Recovery of Ni-Co-Mn Oxides from End-of-Life Lithium-Ion Batteries for the Application of a Negative Temperature Coefficient Sensor
Abstract
:1. Introduction
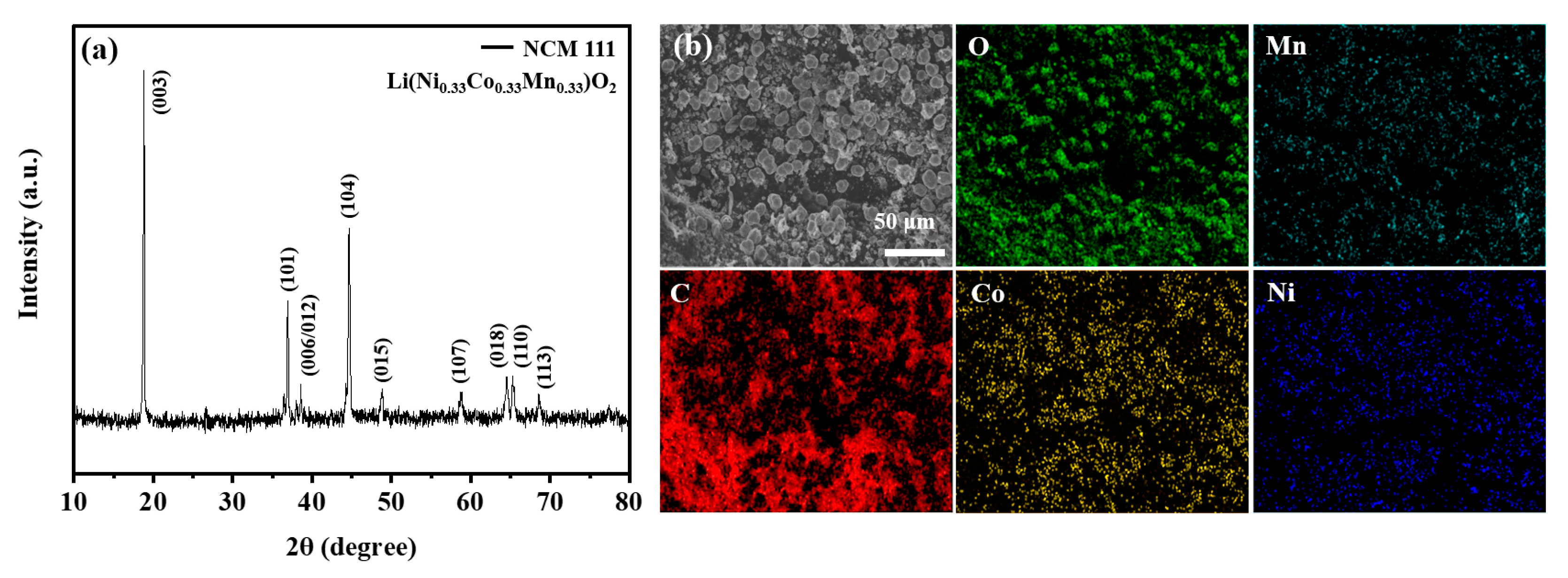
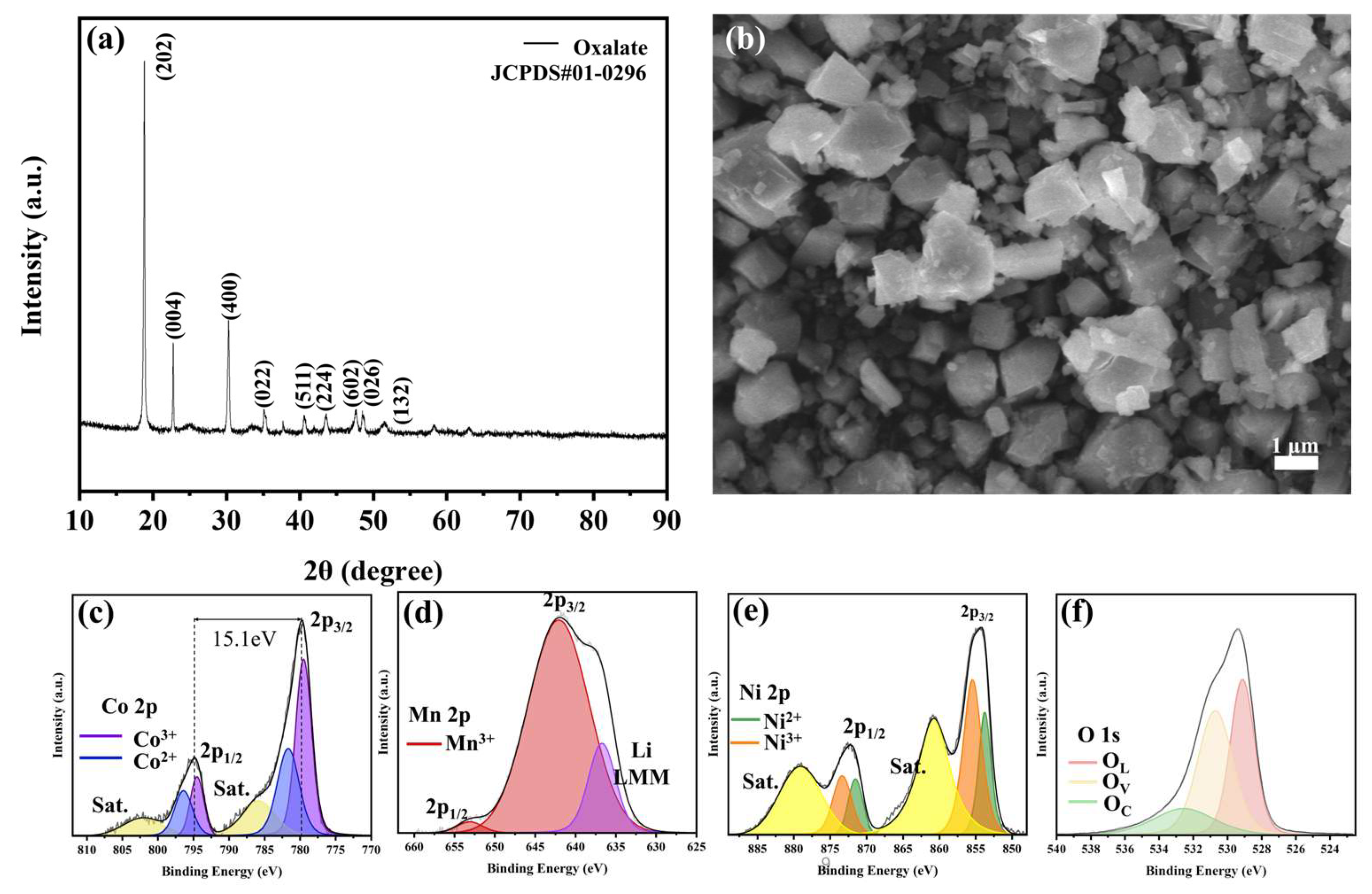
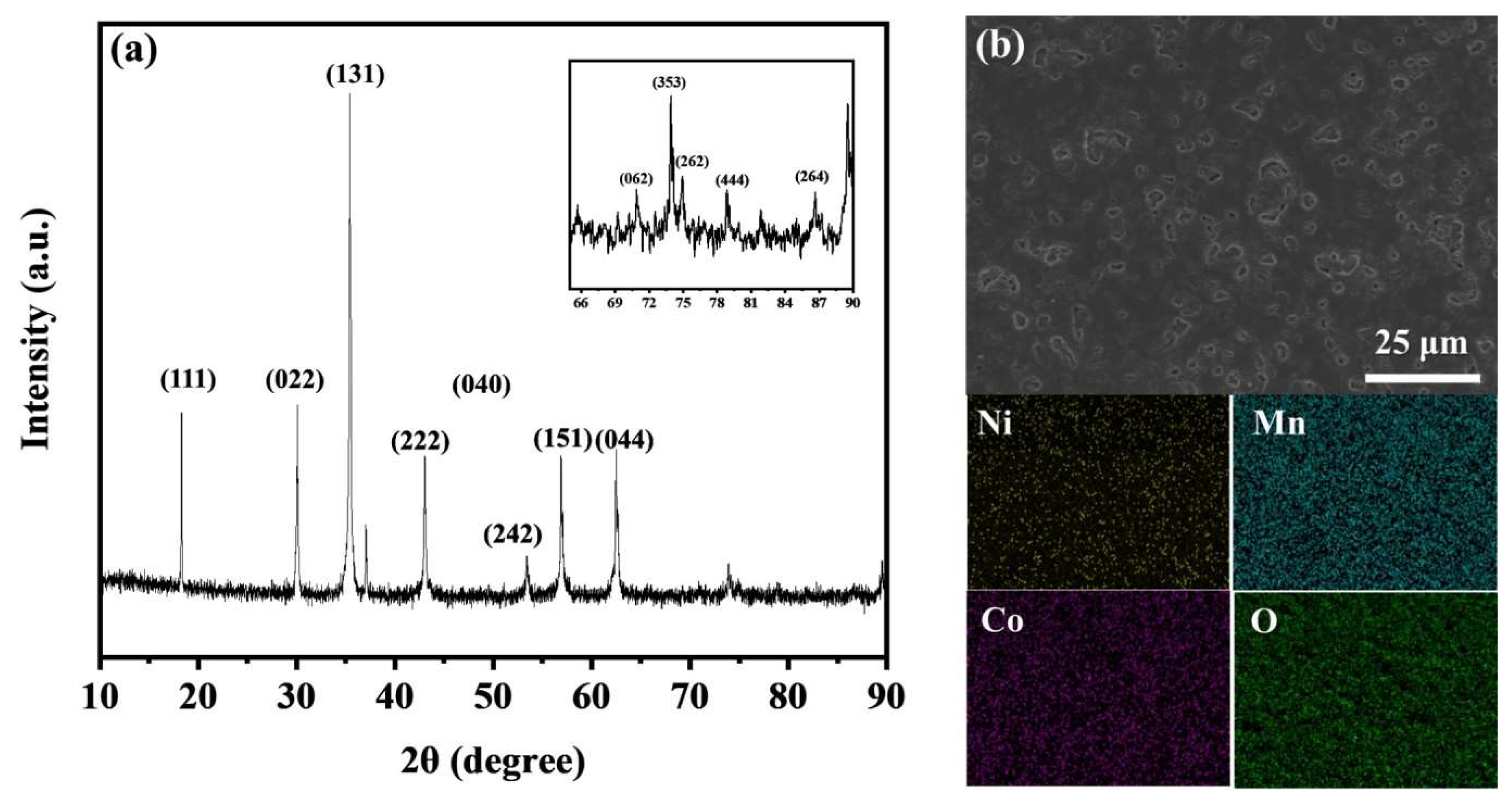
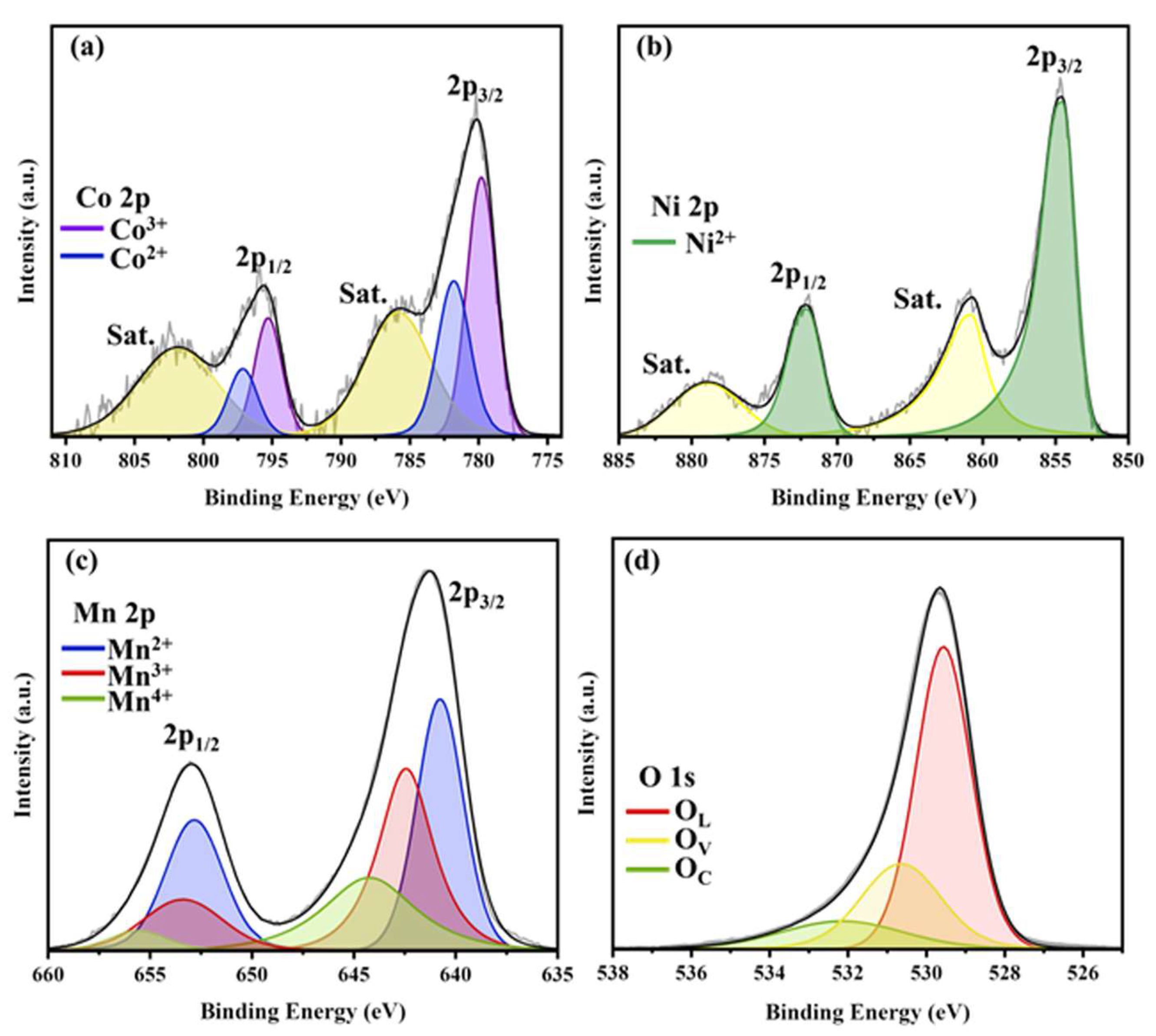
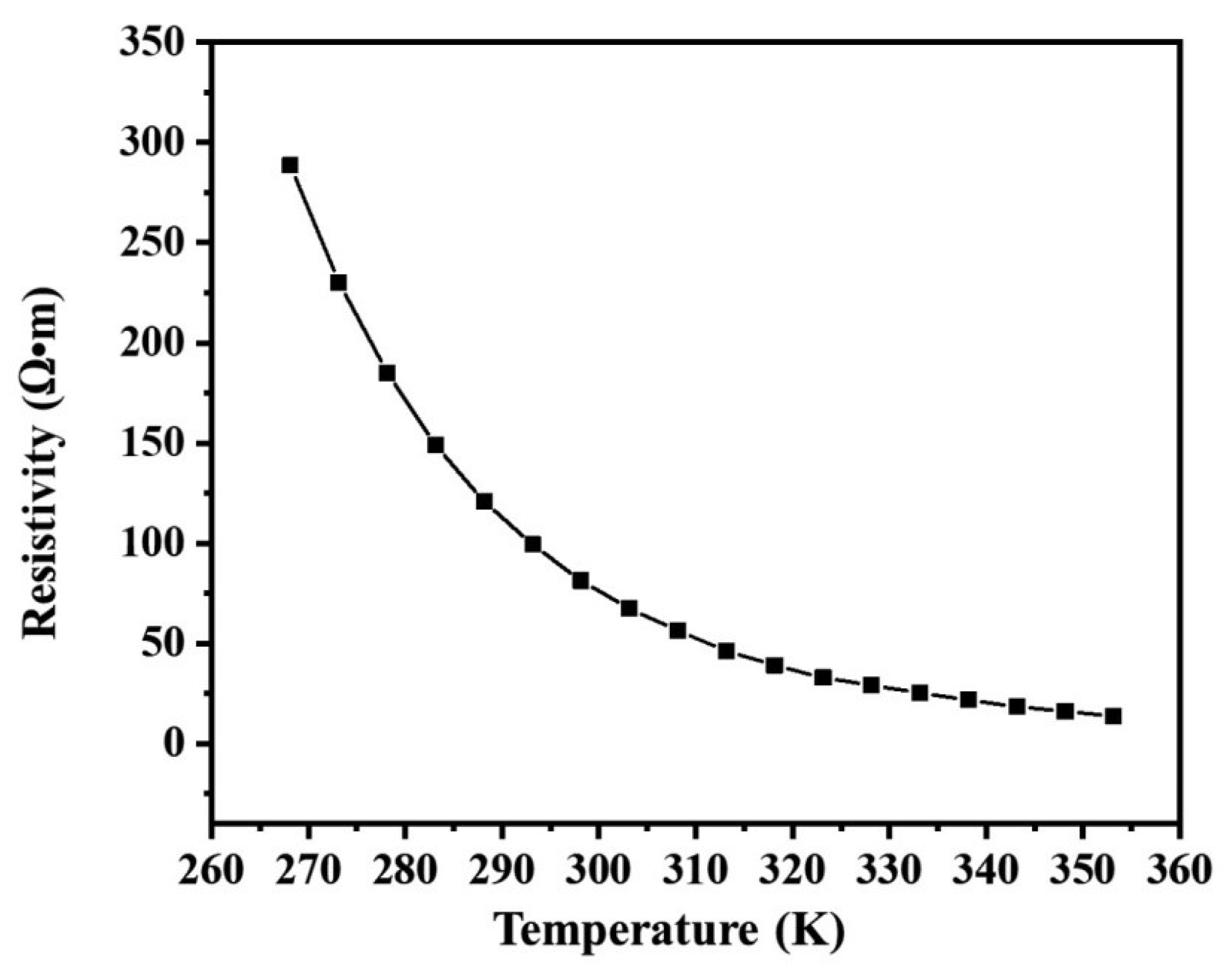
2. Results and Discussion
3. Experimental Procedures
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chan, C.C.; Chau, K.T. An overview of power electronics in electric vehicles. IEEE Trans. Ind. Electron. 1997, 44, 3–13. [Google Scholar] [CrossRef]
- Zahid, T.; Li, W.M. A comparative study based on the least square parameter identification method for state of charge estimation of a LiFePO4 battery pack using three model-based algorithms for electric vehicles. Energies 2016, 9, 720. [Google Scholar] [CrossRef]
- Han, H.; Lee, J.S.; Ryu, J.H.; Kim, K.M.; Jones, J.L.; Lim, J.; Guillemet-Fritsch, S.; Lee, H.C.; Mhin, S. Effect of high cobalt concentration on hopping motion in cobalt manganese spinel oxide (CoxMn3-xO4, x ≥ 2.3). J. Phys. Chem. C 2016, 120, 13667–13674. [Google Scholar] [CrossRef]
