth and spectral properties of Cr, Nd:CaYAlO4 single crystals. J. Cryst. Growth 2014, 404, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.; Xia, Z. Design principles for achieving red emission in Eu2+/Eu3+ doped inorganic solids. J. Appl. Phys. 2021, 129, 200903. [Google Scholar] [CrossRef]
- Terraschke, H.; Wickleder, C. UV, Blue, Green, Yellow, Red, and Small: Newest Developments on Eu2+-Doped Nanophosphors. Chem. Rev. 2015, 115, 11352–11378. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Li, K.; Lian, H.; Shang, M.; Lin, J. Crystal-site engineering control for the reduction of Eu3+ to Eu2+ in CaYAlO4: Structure refinement and tunable emission properties. ACS Appl. Mater. Interfaces 2015, 7, 2715–2725. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, W.; Zhu, Y.; Xu, H.; Zhou, L.; Noh, H.M.; Jeong, J.H.; Liu, X.; Li, L. Eu3+/2+ co-doping system induced by adjusting Al/Y ratio in Eu doped CaYAlO4: Preparation, bond energy, site preference and 5D0–7F4 transition intensity. RSC Adv. 2018, 8, 23981–23989. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, C.; Chen, D. Synthesis of the long-persistence phosphor CaAl2O4:Eu2+, Dy3+, Nd3+ by combustion method and its luminescent properties. Luminescence 2010, 25, 25–29. [Google Scholar] [CrossRef]
- Kumar, K.; Singh, A.K.; Rai, S.B. Laser excited long lasting luminescence in CaAl2O4: Eu3+/Eu2++Nd3+ phosphor. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 102, 212–218. [Google Scholar] [CrossRef]
