Investigating the Shape Memory Effect and Corrosion Resistance of the Fe-(17-2x) Mn-6Si-xNi-yCr-0.3C Alloys (x = 0, 1, 2, 3, 4; y = 0, 1, 3, 5)
Abstract
1. Introduction
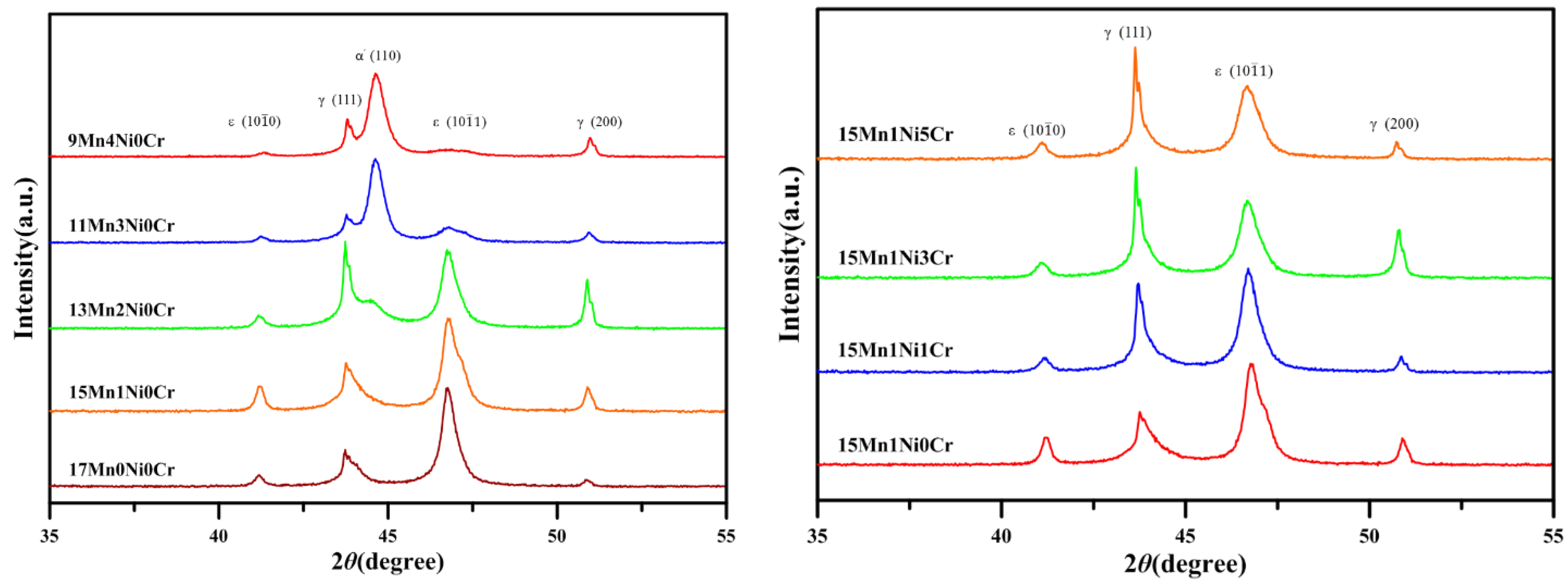
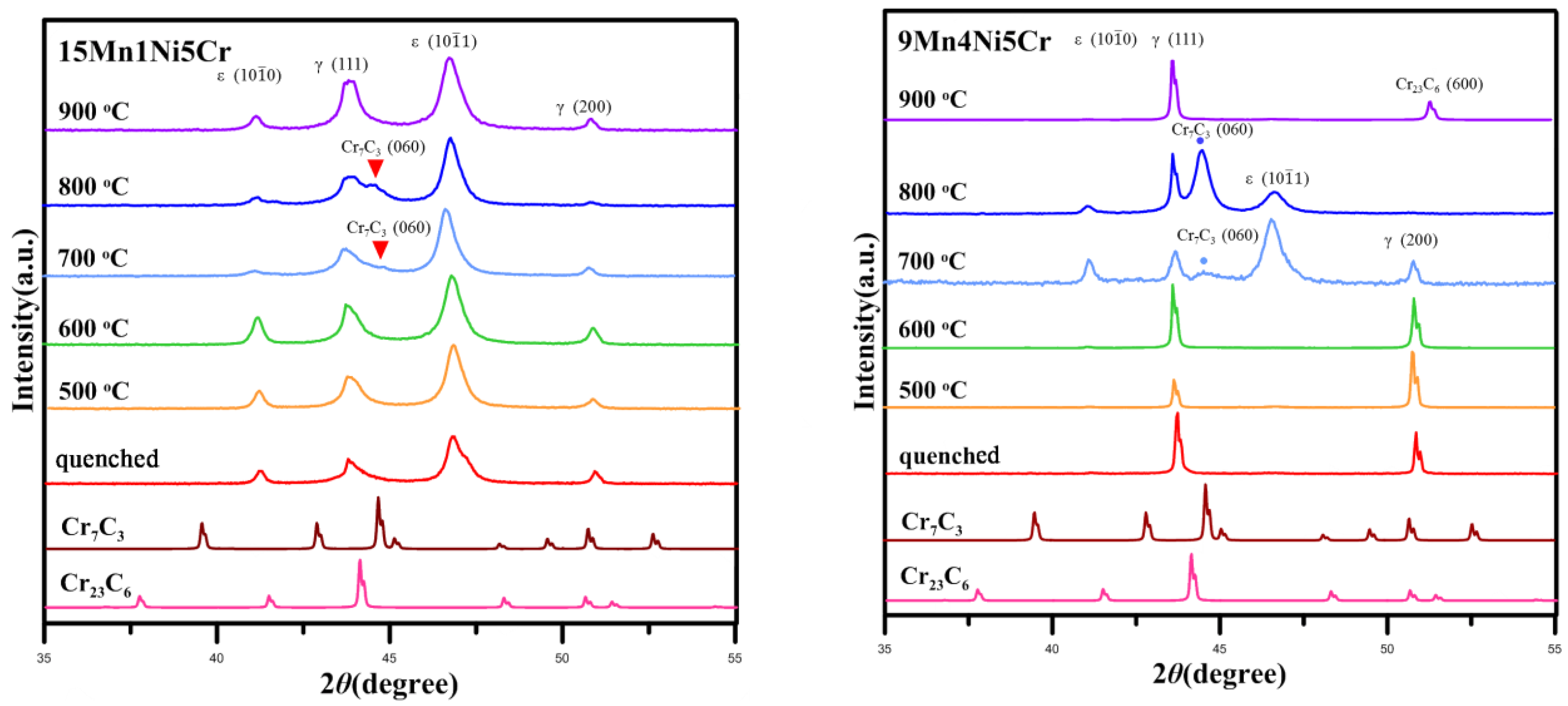
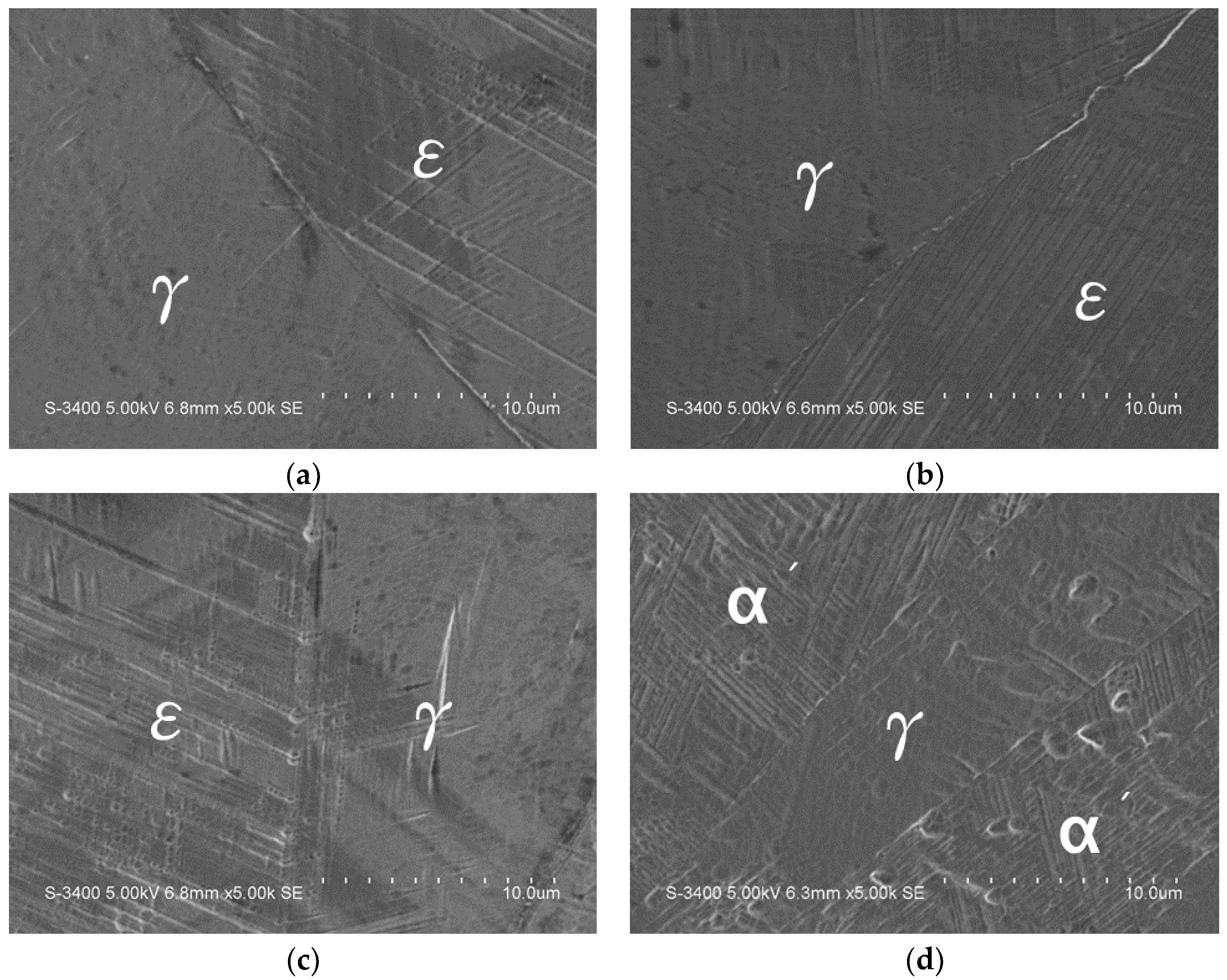
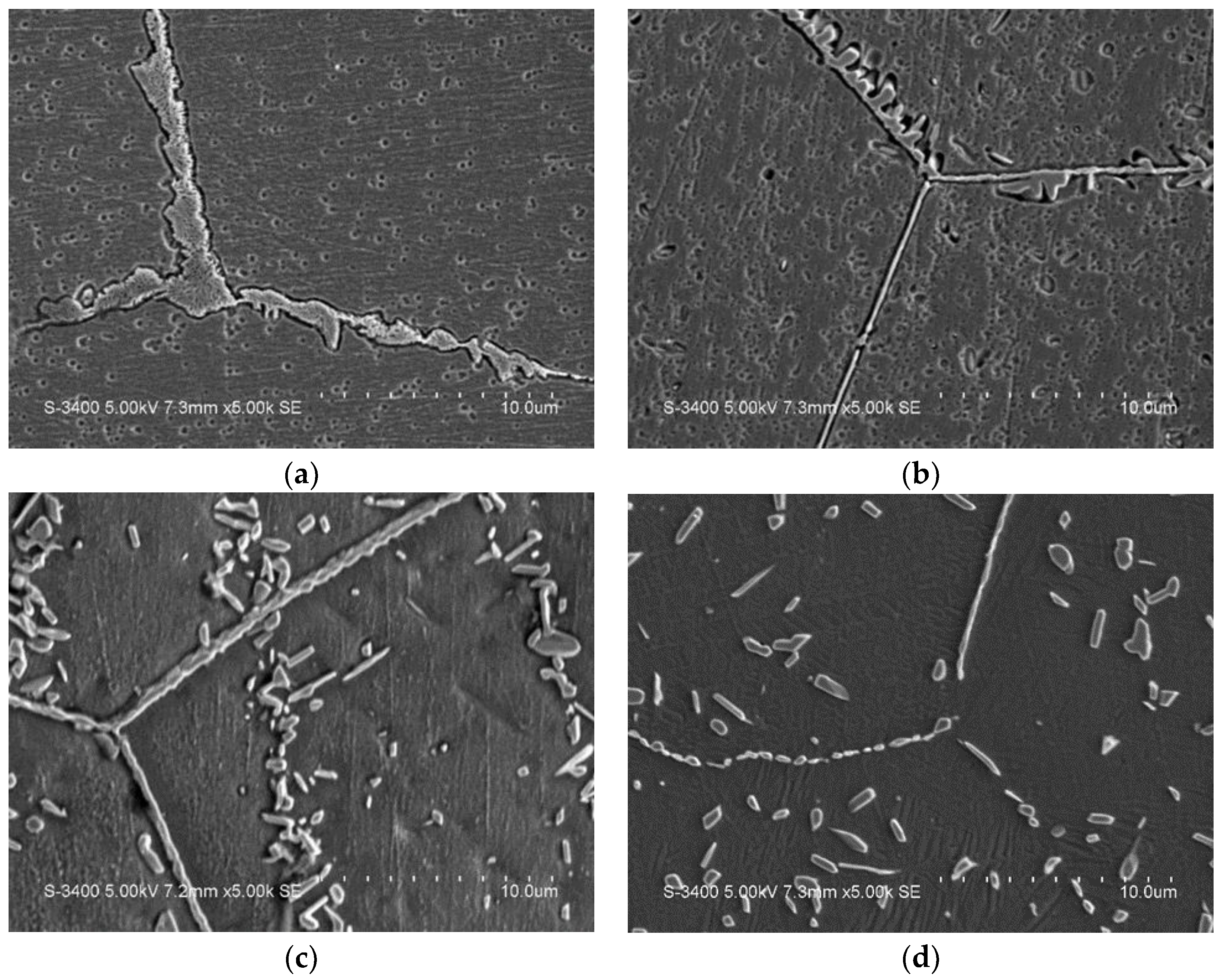
2. Results and Discussion