- Bhargava, A.; Eppstein, R.; Sun, J.; Smeaton, M.A.; Paik, H.; Kourkoutis, L.F.; Schlom, D.G.; Toroker, M.C.; Robinson, R.D. Breakdown of the small-polaron hopping model in higher-order spinels. Adv. Mater. 2020, 32, 2004490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.M.; Chang, A.M.; Peng, C.W. Preparation and characterization of Fe3+-doped Ni0.9Co0.8Mn1.3-xFexO4 (0 <= x <= 0.7) negative temperature coefficient ceramic materials. Microelectron. Eng. 2011, 88, 2934–2940. [Google Scholar]
- Muralidharan, M.N.; Sunny, E.K.; Dayas, K.R.; Seema, A.; Resmi, K.R. Optimization of process parameters for the production of Ni-Mn-Co-Fe based NTC chip thermistors through tape casting route. J. Alloys Compd. 2011, 509, 9363–9371. [Google Scholar] [CrossRef]
- Han, H.; Mhin, S.; Park, K.R.; Kim, K.M.; Lee, J.I.; Ryu, J.H. Fe doped Ni-Mn-Co-O ceramics with varying Fe content as negative temperature coefficient sensors. Ceram. Int. 2017, 43, 10528–10532. [Google Scholar] [CrossRef]
- Yokoyama, T.; Meguro, T.; Nakamura, M.; Tatami, J.; Wakihara, T.; Komeya, K. Fabrication and electrical properties of sintered bodies composed of Mn(1.75-1.25X)Co2.5XNi1.25(1-X)O4 (0 ≤ X ≤ 0.6) with a cubic spinel structure. J. Ceram. Process. Res. 2009, 10, 683–688. [Google Scholar]