- Zhong, J.; Chen, D.; Yuan, S.; Liu, M.; Yuan, Y.; Zhu, Y.; Li, X.; Ji, Z. Tunable Optical Properties and Enhanced Thermal Quenching of Non-Rare-Earth Double-Perovskite (Ba1-xSrx)2YSbO6:Mn4+ Red Phosphors Based on Composition Modulation. Inorg. Chem. 2018, 57, 8978–8987. [Google Scholar] [CrossRef]
- Yuan, G.; Cui, R.; Zhang, J.; Zhang, X.; Qi, X.; Deng, C. Photoluminescence evolution and high thermal stability of orange red-emitting Ba3-xSrxZnNb2O9:Eu3+ phosphors. J. Solid State Chem. 2021, 303, 122447. [Google Scholar] [CrossRef]
- Bai, S.; Liu, Y.; Tan, G.; Liu, W.; Liu, D.; Wang, R.; Zhu, Y.; Ye, S.; Ren, H. Enhanced quantum efficiency and thermal stability in CaWO4:Eu3+ phosphor based on structural modification induced by co-doping Al3+. J. Lumin. 2020, 225, 117351. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Qiao, J.; Molokeev, M.S.; Xia, Z. Rapid Synthesis of Red-Emitting Sr2Sc0.5Ga1.5O5:Eu2+ Phosphors and the Tunable Photoluminescence Via Sr/Ba Substitution. Adv. Opt. Mater. 2021, 9, 2100131. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Zheng, B.; Sun, Q.; Zheng, Z.; Shi, Z.; Song, Y.; Zou, H. Li+ Ion Induced Full Visible Emission in Single Eu2+-Doped White Emitting Phosphor: Eu2+ Site Preference Analysis, Luminescence Properties, and WLED Applications. Adv. Opt. Mater. 2021, 9, 2100337. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Geng, D.; Li, G.; Shang, M.; Peng, C.; Zhang, Y.; Cheng, Z.; Lin, J. Nanocrystalline CaYAlO4:Tb3+/Eu3+ as promising phosphors for full-color field emission displays. Dalton Trans. 2012, 41, 3078–3086. [Google Scholar] [CrossRef]