2.1. Microstructural Observation
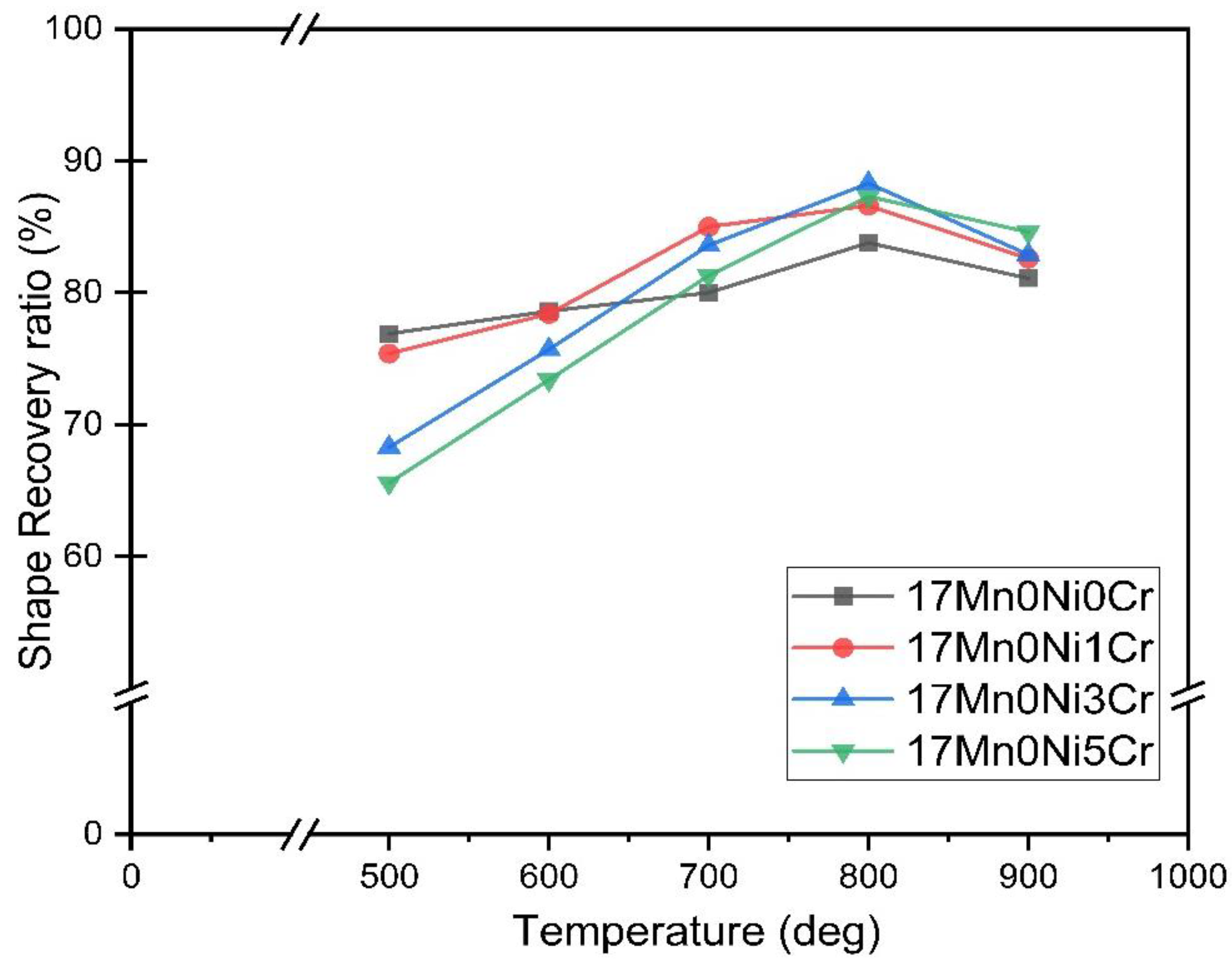
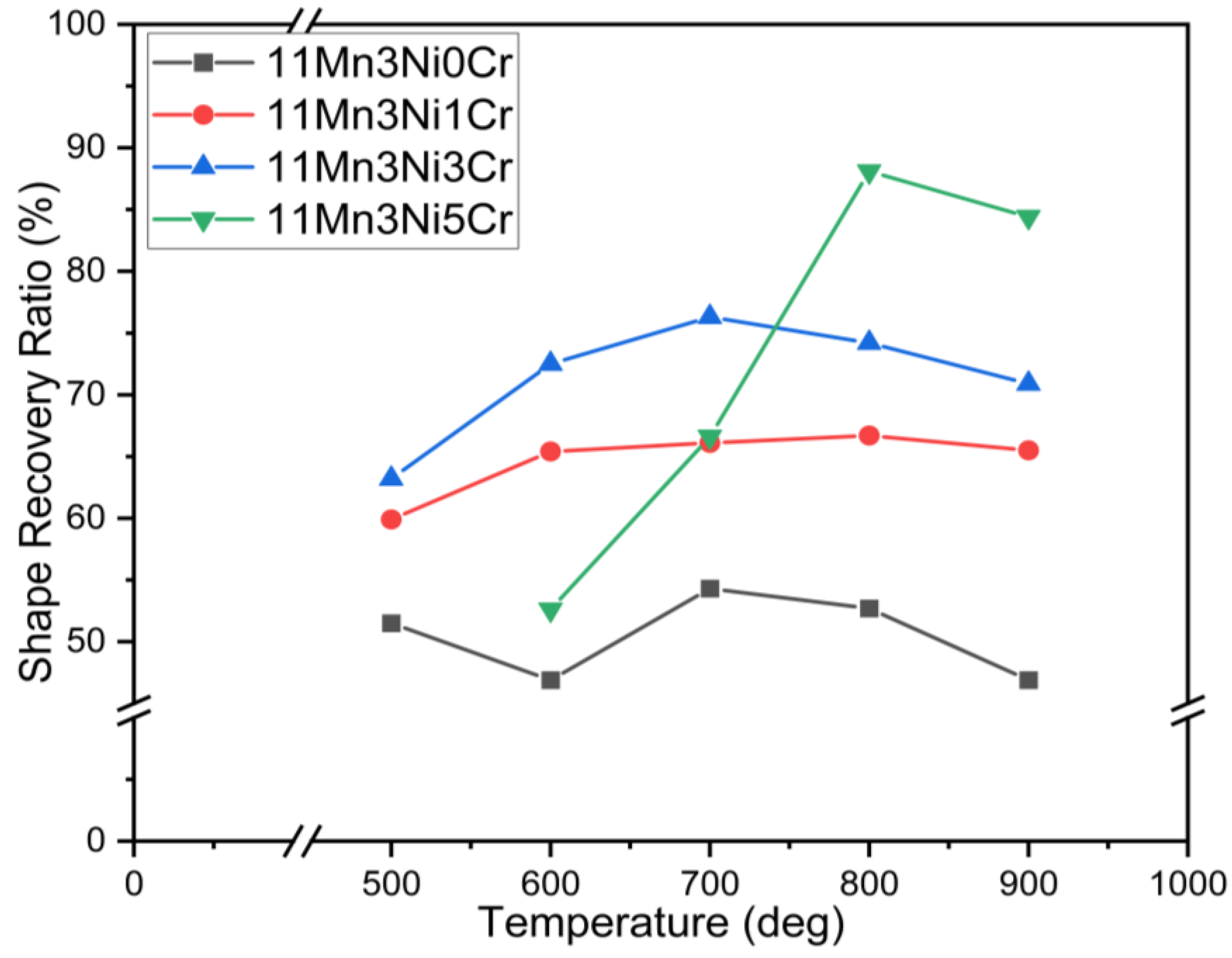
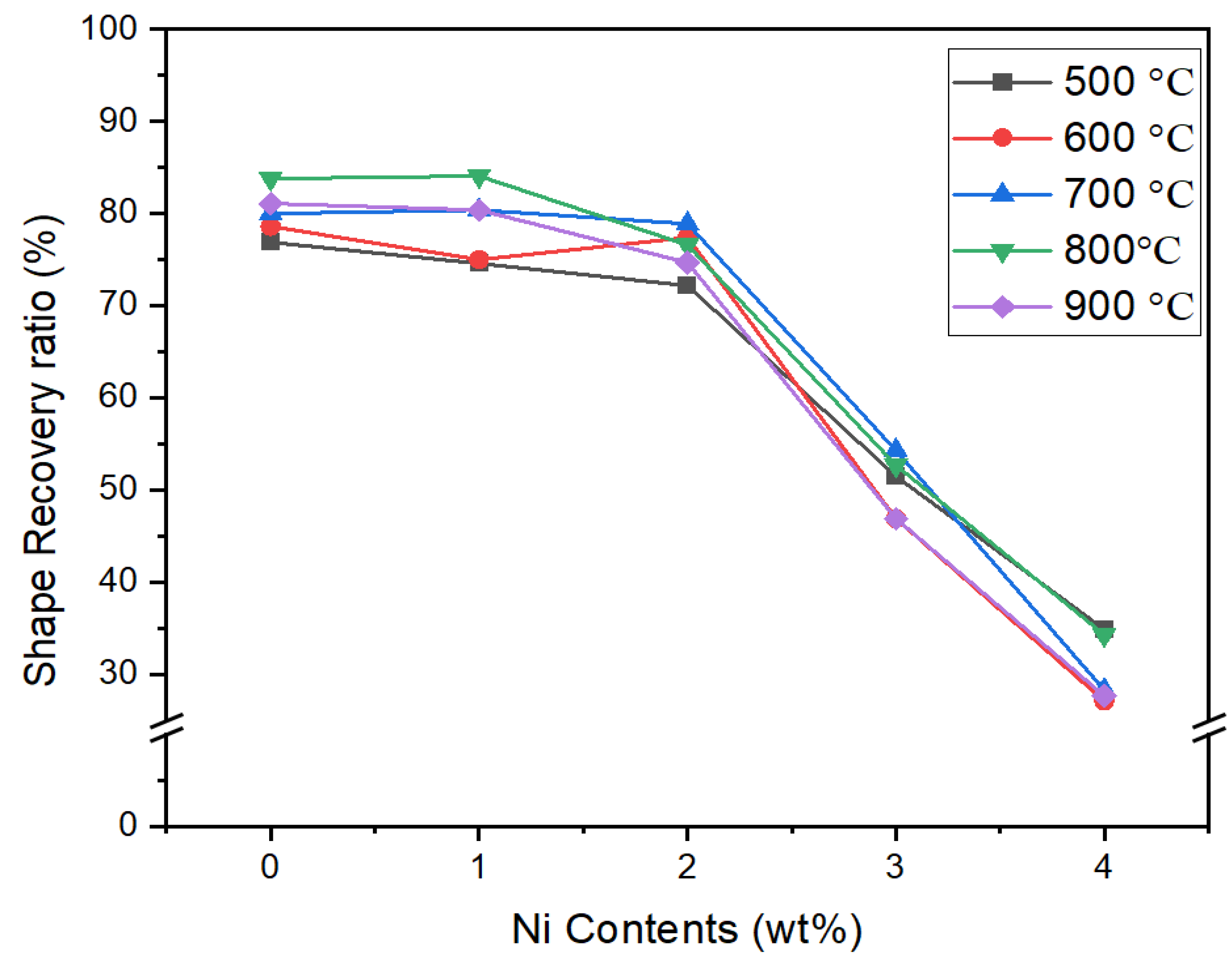
