Abstract
Lead contamination in water poses significant health risks, making its removal imperative. In this study, magnetic strontium ferrite (SrFe12O19) nanoparticles were facilely synthesized by the Pechini sol–gel method and subsequently functionalized with a novel chitosan–Schiff base ligand to obtain a novel inorganic/organic nanocomposite for removing Pb(II) ions from aqueous solutions. The chitosan–Schiff base ligand was synthesized through the reaction of chitosan with 2,4,5-trihydroxybenzaldehyde. The presence of two X-ray diffraction (XRD) peaks at 2Ɵ = 10.5° and 2Ɵ = 20.5°, alongside the characteristic SrFe12O19 peaks, confirmed the functionalization of the nanoparticles with the ligand. Additionally, a significant decrease in the saturation magnetization value from 40.29 emu/g in pure SrFe12O19 nanoparticles to 17.32 emu/g in the nanocomposite further verified the functionalization. The presence of carbon (C) and nitrogen (N) atoms in the energy-dispersive X-ray (EDX) pattern of the nanocomposite, in addition to iron (Fe), strontium (Sr), and oxygen (O), also confirmed the functionalization. The nanocomposite’s maximum adsorption capacity for Pb(II) ions was 390.63 mg/g. Moreover, the adsorption process is endothermic, spontaneous, and chemical, occurring via complexation with -C=N and -OH groups, and it fits well with the Langmuir equilibrium isotherm and the pseudo-second-order kinetic equation.
1. Introduction
Heavy-metal contamination in waterways has become a global concern due to its severe health risks and environmental impact [1,2,3,4]. Among heavy metals, lead (Pb(II)) is highly toxic, stable, and resistant to biodegradation, leading to its accumulation in the human body and the environment. This contamination primarily arises from industrial activities such as battery production, mining, and pigment manufacturing [5,6]. To mitigate these hazards, effective methods for removing Pb(II) ions from water are imperative. Several techniques have been employed for heavy–metal removal, including precipitation [7], electrocoagulation [8], membrane filtration [9], and adsorption [10]. Precipitation is advantageous due to its simplicity and cost-effectiveness, but it often results in the generation of large volumes of sludge that require further disposal. Electrocoagulation is effective for a wide range of contaminants and produces less sludge, but it requires high operational costs and electrode maintenance. Membrane filtration, while offering high efficiency and the ability to remove a variety of pollutants, faces limitations due to membrane fouling and high operational costs. Adsorption has gained significant attention due to its simplicity, cost-effectiveness, high efficiency, and reusability [11]. Among the various adsorbents, composite materials synthesized using organic templates have shown promise. Organic templates like chitosan, a biopolymer derived from chitin [12], are particularly effective due to their biodegradability, biocompatibility, and functional groups (-NH2 and -OH) that enhance metal ion adsorption [13,14,15]. Furthermore, natural organic materials such as cellulose, alginate, and gelatin have also been utilized as templates for the preparation of composite materials for water pollution treatment [16,17,18]. Chitosan alone can be soluble in acidic conditions, which limits its practical application. Enhancing chitosan through physical and chemical methods, including grafting, cross-linking, and integration with materials like metal oxides, zeolite, and graphene, while preserving its fundamental structure, significantly enhances the stability and durability of the chitosan biopolymer within the resulting composite. This makes the resulting composite more resistant to harsh environmental conditions and suitable for a broader range of pH values [19,20,21]. Chitosan Schiff bases offer active coordination sites for the complexation of Pb(II) ions. For instance, Yuvaraja et al. developed aminated Schiff base–chitosan–ZnO and applied it as an effective adsorbent for Pb(II), demonstrating a maximum uptake capability of 55.55 mg/g [22]. Also, Yan et al. developed epichlorohydrin cross-linked Schiff base–chitosan–Fe3O4, which has been identified as a potential candidate for adsorption for removing Pb(II) ions, exhibiting a maximum adsorption capacity of 86.20 mg/g [23]. A study by Ortiz-Oliveros et al. evaluated the removal of Pb(II) ions from six different adsorbents: chitosan–vanillin Schiff base with a maximum adsorption capacity of 23.3 mg/g; chitosan–O–vanillin Schiff base with a maximum adsorption capacity of 66.23 mg/g; alginate with cysteine with a maximum adsorption capacity of 770 mg/g; Bacillus subtilis with a maximum adsorption capacity of 17.34 mg/g; moss peat with a maximum adsorption capacity of 117.58 mg/g; and marine green algae with a maximum adsorption capacity of 181.82 mg/g [24]. Nanoparticles of hexagonal strontium ferrite (SrFe12O19), known for its superior hard magnetic properties, have attracted considerable attention for diverse uses due to their cost-effectiveness, pronounced magneto–crystalline anisotropy, and high Curie temperature [25]. For instance, Sahoo et al. utilized a sonochemical method to produce hexagonal strontium ferrite, exploring its potential for both malachite green dye adsorption and antibacterial activity [26]. Recently, there have been challenges associated with the adsorption capacity, separation, and reusability of conventional adsorbents like activated carbon and ionic-exchange resins. In this study, we focused on developing a novel nanocomposite for the removal of Pb(II) ions from aqueous media, combining the magnetic properties of strontium ferrite (SrFe12O19) nanoparticles with the adsorption capabilities of a chitosan–Schiff base ligand. Strontium ferrite was chosen due to its superior magnetic properties, cost-effectiveness, and ease of separation from treated solutions using a magnetic field. The chitosan–Schiff base ligand was synthesized by reacting chitosan with 2,4,5-trihydroxybenzaldehyde, introducing active sites (-C=N and -OH) for Pb(II) complexation. Hence, the primary advantages of this nanocomposite include its high adsorption capacity, stability, non-toxicity, and ease of separation and reuse.