- Kim, H.; Nam, K.; Park, J.; Kang, M.; Bae, J.-S.; Hong, W.T.; Yang, H.K.; Jeong, J.H.; Oh, J.H.; Lee, S. Hydrogen-mediated manipulation of luminescence color in single-component Eu doped CaYAlSiO4 by defect passivation. J. Alloys Compd. 2023, 932, 167610. [Google Scholar] [CrossRef]
- Judd, B.R. Optical absorption intensities of rare-earth ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. A General Introduction to Luminescent Materials. In Luminescent Materials; Blasse, G., Grabmaier, B.C., Eds.; Springer: Berlin, Heidelberg, 1994; pp. 1–9. [Google Scholar]
- Ofelt, G.S. Intensities of Crystal Spectra of Rare-Earth Ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Perrella, R.V.; Júnior, C.S.N.; Góes, M.S.; Pecoraro, E.; Schiavon, M.A.; Paiva-Santos, C.O.; Lima, H.; Couto dos Santos, M.A.; Ribeiro, S.J.L.; Ferrari, J.L. Structural, electronic and photoluminescence properties of Eu3+-doped CaYAlO4 obtained by using citric acid complexes as precursors. Opt. Mater. 2016, 57, 45–55. [Google Scholar] [CrossRef]
- Unal, F.; Kaya, F.; Kazmanli, K. Effects of dopant rate and calcination parameters on photoluminescence emission of Y2O3:Eu3+ phosphors: A statistical approach. Ceram. Int. 2019, 45, 17818–17825. [Google Scholar] [CrossRef]
- Camenzind, A.; Strobel, R.; Pratsinis, S.E. Cubic or monoclinic Y2O3:Eu3+ nanoparticles by one step flame spray pyrolysis. Chem. Phys. Lett. 2005, 415, 193–197. [Google Scholar] [CrossRef]
- Yan, T.; Zhang, D.; Shi, L.; Li, H. Facile synthesis, characterization, formation mechanism and photoluminescence property of Eu2O3 nanorods. J. Alloys Compd. 2009, 487, 483–488. [Google Scholar] [CrossRef]
