A Novel TiO2-Cuttlebone Photocatalyst for Highly Efficient Catalytic Degradation of Tetracycline Hydrochloride
Abstract
1. Introduction
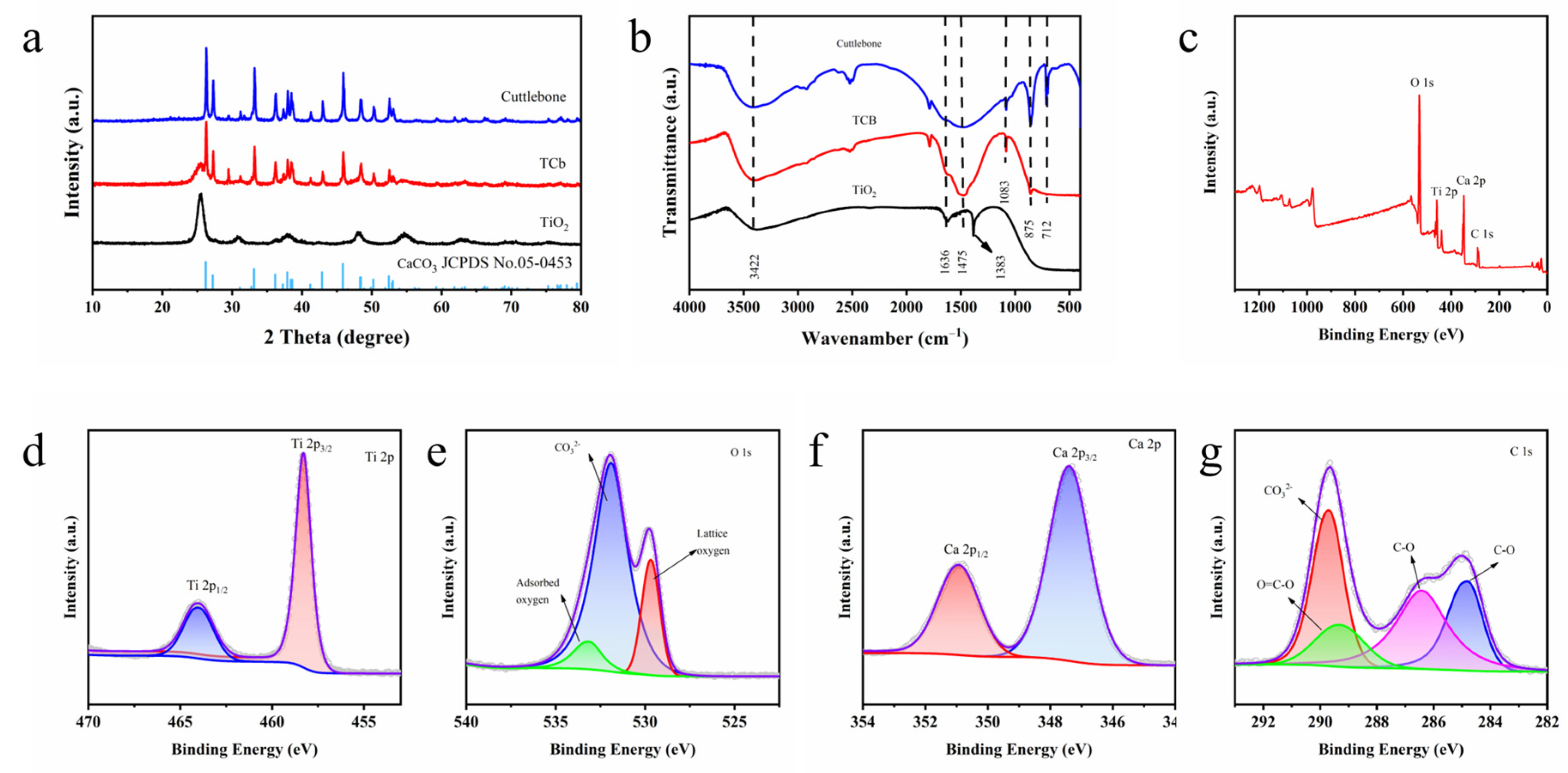
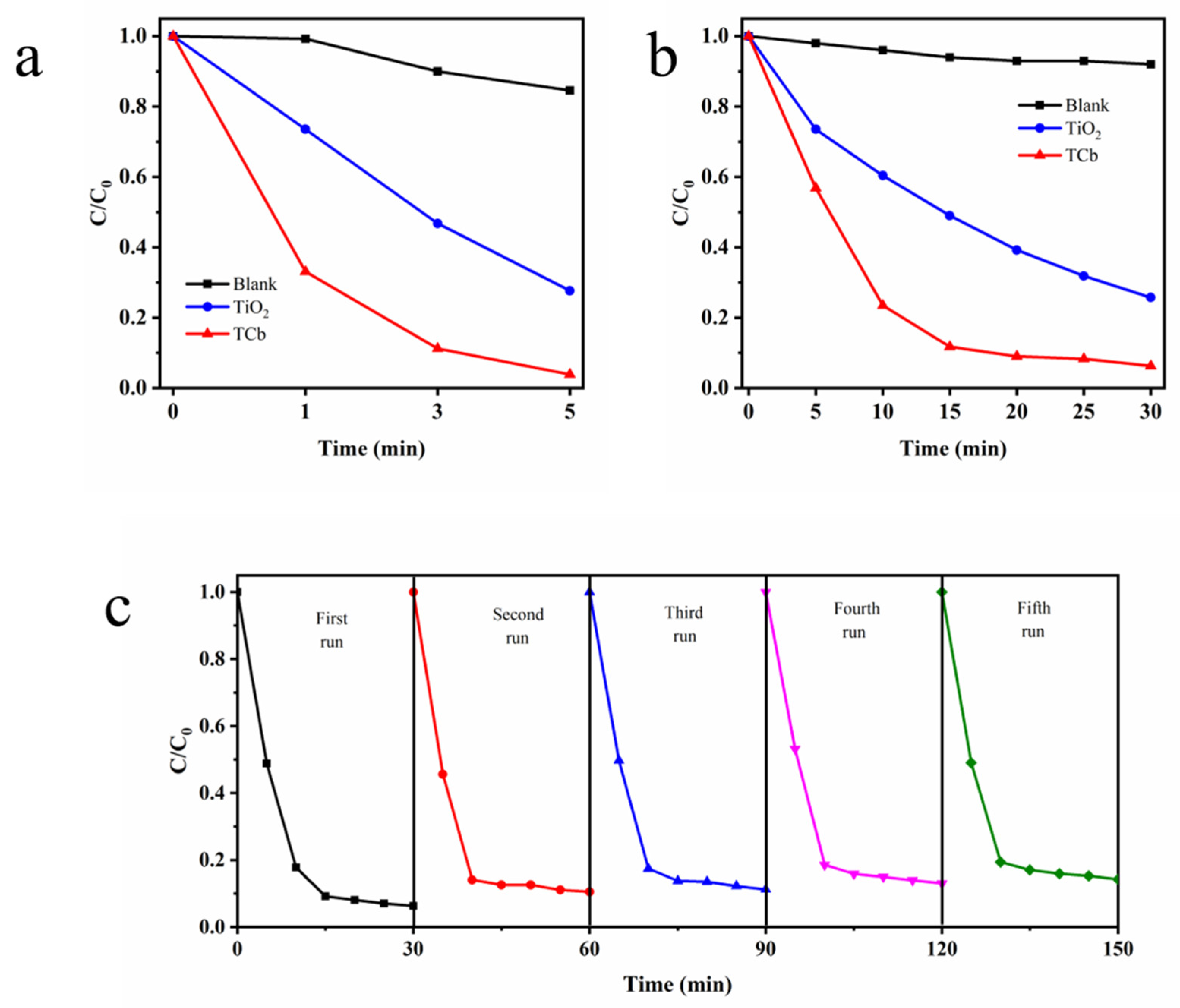
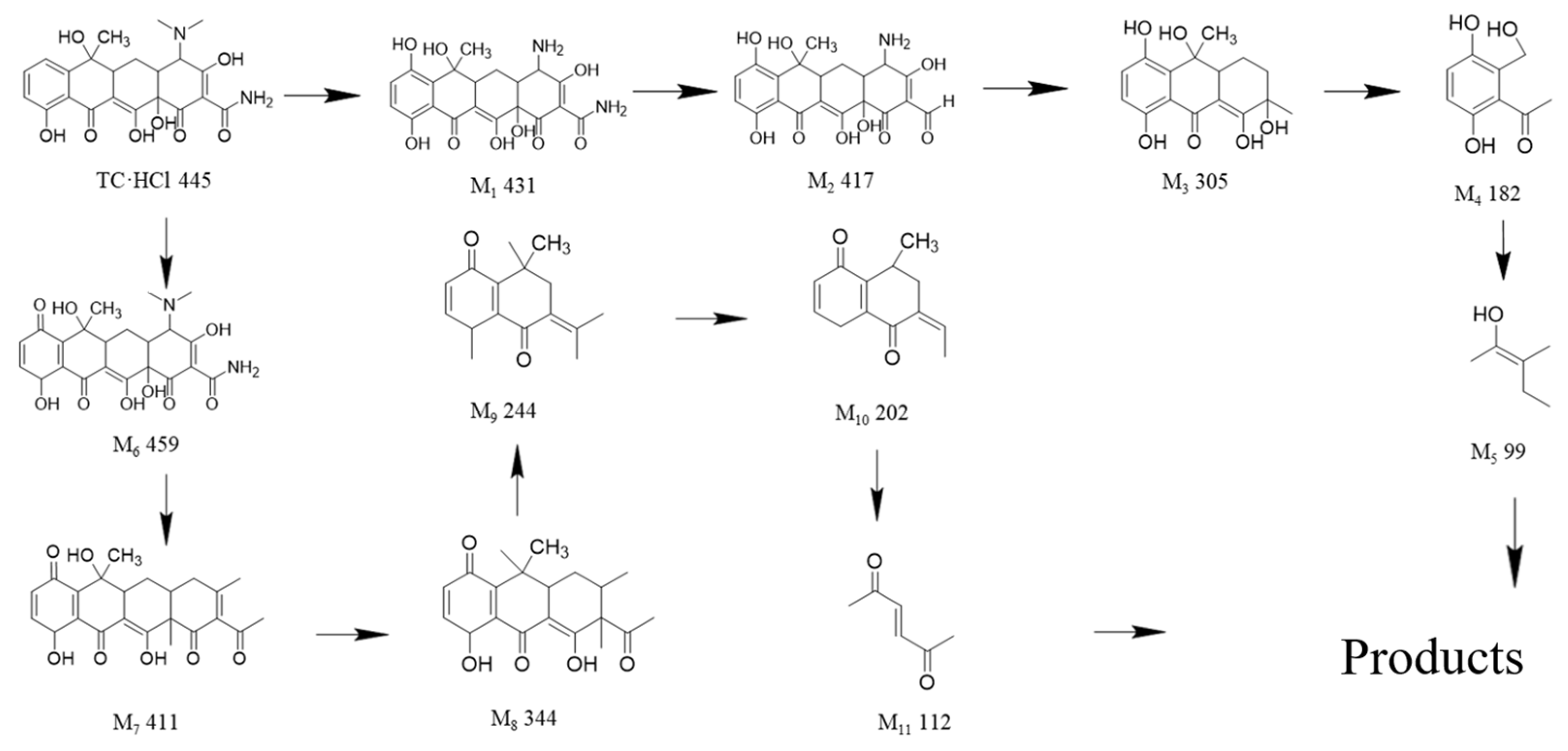
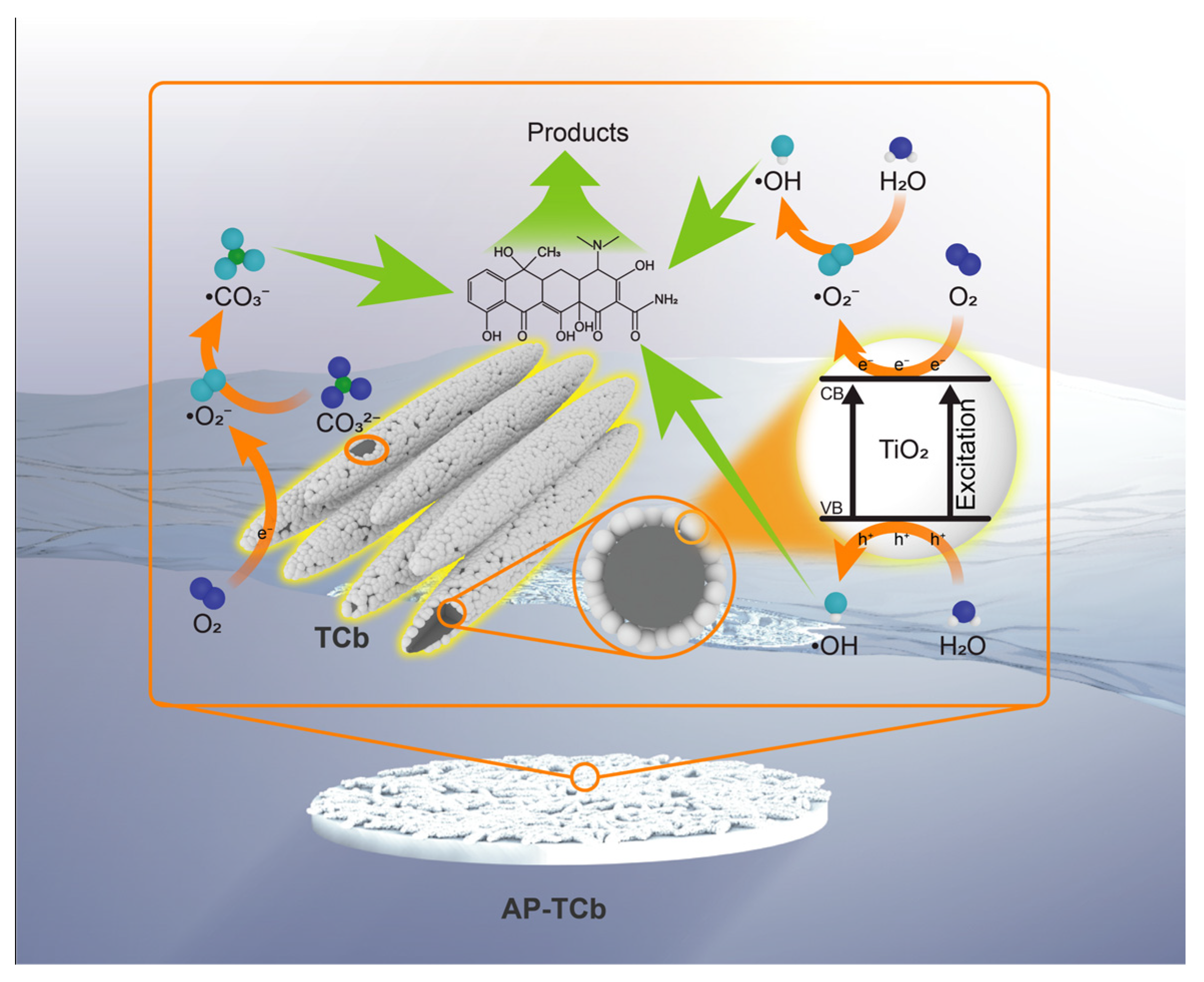
2. Results and Discussion
3. Methods and Materials
3.1. Chemical and Reagents
3.2. Synthesis Process
3.3. Characterization and Analytical Methods
3.4. Photochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kafaei, R.; Papari, F.; Seyedabadi, M.; Sahebi, S.; Tahmasebi, R.; Ahmadi, M.; Sorial, G.A.; Asgari, G.; Ramavandi, B. Occurrence, distribution, and potential sources of antibiotics pollution in the water-sediment of the northern coastline of the persian gulf, Iran. Sci. Total Environ. 2018, 627, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Gambino, D.; Savoca, D.; Sucato, A.; Gargano, V.; Gentile, A.; Pantano, L.; Vicari, D.; Alduina, R. Occurrence of antibiotic resistance in the mediterranean sea. Antibiotics 2022, 11, 332. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Xu, X.F.; Zhang, Z.L.; Ding, Y.Z.; Zhang, K.Q.; Zhi, S.L. A Comprehensive Contamination Investigation of Bohai Bay Seawater: Antibiotics Occurrence, Distribution, Ecological Risks and Their Interactive Factors. Int. J. Environ. Res. Public Health 2023, 20, 1599. [Google Scholar] [CrossRef] [PubMed]
- Qian, G.S.; Shao, J.Y.; Hu, P.; Tang, W.T.; Xiao, Y.H.; Hao, T.W. From micro to macro: The role of seawater in maintaining structural integrity and bioactivity of granules in treating antibiotic-laden mariculture wastewater. Water Res. 2023, 246, 120702. [Google Scholar] [CrossRef]