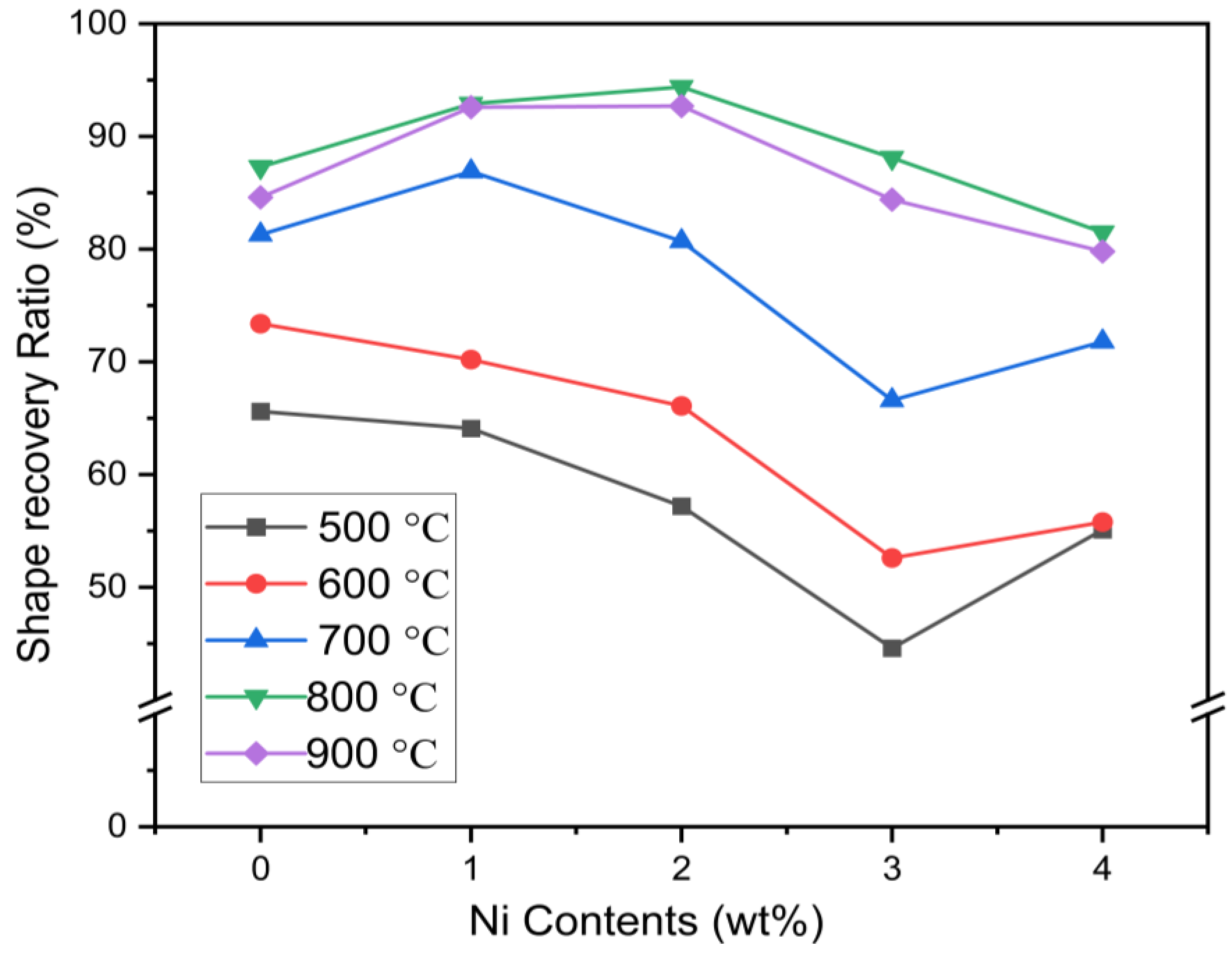
2.2. Shape Memory Effects
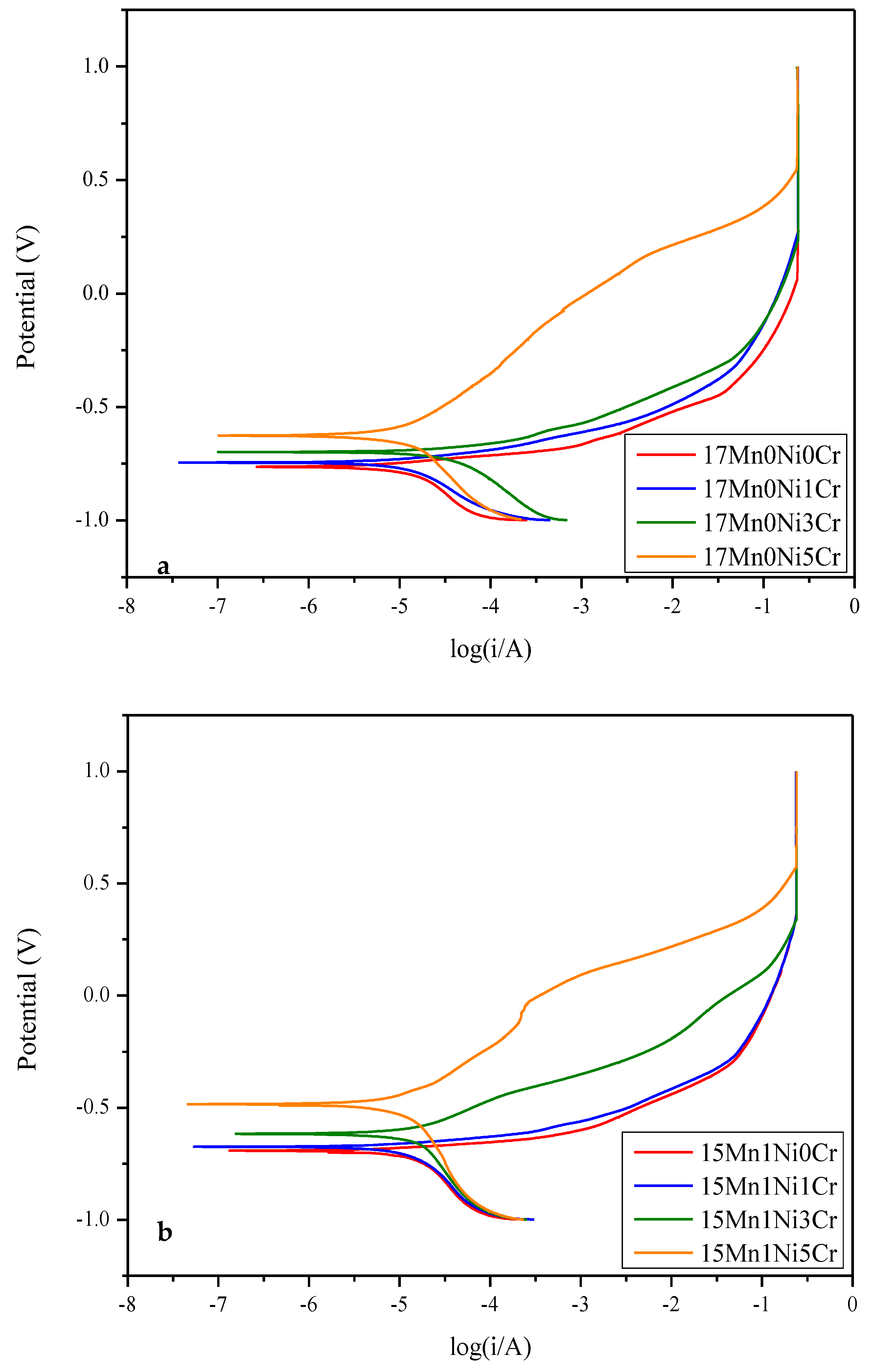
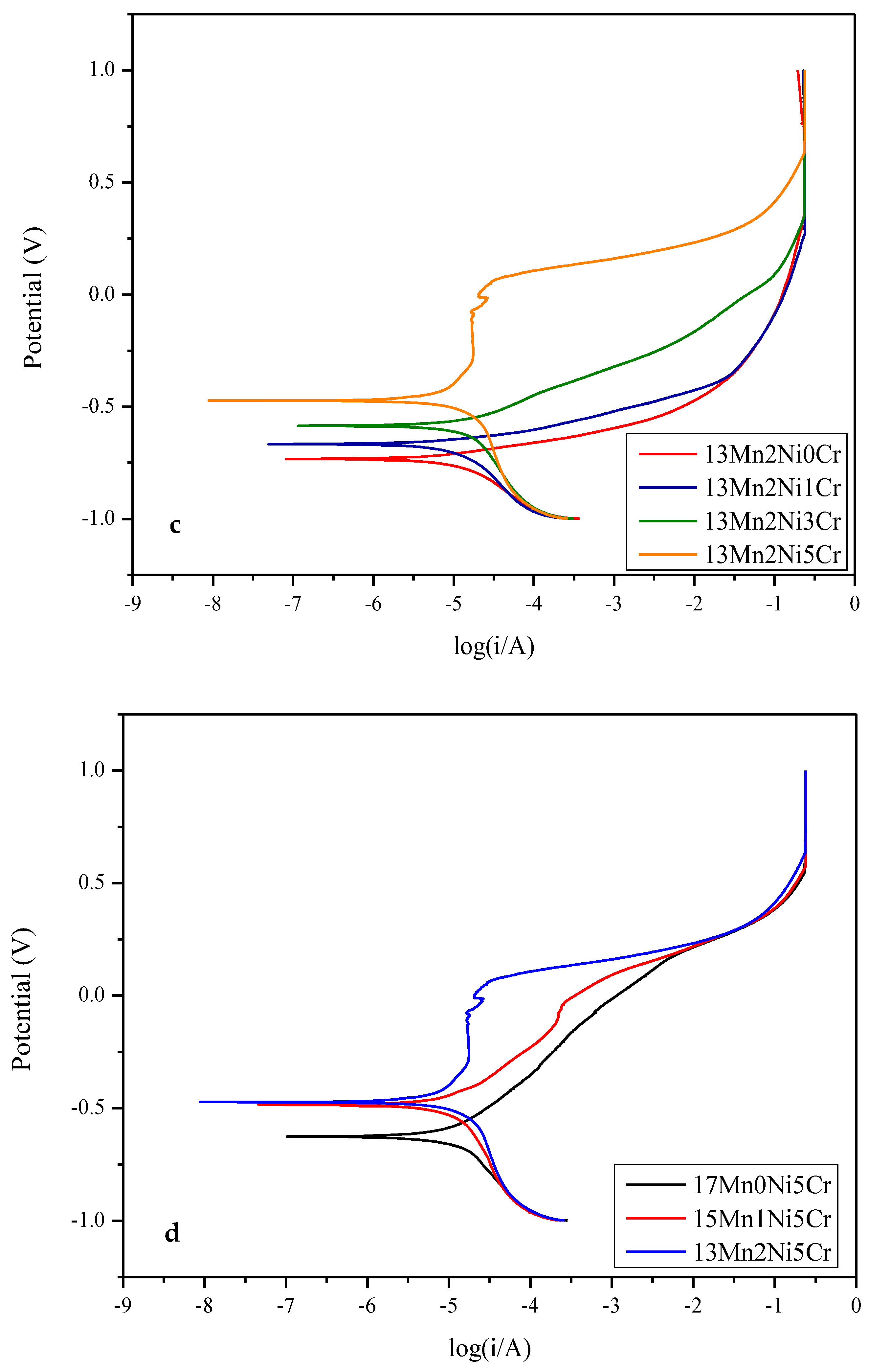
2.3. Electrochemical Corrosion
3. Experimental Method and Process
3.1. Alloy Melting and Preparation
3.2. Microstructural Analysis
3.3. Corrosion Analysis
3.4. Shape Recovery Rate Measurement
4. Conclusions
- 1.