- da Silva Viana, R.; Lago Falcão, E.H.; Lisboa Dutra, J.D.; da Costa, N.B.; Freire, R.O.; Alves, S. New experimental and theoretical approach in Eu2O3 microspheres: From synthesis to a study of the energy transfer. J. Photochem. Photobiol. A Chem. 2014, 281, 1–7. [Google Scholar] [CrossRef]
- Pokhrel, M.; Wahid, K.; Mao, Y. Systematic Studies on RE2Hf2O7:5%Eu3+ (RE = Y, La, Pr, Gd, Er, and Lu) Nanoparticles: Effects of the A-Site RE3+ Cation and Calcination on Structure and Photoluminescence. J. Phys. Chem. C 2016, 120, 14828–14839. [Google Scholar] [CrossRef]
- Singh, J.; Manam, J. Structural and spectroscopic behaviour of Eu3+-doped SrGd2O4 modified by thermal treatments. J. Mater. Sci. 2016, 51, 2886–2901. [Google Scholar] [CrossRef]
- Kolesnikov, I.E.; Povolotskiy, A.V.; Mamonova, D.V.; Kolesnikov, E.Y.; Kurochkin, A.V.; Lähderanta, E.; Mikhailov, M.D. Asymmetry ratio as a parameter of Eu3+ local environment in phosphors. J. Rare Earths 2018, 36, 474–481. [Google Scholar] [CrossRef]
- Wang, Y.; Song, M.; Xiao, L.; Li, Q. Upconversion luminescence of Eu3+ and Sm3+ single-doped NaYF4 and NaY(MoO4)2. J. Lumin. 2021, 238, 118203. [Google Scholar] [CrossRef]
- Boukerika, A.; Guerbous, L. Annealing effects on structural and luminescence properties of red Eu3+-doped Y2O3 nanophosphors prepared by sol–gel method. J. Lumin. 2014, 145, 148–153. [Google Scholar] [CrossRef]
- Singh, R.; King, A.; Nayak, B.B. Influence of dopant concentration on powder morphology and photoluminescence characteristics of red-emitting Eu3+-doped ZnO. Optik 2021, 247, 167870. [Google Scholar] [CrossRef]