- Chen, Y.C.; Liu, J.T.; Zeng, Q.B.; Liang, Z.X.; Ye, X.X.; Lv, Y.C.; Liu, M.H. Preparation of Eucommia ulmoides lignin-based high-performance biochar containing sulfonic group: Synergistic pyrolysis mechanism and tetracycline hydrochloride adsorption. Bioresour. Technol. 2021, 329, 124432. [Google Scholar] [CrossRef]
- Ni, J.W.; Wang, W.; Liu, D.M.; Zhu, Q.; Jia, J.L.; Tian, J.Y.; Li, Z.Y.; Wang, X.; Xing, Z.P. Oxygen vacancy-mediated sandwich-structural TiO2−x/ultrathin g-C3N4/TiO2−x direct Z-scheme heterojunction visible-light-driven photocatalyst for efficient removal of high toxic tetracycline antibiotics. J. Hazard. Mater. 2021, 408, 124432. [Google Scholar] [CrossRef]
- Yang, H.C.; Yu, H.; Wang, J.H.; Ning, T.; Chen, P.; Yu, J.; Di, S.Y.; Zhu, S.K. Magnetic porous biochar as a renewable and highly effective adsorbent for the removal of tetracycline hydrochloride in water. Environ. Sci. Pollut. Res. 2021, 28, 61513–61525. [Google Scholar] [CrossRef]
- Song, X.L.; He, J.L.; Wang, Y.; Wang, J.L.; Zhang, S.W. A novel MIL-125(Ti)-based nanocomposite for enhanced adsorption and catalytic degradation of tetracycline hydrochloride: Synergetic mechanism of calcination and the nitrogen-containing reticulated surface layer. J. Colloid Interface Sci. 2023, 645, 918–932. [Google Scholar] [CrossRef]
- Bhoyar, T.; Vidyasagar, D.; Umare, S.S. Mitigating phytotoxicity of tetracycline by metal-free 8-hydroxyquinoline functionalized carbon nitride photocatalyst. J. Environ. Sci. 2023, 125, 37–46. [Google Scholar] [CrossRef]