- Teichmann, C.; Töpfer, J. Sintering and electrical properties of Cu-substituted Zn-Co-Ni-Mn spinel ceramics for NTC thermistors thick films. J. Eur. Ceram. Soc. 2022, 42, 2261–2267. [Google Scholar] [CrossRef]
- Li, H.; Ley Thayil, I.P.; Ma, X.; Sang, X.; Zhang, H.; Chang, A. Electrical properties and aging behavior of Na-doped Mn1.95Co0.21Ni0.84O4 NTC ceramics. Ceram. Int. 2020, 46, 24365–24370. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Li, M.; Chen, R.; Wu, F.; Amine, K.; Lu, J. The Recycling of Spent Lithium-Ion Batteries: A Review of Current Processes and Technologies. Electrochem. Energy Rev. 2018, 1, 461–482. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, Y.; Liu, L.; Chen, J.; Wang, J. Nanostructured carbon-based cathode materials for non-aqueous Li-O2 batteries. Mater. Lab. 2023, 2, 220015. [Google Scholar]
- Li, H.; Chen, J.; Fang, J. Recent Advances in Wearable Aqueous Metal-Air Batteries: From Configuration Design to Materials Fabrication. Adv. Mater. Technol. 2023, 8, 2201762. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, S.; Ji, L.; Zhou, T.; Miao, Y.; Scott, K.; Li, D.; Yang, J.; Wu, X. Reuse of Ni-Co-Mn oxides from spent Li-ion batteries to prepare bifunctional air electrodes. Resour. Conserv. Recycl. 2018, 129, 135–142. [Google Scholar] [CrossRef]
- Fu, C.C.; Li, G.S.; Luo, D.; Huang, X.S.; Zheng, J.; Li, L.P. One-step calcination-free synthesis of multicomponent spinel assembled microspheres for high-performance anodes of Li-Ion batteries: A case study of MnCo2O4. ACS Appl. Mater. Interfaces 2014, 6, 2439–2449. [Google Scholar] [CrossRef]
- Wakisaka, Y.; Hirata, S.; Mizokawa, T.; Suzuki, Y.; Miyazaki, Y.; Kajitani, T. Electronic structure of Ca3Co4O9 studied by photoemission spectroscopy: Phase separation and charge localization. Phys. Rev. B 2008, 78, 235107. [Google Scholar] [CrossRef]
- Munakata, F.; Takahashi, H.; Akimune, Y.; Shichi, Y.; Tanimura, M.; Inoue, Y.; Itti, R.; Koyama, Y. Electronic state and valence control of LaCoO3: Difference between La-deficient and Sr-substituting effects. Phys. Rev. B 1997, 56, 979–982. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, J.; Cao, G.; Li, Y.; Zhou, Y.; Deng, S.; Hai, C. Flux-free synthesis of single-crystal LiNi0.8Co0.1Mn0.1O2 boosts its electrochemical performance in lithium batteries. J. Power Sources 2007, 174, 965–969. [Google Scholar] [CrossRef]