- Mohamed, S.N.; Sazali, E.S.; Yahya, A.K. Mixed ionic–electronic effect on up–conversion in Er3+/V4+ co–doped Na2O–CaO–B2O3 glasses with enhanced red emission. J. Lumin. 2022, 251, 119135. [Google Scholar] [CrossRef]
- Ferhi, M.; Bouzidi, C.; Horchani-Naifer, K.; Elhouichet, H.; Ferid, M. Judd–Ofelt analysis of spectroscopic properties of Eu3+ doped KLa(PO3)4. J. Lumin. 2015, 157, 21–27. [Google Scholar] [CrossRef]
- Ebendorff-Heidepriem, H.; Ehrt, D. Spectroscopic properties of Eu3+ and Tb3+ ions for local structure investigations of fluoride phosphate and phosphate glasses. J. Non-Cryst. Solids 1996, 208, 205–216. [Google Scholar] [CrossRef]
- Hu, Q.; Jia, Z.; Tang, C.; Lin, N.; Zhang, J.; Jia, N.; Wang, S.; Zhao, X.; Tao, X. The origin of coloration of CaGdAlO4 crystals and its effect on their physical properties. CrystEngComm 2017, 19, 537–545. [Google Scholar] [CrossRef]
- Hutchinson, J.A.; Verdun, H.R.; Chai, B.H.T.; Zandi, B.; Merkle, L.D. Spectroscopic evaluation of CaYA1O4 doped with trivalent Er, Tm, Yb and Ho for eyesafe laser applications. Opt. Mater. 1994, 3, 287–306. [Google Scholar] [CrossRef]
- Zhou, D.; Xu, X.; Chen, X.; Zhu, H.; Li, D.; Di, J.; Xia, C.; Wu, F.; Xu, J. Crystal growth and spectroscopic properties of Er3+-doped CaYAlO4. Phys. Status Solidi (A) 2012, 209, 730–735. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Spectral Intensities of the Trivalent Lanthanides and Actinides in Solution. II. Pm3+, Sm3+, Eu3+, Gd3+, Tb3+, Dy3+, and Ho3+. J. Chem. Phys. 1968, 49, 4412–4423. [Google Scholar] [CrossRef]