- Li, H.B.; Li, H.J.; Zhou, Z.N.; Tong, H.; Long, B.; Liu, W.; Li, W.H. Tailoring hydrophily and composition of BiOI for an ultrafast photodegradation of tetracycline hydrochloride. J. Environ. Chem. Eng. 2021, 9, 106292. [Google Scholar] [CrossRef]
- Yu, G.L.; Yang, K.; Yang, Y.; Li, Y.F.; Sun, Q.F.; Li, P.Y.; Wang, W.M.; Song, F.M.; Ling, T.; Peng, X.J.; et al. Efficient removal of tetracycline hydrochloride through novel Fe/BiOBr/Bi2WO6 photocatalyst prepared by dual-strategy under visible-light irradiation. J. Environ. Sci. 2024, 138, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.T.; Yuan, X.P.; Niu, M.M.; Li, Y.J.; Wang, L.P.; Zhang, M.Y. In situ composite of Co-MOF on a Ti-based material for visible light multiphase catalysis: Synthesis and the photocatalytic degradation mechanism. New J. Chem. 2022, 46, 11341–11349. [Google Scholar] [CrossRef]
- Chen, M.G.; Chang, W.Y.; Zhang, J.; Zhao, W.; Chen, Z. Preparation of a Hybrid TiO2 and 1T/2H-MoS2 Photocatalyst for the Degradation of Tetracycline Hydrochloride. Acs Omega 2023, 8, 15458–15466. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, H.M.; Long, M.Y.; Bai, X.J.; Zhao, Q.Q.; Wen, Q.; Song, F. Synergetic effect of photocatalysis and peroxymonosulfate activation by MIL-53Fe@TiO2 on efficient degradation of tetracycline hydrochloride under visible light irradiation. Crystengcomm 2022, 24, 4283–4293. [Google Scholar] [CrossRef]