- The addition of Ni and Cr has successfully reduced the Mn contents to 13 wt%, and shape memory performance is still better than the 2% recoverable strain required for engineering applications.
- 2.
- The addition of Cr to each series of alloys can effectively improve the stability of the parent γ phase, and the shape memory effect of the alloy in the solid solution state is reduced.
- 3.
- Each series of alloys has the greatest shape memory effect at 800 °C. The shape recovery ratio is 88.3% for 17Mn0Ni3Cr, 94.0% for 15Mn1Ni3Cr, 94.4% for 13Mn2Ni5Cr, 88.1% for 11Mn3Ni5Cr, and 86.8% for 9Mn4Ni7Cr.
- 4.
- The addition of Cr and aging heat treatments at 600 °C~800 °C generates carbides similar to M23C6 and M7C3, which consequently improves the shape memory effects in the alloys.
- 5.
- The results of the electrochemical corrosion test of this alloy system in 3.5 wt% NaCl show that the corrosion resistance of the alloy increases with the increase in Cr content; however, when the content is higher than 5Cr, pitting corrosion will occur in each alloy.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Es-Souni, M.; Es-Souni, M.; Fischer-Brandies, H. Assessing the Biocompatibility of NiTi Shape Memory Alloys Used for Medical Applications. Anal. Bioanal. Chem. 2005, 381, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.S.M.; Madhu, H.C.; Perugu, C.S.; Akash, K.; Mithun, R.; Kumar, P.A.; Kailas, S.V.; Anbarasu, M.; Palani, I.A. Shape Memory Effect, Temperature Distribution and Mechanical Properties of Friction Stir Welded Nitinol. J. Alloys Compd. 2019, 776, 334–345. [Google Scholar] [CrossRef]
- Shiva, S.; Palani, I.A.; Mishra, S.K.; Paul, C.P.; Kukreja, L.M. Investigations on the Influence of Composition in the Development of Ni–Ti Shape Memory Alloy Using Laser Based Additive Manufacturing. Opt. Laser Technol. 2015, 69, 44–51. [Google Scholar] [CrossRef]
- Farber, E.; Zhu, J.-N.; Popovich, A.; Popovich, V. A Review of NiTi Shape Memory Alloy as a Smart Material Produced by Additive Manufacturing. Mater. Today Proc. 2020, 30, 761–767. [Google Scholar] [CrossRef]
- Pan, M.-M.; Zhang, X.-M.; Zhou, D.; Misra, R.D.K.; Chen, P. Fe–Mn–Si–Cr–Ni Based Shape Memory Alloy: Thermal and Stress-Induced Martensite. Mater. Sci. Eng. A 2020, 797, 140107. [Google Scholar] [CrossRef]
- Michiardi, A.; Aparicio, C.; Planell, J.A.; Gil, F.J. New Oxidation Treatment of NiTi Shape Memory Alloys to Obtain Ni-Free Surfaces and to Improve Biocompatibility. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77B, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.S.; Canejo, J.P.H.G.; Martins, R.M.S.; Braz Fernandes, F.M. Effect of Thermal Cycling on the Transformation Temperature Ranges of a Ni–Ti Shape Memory Alloy. Mater. Sci. Eng. A 2004, 378, 92–96. [Google Scholar] [CrossRef]
- Ruiz-Pinilla, J.G.; Montoya-Coronado, L.A.; Ribas, C.; Cladera, A. Finite Element Modeling of RC Beams Externally Strengthened with Iron-Based Shape Memory Alloy (Fe-SMA) Strips, Including Analytical Stress-Strain Curves for Fe-SMA. Eng. Struct. 2020, 223, 111152. [Google Scholar] [CrossRef]
- Vollmer, M.; Bauer, A.; Kriegel, M.J.; Motylenko, M.; Niendorf, T. Functionally Graded Structures Realized Based on Fe–Mn–Al–Ni Shape Memory Alloys. Scr. Mater. 2021, 194, 113619. [Google Scholar] [CrossRef]
- Alaneme, K.K.; Okotete, E.A.; Anaele, J.U. Structural Vibration Mitigation—a Concise Review of the Capabilities and Applications of Cu and Fe Based Shape Memory Alloys in Civil Structures. J. Build. Eng. 2019, 22, 22–32. [Google Scholar] [CrossRef]
- Izadi, M.; Motavalli, M.; Ghafoori, E. Iron-Based Shape Memory Alloy (Fe-SMA) for Fatigue Strengthening of Cracked Steel Bridge Connections. Constr. Build. Mater. 2019, 227, 116800. [Google Scholar] [CrossRef]
- Kikuchi, T.; Kajiwara, S. Shape Memory Effect in an Unausaged Fe-Ni-Co-Ti Alloy. In Ecomaterials; Elsevier: Amsterdam, The Netherlands, 1994; pp. 989–992. [Google Scholar]
- Fritsch, E.; Izadi, M.; Ghafoori, E. Development of Nail-Anchor Strengthening System with Iron-Based Shape Memory Alloy (Fe-SMA) Strips. Constr. Build. Mater. 2019, 229, 117042. [Google Scholar] [CrossRef]
- Adarsh, S.H.; Sampath, V. Prediction of High Temperature Deformation Characteristics of an Fe-Based Shape Memory Alloy Using Constitutive and Artificial Neural Network Modelling. Mater. Today Commun. 2020, 22, 100841. [Google Scholar] [CrossRef]
- Zambrano, O.A.; Logé, R.E. Dynamic Recrystallization Study of a Fe-Mn-Si Based Shape Memory Alloy in Constant and Variable Thermomechanical Conditions. Mater. Charact. 2019, 152, 151–161. [Google Scholar] [CrossRef]