- Kumar, M.; Seshagiri, T.K.; Godbole, S.V. Fluorescence lifetime and Judd–Ofelt parameters of Eu3+ doped SrBPO5. Phys. B Condens. Matter 2013, 410, 141–146. [Google Scholar] [CrossRef]
- Jørgensen, C.K.; Reisfeld, R. Judd-Ofelt parameters and chemical bonding. J. Less Common Met. 1983, 93, 107–112. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, T.; Kan, D.; Zhang, D.; Dong, R.; An, Z.; Song, Y.; Zheng, K.; Sheng, Y.; Shi, Z.; et al. Study on the Local Structure and Luminescence Properties of a Y2Mg2Al2Si2O12:Eu3+ Red Phosphor for White-Light-Emitting Diodes. Inorg. Chem. 2020, 59, 9927–9937. [Google Scholar] [CrossRef]
- Antic-Fidancev, E.; Hölsä, J.; Lastusaari, M.; Lupei, A. Dopant-host relationships in rare-earth oxides and garnets doped with trivalent rare-earth ions. Phys. Rev. B 2001, 64, 195108. [Google Scholar] [CrossRef]
- Xiao, W.; Wu, D.; Zhang, L.; Zhang, X.; Hao, Z.; Pan, G.-H.; Zhang, L.; Ba, X.; Zhang, J. The Inductive Effect of Neighboring Cations in Tuning Luminescence Properties of the Solid Solution Phosphors. Inorg. Chem. 2017, 56, 9938–9945. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Sreena, T.S.; Prabhakar Rao, P.; Raj, A.K.V.; Aju Thara, T.R. Exploitation of Eu3+ red luminescence through order–disorder structural transitions in lanthanide stannate pyrochlores for warm white LED applications. Phys. Chem. Chem. Phys. 2018, 20, 24287–24299. [Google Scholar] [CrossRef]
- Zhong, C.; Zhang, L.; Xu, Y.; Wu, X.; Yin, S.; Zhang, X.; You, H. Design of novel cyan phosphor Ca7NaLu(PO4)6:Eu2+ for full-visible-spectrum white LED: Enhanced thermal stability and tuned emission by neighbor cation effect. Mater. Today Chem. 2022, 26, 101233. [Google Scholar] [CrossRef]