- Han, W.M.; Wu, T.; Wu, Q.S. Fabrication of WO3/Bi2MoO6 heterostructures with efficient and highly selective photocatalytic degradation of tetracycline hydrochloride. J. Colloid Interface Sci. 2021, 602, 544–552. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, L.X.; Liu, F.Y.; Jia, M.; Liu, M.; Li, J.; Lai, Y.Q. Enhanced photoelectrochemical degradation of tetracycline hydrochloride with FeOOH and Au nanoparticles decorated WO3. Chem. Eng. J. 2021, 407, 127195. [Google Scholar] [CrossRef]
- Liang, J.C.; Li, X.Q.; Zuo, J.L.; Lin, J.; Liu, Z.L. Hybrid 0D/2D heterostructures: In-situ growth of 0D g-C3N4 on 2D BiOI for efficient photocatalyst. Adv. Compos. Hybrid Mater. 2021, 4, 1122–1136. [Google Scholar] [CrossRef]
- Liu, S.Y.; Zada, A.; Yu, X.Y.; Liu, F.Z.; Jin, G. NiFe2O4/g-C3N4 heterostructure with an enhanced ability for photocatalytic degradation of tetracycline hydrochloride and antibacterial performance. Chemosphere 2022, 307, 135717. [Google Scholar] [CrossRef]
- Wang, J.Q.; Qian, Q.R.; Chen, Q.H.; Liu, X.P.; Luo, Y.J.; Xue, H.; Li, Z.H. Significant role of carbonate radicals in tetracycline hydrochloride degradation based on solar light-driven TiO2-seashell composites: Removal and transformation pathways. Chin. J. Catal. 2020, 41, 1511–1521. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Mielczarski, E.; Mielczarski, J.; Laub, D.; Buffat, P.; Klehm, U.; Albers, P.; Lee, K.; Kulik, A.; Kiwi-Minsker, L.; et al. Preparation, stabilization and characterization of TiO2 on thin polyethylene films (LDPE). Photocatalytic applications. Water Res. 2007, 41, 862–874. [Google Scholar]