- Ni, L.; Guo, R.; Deng, W.; Wang, B.; Chen, J.; Mei, Y.; Gao, J.; Gao, X.; Yin, S.; Liu, H.; et al. Single-Crystalline Ni-Rich layered cathodes with Super-Stable cycling. Chem. Eng. J. 2022, 431, 133731. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Zhai, W.; Liu, H.; Sakthivel, T.; Guo, S.; Dai, Z. Metastabilizing the Ruthenium Clusters by Interfacial Oxygen Vacancies for Boosted Water Splitting Electrocatalysis. Adv. Energy Mater. 2024, 14, 2400059. [Google Scholar] [CrossRef]
- Zhai, W.; Chen, Y.; Liu, Y.; Sakthivel, T.; Ma, Y.; Qin, Y.; Qu, Y.; Dai, Z. Enlarging the Ni–O Bond Polarizability in a Phosphorene-Hosted Metal–Organic Framework for Boosted Water Oxidation Electrocatalysis. ACS Nano 2023, 17, 17254–17264. [Google Scholar] [CrossRef] [PubMed]
- Zhai, W.; Chen, Y.; Liu, Y.; Ma, Y.; Vijayakumar, P.; Qin, Y.; Qu, Y.; Dai, Z. Covalently Bonded Ni Sites in Black Phosphorene with Electron Redistribution for Efficient Metal-Lightweighted Water Electrolysis. Nano-Micro. Lett. 2024, 16, 115. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Jana, R.; Ganguli, S.; Inta, H.R.; Tudu, G.; Koppisetti, H.V.S.R.M.; Datta, A.; Mahalingam, V. Nickel–cobalt oxalate as an efficient non-precious electrocatalyst for an improved alkaline oxygen evolution reaction. Nanoscale Adv. 2021, 3, 3770–3779. [Google Scholar] [CrossRef]
- Muralidharan, M.; Rohini, P.; Sunny, E.K.; Dayas, K.R.; Seema, A. Effect of Cu and Fe addition on electrical properties of Ni–Mn–Co–O NTC thermistor compositions. Ceram. Int. 2012, 38, 6481–6486. [Google Scholar] [CrossRef]
- Jeon, J.; Park, K.R.; Kim, K.M.; Ahn, C.; Lee, J.; Yu, D.; Bang, J.; Oh, N.; Han, H.; Mhin, S. Effect of Cu/Fe addition on the microstructures and electrical performances of Ni–Co–Mn oxides. J. Alloys Compd. 2021, 859, 157769. [Google Scholar] [CrossRef]
- Gao, C.; Li, Z.; Yang, L.; Peng, D.; Zhang, H. Investigation of electrical and aging properties of Bi-modified (Zn0.4Ni0.6)1-xNaxO ceramic thermistors. J. Eur. Ceram. Soc. 2021, 41, 4160–4166. [Google Scholar] [CrossRef]