- Hallab, N.J.; Vermes, C.; Messina, C.; Roebuck, K.A.; Glant, T.T.; Jacobs, J.J. Concentration- and Composition-Dependent Effects of Metal Ions on Human MG-63 Osteoblasts. J. Biomed. Mater. Res. 2002, 60, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, J.; Zhong, M.; Xiang, W.; Wu, Z. Martensitic Transformation Behaviour and Mechanical Property of Dual-Phase Ni-Co-Mn-Sn-Fe Ferromagnetic Shape Memory Alloys. J. Magn. Magn. Mater. 2021, 521, 167540. [Google Scholar] [CrossRef]
- Walnsch, A.; Kriegel, M.J.; Motylenko, M.; Korpala, G.; Prahl, U.; Leineweber, A. Thermodynamics of Martensite Formation in Fe–Mn–Al–Ni Shape Memory Alloys. Scr. Mater. 2021, 192, 26–31. [Google Scholar] [CrossRef]
- Min, X.H.; Sawaguchi, T.; Ogawa, K.; Maruyama, T.; Yin, F.X.; Tsuzaki, K. Shape Memory Effect in Fe–Mn–Ni–Si–C Alloys with Low Mn Contents. Mater. Sci. Eng. A 2011, 528, 5251–5258. [Google Scholar] [CrossRef]
- Min, X.H.; Sawaguchi, T.; Ogawa, K.; Maruyama, T.; Yin, F.X.; Tsuzaki, K. An Attempt to Lower Mn Content of Fe–17Mn–6Si–0.3C Shape Memory Alloy. J. Alloys Compd. 2013, 577, S478–S482. [Google Scholar] [CrossRef]
- Chen, J.; Peng, H.B.; Yang, Q.; Wang, S.L.; Song, F.; Wen, Y.H. Effect of Carbon Content on Shape Memory Effect of Fe-Mn-Si-Cr-Ni-Based Alloys at Different Deformation Temperatures. Mater. Sci. Eng. A 2016, 677, 133–139. [Google Scholar] [CrossRef]
- Malamud, F.; Guerrero, L.M.; La Roca, P.; Sade, M.; Baruj, A. Role of Mn and Cr on Structural Parameters and Strain Energy during FCC-HCP Martensitic Transformation in Fe-Mn-Cr Shape Memory Alloys. Mater. Des. 2018, 139, 314–323. [Google Scholar] [CrossRef]
- Guerrero, L.M.; La Roca, P.; Malamud, F.; Baruj, A.; Sade, M. A Short Review on the Effect of Cr on the Fcc–Hcp Phase Transition in Fe–Mn-Based Alloys. Shape Mem. Superelasticity 2020, 6, 202–212. [Google Scholar] [CrossRef]
- Dias, D.; Nakamatsu, S.; Della Rovere, C.A.; Otubo, J.; Mariano, N.A. Characterization and Corrosion Resistance Behavior of Shape Memory Stainless Steel Developed by Alternate Routes. Metals 2019, 10, 13. [Google Scholar] [CrossRef]
- Otsuka, H.; Yamada, H.; Maruyama, T.; Tanahashi, H.; Matsuda, S.; Murakami, M. Effects of Alloying Additions on Fe-Mn-Si Shape Memory Alloys. ISIJ Int. 1990, 30, 674–679. [Google Scholar] [CrossRef]
- Hsu, T.Y. Fe-Mn-Si Based Shape Memory Alloys. Mater. Sci. Forum 2000, 327–328, 199–206. [Google Scholar] [CrossRef]
- Saito, T.; Kapusta, C.; Takasaki, A. Synthesis and Characterization of Fe–Mn–Si Shape Memory Alloy by Mechanical Alloying and Subsequent Sintering. Mater. Sci. Eng. A 2014, 592, 88–94. [Google Scholar] [CrossRef]
- Li, C.L.; Cheng, D.J.; Jin, Z.H. Influence of Deformation Temperature on Shape Memory Effect of Fe–Mn–Si–Ni–Cr Alloy. Mater. Sci. Eng. A 2002, 325, 375–379. [Google Scholar] [CrossRef]
- Aksu Canbay, C.; Karagoz, Z. The Effect of Quaternary Element on the Thermodynamic Parameters and Structure of CuAlMn Shape Memory Alloys. Appl. Phys. A 2013, 113, 19–25. [Google Scholar] [CrossRef]
- Liu, A.; Meng, X.; Cai, W.; Zhao, L.C. Effect of Ce Addition on Martensitic Transformation Behavior of TiNi Shape Memory Alloys. Mater. Sci. Forum 2005, 475–479, 1973–1976. [Google Scholar] [CrossRef]
- Shen, Y.; Sun, W.; Wei, Z.; Li, J.; Guo, J.; Wang, K.; Zhang, Y.; Liu, J. Influence of Microstructure on Elastocaloric and Shape Memory Effects in Mn50Ni32Sn7Co11 Alloys. J. Alloys Compd. 2020, 832, 154830. [Google Scholar] [CrossRef]
- Koster, M.; Lee, W.J.; Schwarzenberger, M.; Leinenbach, C. Cyclic Deformation and Structural Fatigue Behavior of an FE–Mn–Si Shape Memory Alloy. Mater. Sci. Eng. A 2015, 637, 29–39. [Google Scholar] [CrossRef]
- Alcantar-Mondragón, N.; Reyes-Calderón, F.; García-García, V.; Vázquez-Gómez, O.; Salgado-López, J.M. Effect of PWHT on the Dissolution of δ-Ferrite in the Welded Joint of 12Cr–1Mo Steels for Steam Turbines. J. Mater. Res. Technol. 2021, 10, 1262–1279. [Google Scholar] [CrossRef]
- Łukaszczyk, A.; Banaś, J.; Pisarek, M.; Seyeux, A.; Marcus, P.; Światowska, J. Effect of Cr Content on Corrosion Resistance of Low-Cr Alloy Steels Studied by Surface and Electrochemical Techniques. Electrochem 2021, 2, 546–562. [Google Scholar] [CrossRef]
- Zheng, Z.; Chen, S.; Long, J.; Zheng, K.; Wang, H.; Li, H. Effect of Chromium Content on the Erosion-Corrosion Behavior of Fe-Cr Alloy Produced by Ball Milling Liner in Weakly Alkaline Slurry. Mater. Res. Express 2020, 7, 036510. [Google Scholar] [CrossRef]
- Abbas, A.; Hsu, J.-W.; Naqvi, S.M.A.; Lin, H.-C.; Lin, K.-M. Effects of Ti and Nb Additions on Microstructure and Shape Memory Effect of Fe-10Mn-6Si-4Ni-7Cr-0.3C Shape Memory Alloy. JOM 2024. [Google Scholar] [CrossRef]