- Balakrishnan, A.; Appunni, S.; Gopalram, K. Immobilized TiO2/chitosan beads for photocatalytic degradation of 2,4-dichlorophenoxyacetic acid. Int. J. Biol. Macromol. 2020, 161, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Garg, S.; Chandra, A.; Hernadi, K. Immobilization of green BiOX (X = cl, Br and I) photocatalysts on ceramic fibers for enhanced photocatalytic degradation of recalcitrant organic pollutants and efficient regeneration process. Ceram. Int. 2019, 45, 17715–17722. [Google Scholar] [CrossRef]
- Chang, C.J.; Chen, J.K.; Lin, K.S.; Wei, Y.H.; Chao, P.Y.; Huang, C.Y. Enhanced visible-light-driven photocatalytic degradation by metal wire-mesh supported Ag/flower-like Bi2WO6 photocatalysts. J. Alloys Compd. 2020, 813, 152186. [Google Scholar] [CrossRef]
- Martin de Vidales, M.J.; Nieto-Marquez, A.; Morcuende, D.; Atanes, E.; Blaya, F.; Soriano, E.; Fernandez-Martinez, F. 3D printed floating photocatalysts for wastewater treatment. Catal. Today 2019, 328, 157–163. [Google Scholar] [CrossRef]
- Liu, Z.; Xia, X.S.; Li, W.; Xiao, L.R.; Sun, X.L.; Luo, F.B.; Chen, Q.H.; Qian, Q.R. In Situ Growth of Ca2+-Based Metal–Organic Framework on CaSiO3/ABS/TPU 3D Skeleton for Methylene Blue Removal. Materials 2020, 13, 4403. [Google Scholar] [CrossRef]
- Zhao, Z.A.; Mao, J.Y.; Lu, C.Y.; Yang, S.Q.; Qian, Q.R.; Chen, Q.H.; Xue, H.; Sun, X.L.; Yang, M.Q. Design and fabrication of self-suspending aluminum-plastic/semiconductor photocatalyst devices for solar energy conversion. J. Environ. Sci. 2024, 136, 615–625. [Google Scholar] [CrossRef]