- Umair, M.M.; Yahya, A.K.; Halimah, M.K.; Sidek, H.A.A. Effects of Increasing Tungsten on Structural, Elastic and Optical Properties of xWO3–(40−x)Ag2O–60Te2O Glass System. J. Mater. Sci. Technol. 2015, 31, 83–90. [Google Scholar] [CrossRef]
- Karunadasa, K.S.P.; Manoratne, C.H.; Pitawala, H.M.T.G.A.; Rajapakse, R.M.G. Thermal decomposition of calcium carbonate (calcite polymorph) as examined by in-situ high-temperature X-ray powder diffraction. J. Phys. Chem. Solids 2019, 134, 21–28. [Google Scholar] [CrossRef]
- Singh, N.B.; Singh, N.P. Formation of CaO from thermal decomposition of calcium carbonate in the presence of carboxylic acids. J. Therm. Anal. Calorim. 2007, 89, 159–162. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, S.; Liu, W.; Ding, H.; Zhang, J. Synthesis of Perovskite by Solid-Phase Method with Metatitanic Acid and Calcium Carbonate and Its Pigment Properties Investigation. Materials 2020, 13, 1508. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Wu, W.; Xing, Z.; Zhou, X.; Yao, A.; Hu, S.; Hong, Y.; Wang, B.; Lu, S.; Wang, Y. Red luminescence properties of Te4+-doped CaYAlO4 phosphors. Ceram. Int. 2021, 47, 17286–17292. [Google Scholar] [CrossRef]
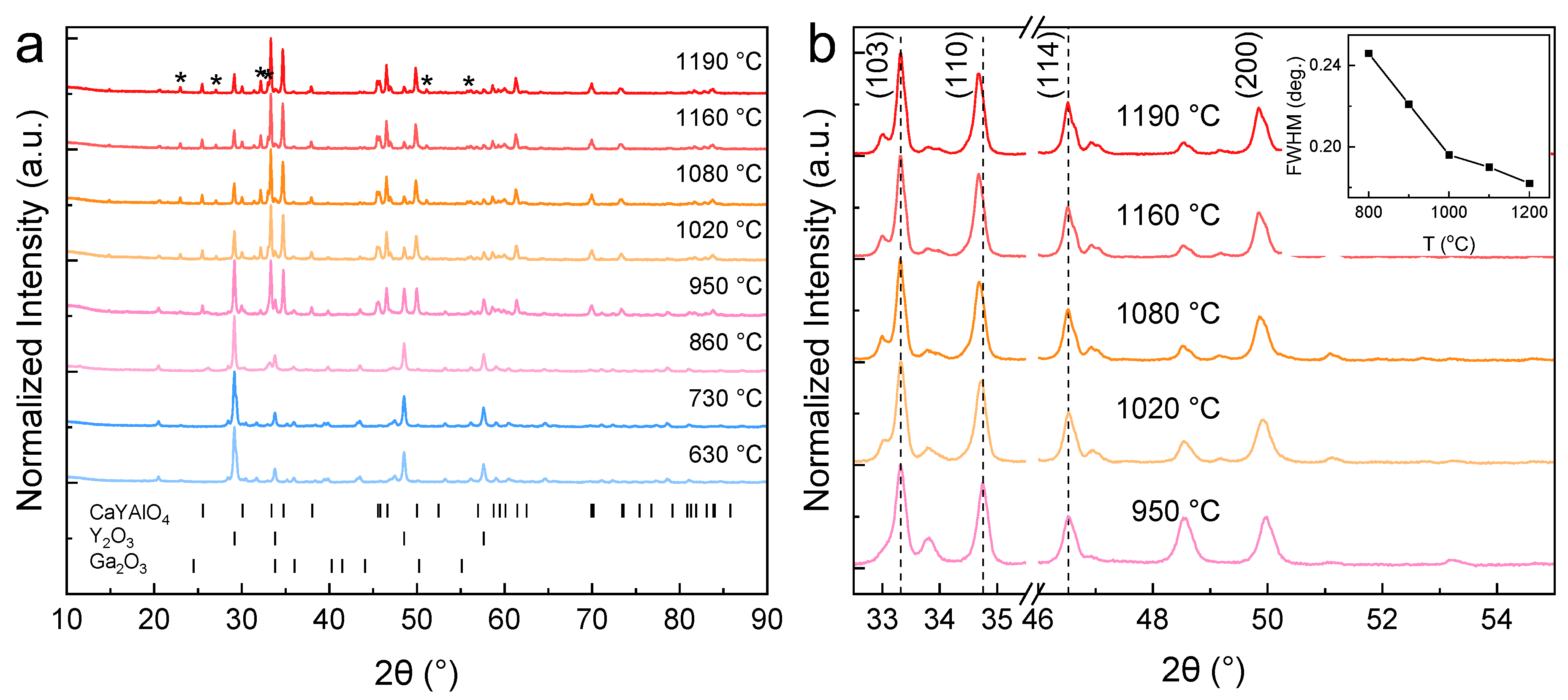
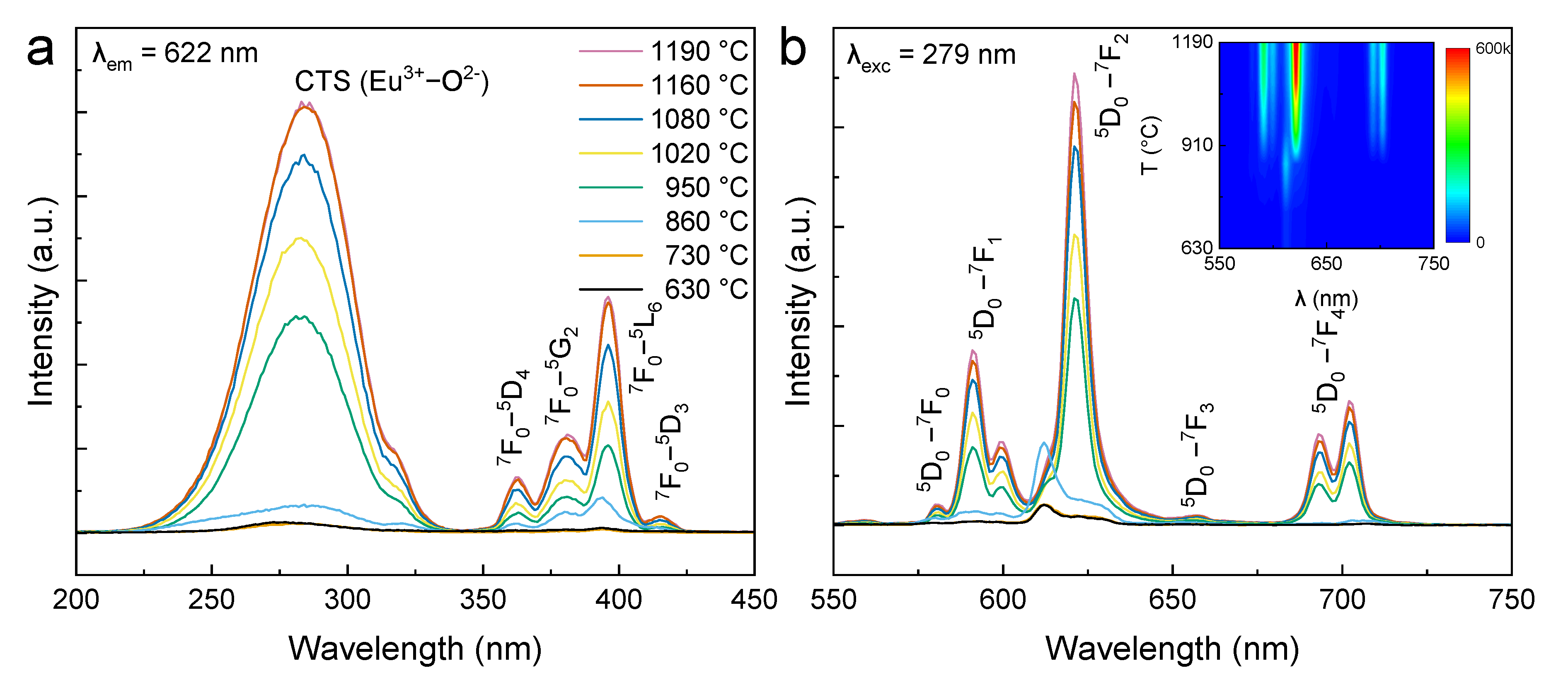
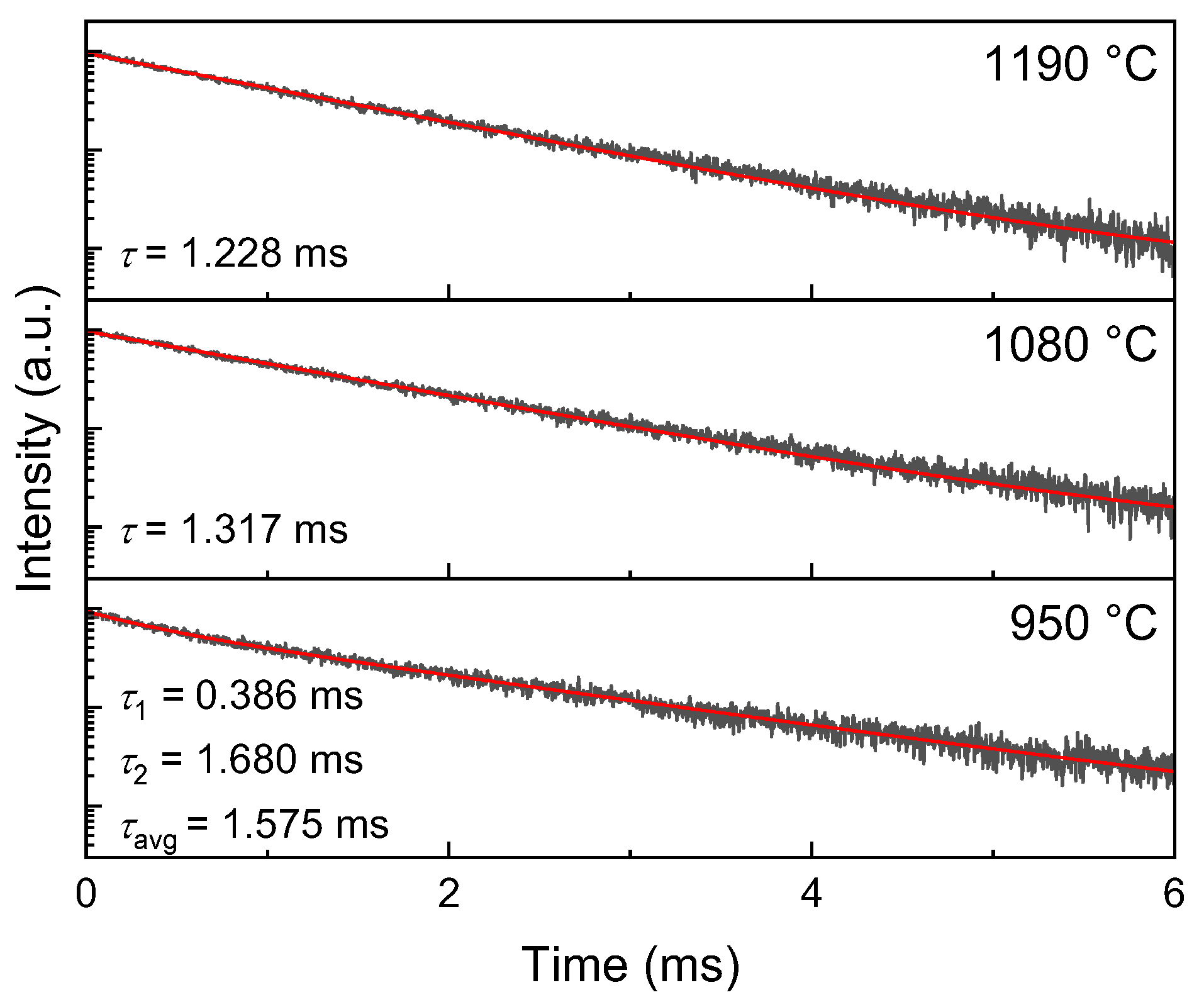
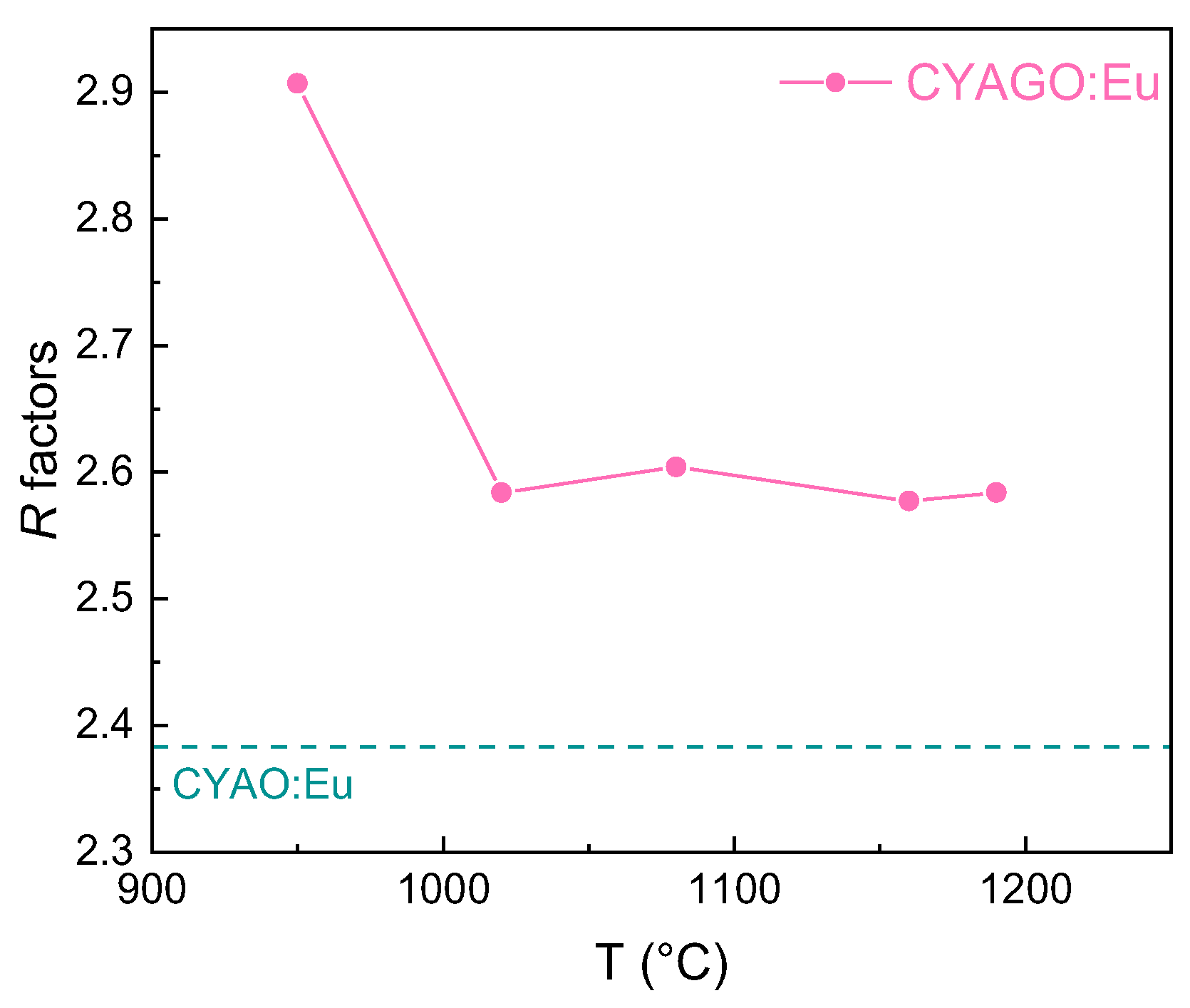
| Samples | Syn. Temp. (°C) | a = b (Å) | c (Å) | Volume (Å3) | Grain Size (nm) |