- Alharthi, N.; Sherif, E.-S.M.; Abdo, H.S.; Zein El Abedin, S. Effect of Nickel Content on the Corrosion Resistance of Iron-Nickel Alloys in Concentrated Hydrochloric Acid Pickling Solutions. Adv. Mater. Sci. Eng. 2017, 2017, 1–8. [Google Scholar] [CrossRef]





| Alloy | Fe | Mn | Si | Cr | Ni | C |
|---|---|---|---|---|---|---|
| 17Mn0Ni0Cr | bal. | 17.3 | 5.58 | - | - | 0.3 |
| 17Mn0Ni1Cr | bal. | 17.3 | 5.51 | 1.03 | - | * |
| 17Mn0Ni3Cr | bal. | 16.8 | 5.47 | 3.03 | - | * |
| 17Mn0Ni5Cr | bal. | 17.2 | 5.62 | 5.08 | - | * |
| 15Mn0Ni0Cr | bal. | 15.2 | 5.56 | - | 1.20 | 0.3 |
| 15Mn1Ni1Cr | bal. | 14.8 | 5.63 | 1.03 | 1.03 | * |
| 15Mn1Ni3Cr | bal. | 14.7 | 5.63 | 3.03 | 1.01 | * |
| 15Mn1Ni5Cr | bal. | 14.7 | 5.62 | 5.04 | 1.01 | * |
| 13Mn2Ni0Cr | bal. | 13.1 | 5.79 | - | 2.06 | 0.3 |
| 13Mn2Ni1Cr | bal. | 13.0 | 5.60 | 1.04 | 2.04 | * |
| 13Mn2Ni3Cr | bal. | 13.1 | 5.42 | 3.06 | 2.04 | * |
| 13Mn2Ni5Cr | bal. | 12.8 | 5.63 | 5.05 | 2.01 | * |
| 11Mn3Ni0Cr | bal. | 10.6 | 5.55 | - | 3.04 | 0.3 |
| 11Mn3Ni1Cr | bal. | 10.6 | 5.55 | 1.03 | 3.05 | * |
| 11Mn3Ni3Cr | bal. | 10.7 | 5.70 | 3.06 | 3.02 | * |
| 11Mn3Ni5Cr | bal. | 10.2 | 5.54 | 4.82 | 2.98 | * |
| 9Mn4Ni0Cr | bal. | 8.97 | 5.60 | - | 3.88 | 0.3 |
| 9Mn4Ni5Cr | bal. | 8.73 | 5.56 | 5.03 | 3.98 | * |
| 9Mn4Ni7Cr | bal. | 8.72 | 5.48 | 6.96 | 4.02 | * |
| Alloy | Solid Solution Treatment | 500 °C | 600 °C | 700 °C | 800 °C | 900 °C |
|---|---|---|---|---|---|---|
| 17Mn0Ni0Cr | 74.5 | 76.9 | 78.6 | 80.0 | 83.8 | 81.1 |
| 17Mn0Ni1Cr | 72.2 | 75.4 | 78.4 | 85.0 | 86.6 | 82.6 |
| 17Mn0Ni3Cr | 65.9 | 68.3 | 75.7 | 83.6 | 88.3 | 82.9 |
| 17Mn0Ni5Cr | 59.8 | 65.6 | 73.4 | 81.3 | 87.3 | 84.6 |
| 15Mn1Ni0Cr | 72.9 | 74.6 | 75.0 | 80.4 | 84.1 | 80.4 |
| 15Mn1Ni1Cr | 74.1 | 75.9 | 81.6 | 91.0 | 90.3 | 82.2 |
| 15Mn1Ni3Cr | 61.8 | 75.8 | 78.1 | 91.6 | 94.0 | 83.3 |
| 15Mn1Ni5Cr | 57.6 | 64.1 | 70.2 | 86.9 | 92.9 | 92.6 |
| 13Mn2Ni0Cr | 71.0 | 72.2 | 77.4 | 78.9 | 76.6 | 74.7 |
| 13Mn2Ni1Cr | 71.3 | 71.4 | 73.6 | 82.0 | 85.9 | 79.4 |
| 13Mn2Ni3Cr | 70.4 | 73.1 | 76.4 | 90.4 | 91.4 | 81.3 |
| 13Mn2Ni5Cr | 53.9 | 57.2 | 66.1 | 80.7 | 94.4 | 92.7 |
| 11Mn3Ni0Cr | 53.5 | 51.5 | 46.9 | 54.3 | 52.7 | 46.9 |
| 11Mn3Ni1Cr | 61.2 | 59.9 | 65.4 | 66.1 | 66.7 | 65.5 |
| 11Mn3Ni3Cr | 64.1 | 63.2 | 72.5 | 76.3 | 74.2 | 70.9 |
| 11Mn3Ni5Cr | 47.3 | 44.6 | 52.6 | 66.6 | 88.1 | 84.4 |
| 9Mn4Ni0Cr | 34.8 | 34.9 | 27.1 | 28.3 | 34.3 | 27.7 |
| 9Mn4Ni5Cr | 56.3 | 55.1 | 55.8 | 71.8 | 81.5 | 79.8 |
| 9Mn4Ni7Cr | 48.1 | 37.5 | 48.1 | 61.6 | 86.8 | 83.6 |
| Alloy | Ms | As |
|---|---|---|
| 17Mn0Ni0Cr | −49.5 °C | 198.2 °C |
| 15Mn1Ni0Cr | −12.4 °C | 188.4 °C |
| 15Mn1Ni1Cr | −15.7 °C | 111.3 °C |
| 15Mn1Ni3Cr | −17.9 °C | 110.4 °C |
| 15Mn1Ni5Cr | −18.7 °C | 109.7 °C |
| 13Mn2Ni0Cr | −10.5 °C | 183.5 °C |
| 11Mn3Ni0Cr | −8.5 °C | 177.5 °C |
| 9Mn4Ni0Cr | −3.6 °C | 110.9 °C |
| Alloy | Ecorr (V) | Icorr (A) |
|---|---|---|
| 17Mn0Ni0Cr | −0.765 | 2.617 × 10−5 |
| 17Mn0Ni1Cr | −0.746 | 1.557 10−5 |
| 17Mn0Ni3Cr | −0.700 | 1.384 10−5 |
| 17Mn0Ni5Cr | −0.627 | 1.266 10−5 |
| 15Mn1Ni0Cr | −0.693 | 2.351 10−5 |
| 15Mn1Ni1Cr | −0.675 | 1.289 × 10−5 |
| 15Mn1Ni3Cr | −0.617 | 1.265 × 10−5 |
| 15Mn1Ni5Cr | −0.485 | 1.236 × 10−5 |
| 13Mn2Ni0Cr | −0.734 | 1.454 10−5 |
| 13Mn2Ni1Cr | −0.667 | 1.275 10−5 |
| 13Mn2Ni3Cr | −0.586 | 1.247 10−5 |
| 13Mn2Ni5Cr | −0.474 | 1.231 10−5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, A.; Chang, K.-C.; Lin, K.-M.; Lin, H.-C. Investigating the Shape Memory Effect and Corrosion Resistance of the Fe-(17-2x) Mn-6Si-xNi-yCr-0.3C Alloys (x = 0, 1, 2, 3, 4; y = 0, 1, 3, 5). Inorganics 2024, 12, 262. https://doi.org/10.3390/inorganics12100262
Abbas A, Chang K-C, Lin K-M, Lin H-C. Investigating the Shape Memory Effect and Corrosion Resistance of the Fe-(17-2x) Mn-6Si-xNi-yCr-0.3C Alloys (x = 0, 1, 2, 3, 4; y = 0, 1, 3, 5). Inorganics. 2024; 12(10):262. https://doi.org/10.3390/inorganics12100262
Chicago/Turabian StyleAbbas, Aqeel, Kai-Cheng Chang, Kun-Ming Lin, and Hsin-Chih Lin. 2024. "Investigating the Shape Memory Effect and Corrosion Resistance of the Fe-(17-2x) Mn-6Si-xNi-yCr-0.3C Alloys (x = 0, 1, 2, 3, 4; y = 0, 1, 3, 5)" Inorganics 12, no. 10: 262. https://doi.org/10.3390/inorganics12100262
APA StyleAbbas, A., Chang, K.-C., Lin, K.-M., & Lin, H.-C. (2024). Investigating the Shape Memory Effect and Corrosion Resistance of the Fe-(17-2x) Mn-6Si-xNi-yCr-0.3C Alloys (x = 0, 1, 2, 3, 4; y = 0, 1, 3, 5). Inorganics, 12(10), 262. https://doi.org/10.3390/inorganics12100262