- Wu, W.; Song, H.W.; Gan, Q.Q.; Liu, D.X. Decomposition of dimethyl methylphosphonate vapor on ultrathin-film titania photocatalytic light absorber. Chemosphere 2021, 274, 129719. [Google Scholar] [CrossRef]
- Torane, A.P.; Ubale, A.B.; Kanade, K.G.; Pagare, P.K. Photocatalytic dye degradation study of TiO2 material. Mater. Today Proc. 2020, 43, 2738–2741. [Google Scholar] [CrossRef]
- Huang, Z.Q.; Wang, J.Q.; Yang, M.Q.; Qian, Q.R.; Liu, X.P.; Xiao, L.R.; Xue, H. Construction of TiO2-Eggshell for Efficient Degradation of Tetracycline Hydrochloride: Sunlight Induced In-situ Formation of Carbonate Radical. Materials 2021, 14, 1598. [Google Scholar] [CrossRef]
- Xue, H.; Chen, Y.; Liu, X.; Qian, Q.; Luo, Y.; Cui, M.; Chen, Y.; Yang, D.P.; Chen, Q. Visible light-assisted efficient degradation of dye pollutants with biomass-supported TiO2 hybrids. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 197–203. [Google Scholar] [CrossRef]
- Dai, Y.Z.; Zou, H.F.; Zhu, H.F.; Zhou, X.Q.; Song, Y.H.; Zheng, K.Y.; Shi, Z.; Sheng, Y. Facile surfactant- and template-free synthesis and luminescence properties of needle-like calcite CaCO3:Eu3+ phosphors. Crystengcomm 2018, 20, 496–504. [Google Scholar] [CrossRef]
- Li, Q.H.; Dong, M.; Li, R.; Cui, Y.Q.; Xie, G.X.; Wang, X.X.; Long, Y.Z. Enhancement of Cr(VI) removal efficiency via adsorption/photocatalysis synergy using electrospun chitosan/g-C3N4/TiO2 nanofibers. Carbohydr. Polym. 2021, 253, 117200. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.H.; Wu, J.M.; Xiao, J.Z.; Zhao, Y.P.; Dong, W.W.; Fang, X.D. Conformal growth of ZnO on TiO2 nanowire array for enhanced photocatalytic activity. Appl. Surf. Sci. 2013, 279, 324–328. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.X.; Li, Y.Y.; Sun, X.; Xu, L.M.; Huang, W.X. Facile defect construction of TiO2 nanotube for excellent photocatalytic degradation of tetracycline under visible light. J. Photochem. Photobiol. A 2023, 437, 114475. [Google Scholar] [CrossRef]
- Tian, J.L.; Wei, L.X.; Ren, Z.Q.; Lu, J.F.; Ma, J. The facile fabrication of Z-scheme Bi2WO6-P25 heterojunction with enhanced photodegradation of antibiotics under visible light. J. Environ. Chem. Eng. 2021, 9, 106167. [Google Scholar] [CrossRef]
- Liu, H.J.; Hou, M.C.; Fu, H.; Hu, A.J.; Zhai, Y.L.; Wang, L.W.; Zhai, D.; Zhang, S.L.; Wang, S.P. 2D/1D BiOBr/TiO2 flexible nanofibrous film heterojunction photocatalyst for tetracycline degradation. Surf. Interfaces 2024, 44, 103795. [Google Scholar] [CrossRef]
- Li, H.X.; Liang, L.; Niu, X.H.; Zhang, D.Y.; Fan, H.Y.; Wang, K.J. Construction of a Bi2WO6/TiO2 heterojunction and its photocatalytic degradation performance. New J. Chem. 2022, 46, 8185–8194. [Google Scholar] [CrossRef]
- Zhang, X.L.; Han, D.F.; Dai, M.J.; Chen, K.; Han, Z.Y.; Fan, Y.Y.; He, Y.; Han, D.X.; Niu, L. Enhanced photocatalytic degradation of tetracycline by constructing a controllable Cu2O-TiO2 heterojunction with specific crystal facets. Catal. Sci. Technol. 2021, 11, 6248–6256. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, Y.X.; Wang, X.Q.; Zhan, X.Y.; Xu, J.C.; Jin, A.; Hong, B. Sea-Urchin carbon nitride with carbon vacancies (C-v) and oxygen substitution (O-s) for photodegradation of Tetracycline: Performance, mechanism insight and pathways. Chem. Eng. J. 2022, 446, 137053. [Google Scholar] [CrossRef]