|---|---|---|---|---|---|
| CYAO:Eu | 1190 | 3.640(2) | 11.87(9) | 157.4(1) | 53.86 |
| CYAGO:Eu | 950 | 3.648(2) | 11.92(1) | 158.6(6) | 39.49 |
| 1020 | 3.651(6) | 11.90(3) | 158.7(1) | 44.82 | |
| 1080 | 3.654(0) | 11.89(8) | 158.8(5) | 51.82 | |
| 1160 | 3.654(8) | 11.89(2) | 158.8(5) | 53.84 | |
| 1190 | 3.654(6) | 11.89(1) | 158.8(1) | 56.79 |
| Samples | Syn. Temp. (℃) | Ω2 (10−20 cm2) | Ω4 (10−20 cm2) |
|---|---|---|---|
| CYAO:Eu | 1190 | 3.630 | 2.512 |
| CYAGO:Eu | 950 | 4.258 | 2.780 |
| 1020 | 3.847 | 2.536 | |
| 1080 | 3.824 | 2.580 | |
| 1160 | 3.798 | 2.604 | |
| 1190 | 3.806 | 2.619 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, J.H.; Kim, H.; Kang, M.; Lee, S. Influence of Ga Substitution on the Local Structure and Luminescent Properties of Eu-Doped CaYAlO4 Phosphors. Inorganics 2023, 11, 329. https://doi.org/10.3390/inorganics11080329
Oh JH, Kim H, Kang M, Lee S. Influence of Ga Substitution on the Local Structure and Luminescent Properties of Eu-Doped CaYAlO4 Phosphors. Inorganics. 2023; 11(8):329. https://doi.org/10.3390/inorganics11080329
Chicago/Turabian StyleOh, Ju Hyun, Hyunwoo Kim, Mijeong Kang, and Seunghun Lee. 2023. "Influence of Ga Substitution on the Local Structure and Luminescent Properties of Eu-Doped CaYAlO4 Phosphors" Inorganics 11, no. 8: 329. https://doi.org/10.3390/inorganics11080329
APA StyleOh, J. H., Kim, H., Kang, M., & Lee, S. (2023). Influence of Ga Substitution on the Local Structure and Luminescent Properties of Eu-Doped CaYAlO4 Phosphors. Inorganics, 11(8), 329. https://doi.org/10.3390/inorganics11080329