- Hardian, A.; Greshela, S.; Yuliana, T.; Budiman, S.; Murniati, A.; Kusumaningtyas, V.A.; Syarif, D.G. Cu-Mn Co-doped NiFe2O4 based thick ceramic film as negative temperature coefficient thermistors. Earth Environ. Sci. 2021, 882, 012017. [Google Scholar] [CrossRef]

| Sample No. | Unit | Ni | Mn | Co |
|---|---|---|---|---|
| 1 | wt% | 15.8 | 16.8 | 13.5 |
| 2 | 15.5 | 16.2 | 13.2 | |
| 3 | 15.7 | 16.7 | 13.4 |
| Element | Wt% | At% |
|---|---|---|
| C | 22.68 | 41.76 |
| O | 26.52 | 38.24 |
| Mn | 3.40 | 1.43 |
| Co | 21.31 | 8.34 |
| Ni | 26.08 | 10.25 |
| Total | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mhin, S. Recovery of Ni-Co-Mn Oxides from End-of-Life Lithium-Ion Batteries for the Application of a Negative Temperature Coefficient Sensor. Inorganics 2024, 12, 105. https://doi.org/10.3390/inorganics12040105
Mhin S. Recovery of Ni-Co-Mn Oxides from End-of-Life Lithium-Ion Batteries for the Application of a Negative Temperature Coefficient Sensor. Inorganics. 2024; 12(4):105. https://doi.org/10.3390/inorganics12040105
Chicago/Turabian StyleMhin, Sungwook. 2024. "Recovery of Ni-Co-Mn Oxides from End-of-Life Lithium-Ion Batteries for the Application of a Negative Temperature Coefficient Sensor" Inorganics 12, no. 4: 105. https://doi.org/10.3390/inorganics12040105