- Yan, J.T.; Chai, B.; Liu, Y.Y.; Fan, G.Z.; Song, G.S. Construction of 3D/2D ZnFe2O4/g-C3N4 S-scheme heterojunction for efficient photo-Fenton degradation of tetracycline hydrochloride. Appl. Surf. Sci. 2023, 607, 155088. [Google Scholar] [CrossRef]
- Burns, J.M.; Cooper, W.J.; Ferry, J.L.; King, D.W.; DiMento, B.P.; McNeill, K.; Miller, C.J.; Miller, W.L.; Peake, B.M.; Rusak, S.A.; et al. Methods for reactive oxygen species (ROS) detection in aqueous environments. Aquat. Sci. 2012, 74, 683–734. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Liu, P.; Lin, H.; Xue, H.; Mao, J. A Novel TiO2-Cuttlebone Photocatalyst for Highly Efficient Catalytic Degradation of Tetracycline Hydrochloride. Inorganics 2024, 12, 319. https://doi.org/10.3390/inorganics12120319
Li Q, Liu P, Lin H, Xue H, Mao J. A Novel TiO2-Cuttlebone Photocatalyst for Highly Efficient Catalytic Degradation of Tetracycline Hydrochloride. Inorganics. 2024; 12(12):319. https://doi.org/10.3390/inorganics12120319
Chicago/Turabian StyleLi, Qing, Penghui Liu, Huizhen Lin, Hun Xue, and Jingyun Mao. 2024. "A Novel TiO2-Cuttlebone Photocatalyst for Highly Efficient Catalytic Degradation of Tetracycline Hydrochloride" Inorganics 12, no. 12: 319. https://doi.org/10.3390/inorganics12120319
APA StyleLi, Q., Liu, P., Lin, H., Xue, H., & Mao, J. (2024). A Novel TiO2-Cuttlebone Photocatalyst for Highly Efficient Catalytic Degradation of Tetracycline Hydrochloride. Inorganics, 12(12), 319. https://doi.org/10.3390/inorganics12120319