2. Results and Discussion
2.1. Characterization of the Chitosan–Schiff Base–SrFe12O19 Nanocomposite
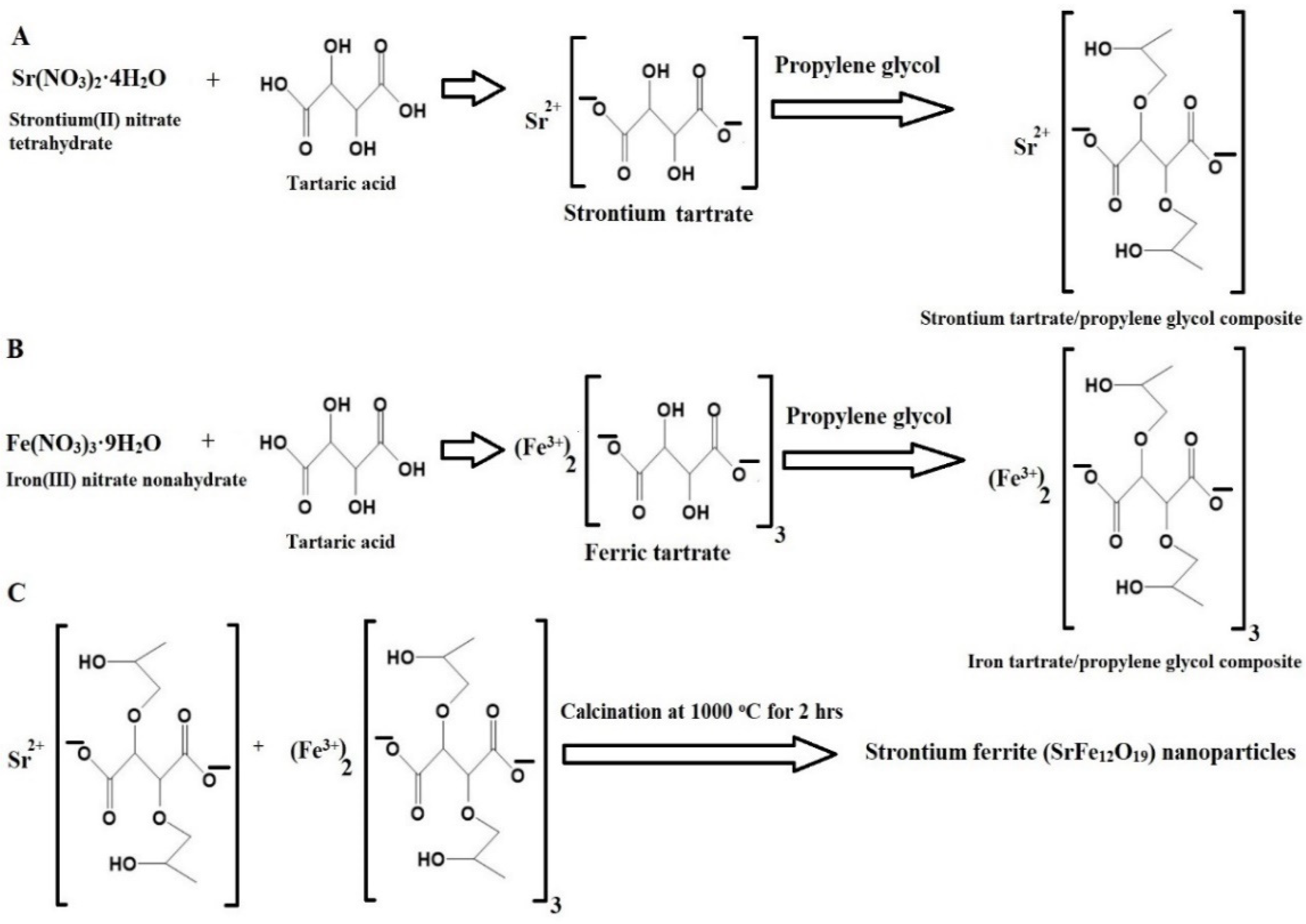
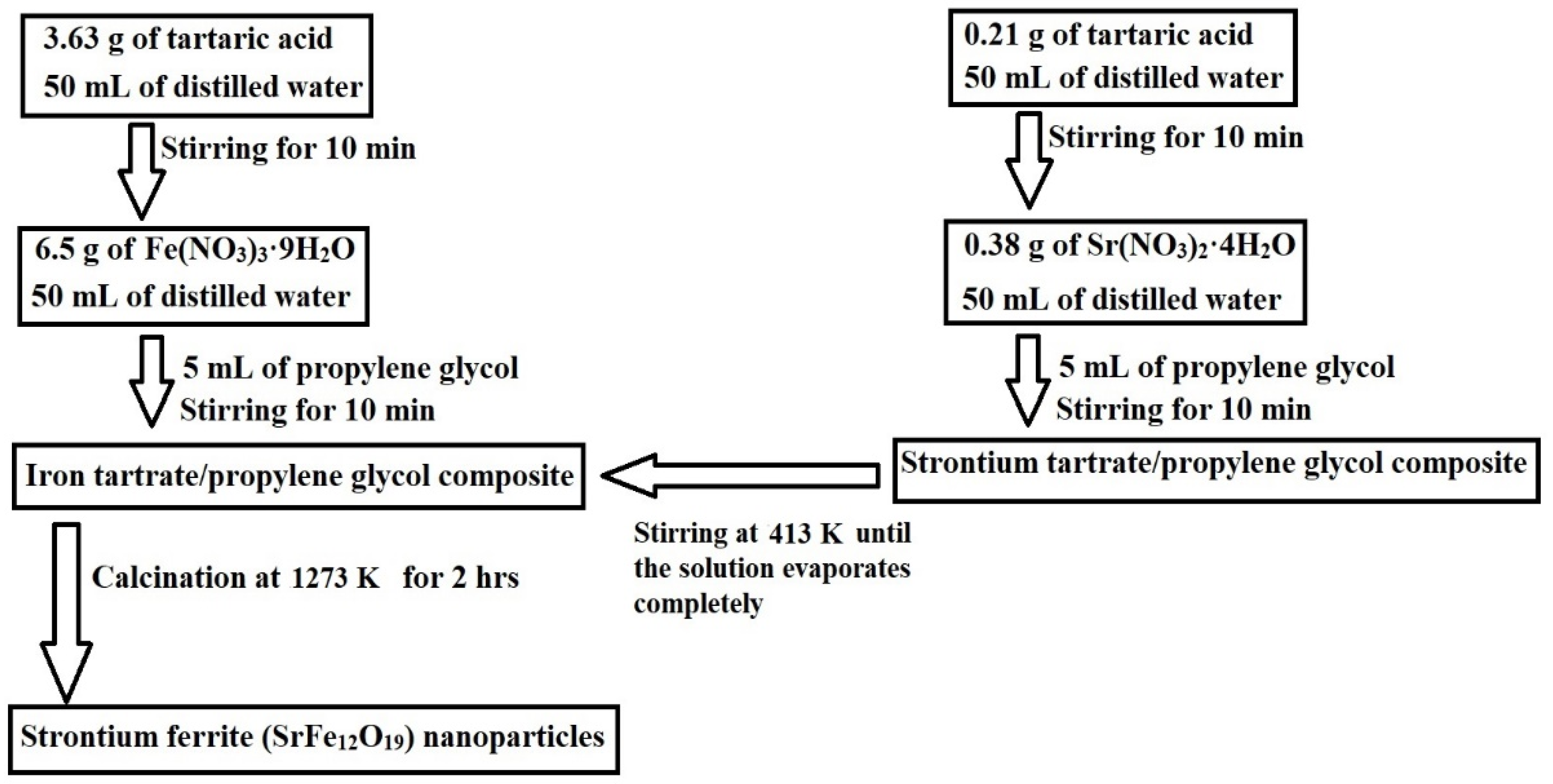
Scheme 1 depicts the synthesis of SrFe12O19 nanoparticles via the Pechini sol–gel method. Strontium(II) nitrate tetrahydrate reacted with tartaric acid and propylene glycol to form a strontium tartrate/propylene glycol composite, while iron(III) nitrate nonahydrate reacted similarly to form an iron tartrate/propylene glycol composite. These composites were then calcined at 1000 °C to remove the organic moiety and produce the SrFe12O19 nanoparticles. This step-by-step process aligned with established protocols found in similar studies on the Pechini sol–gel method [27,28].
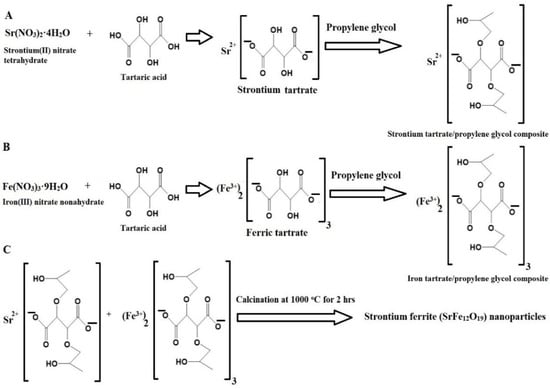
Scheme 1.
Synthesis of strontium ferrite (SrFe12O19) nanoparticles by the Pechini sol–gel method. (A) Formation of strontium tartrate/propylene glycol composite (B) Formation of iron tartrate/propylene glycol composite (C) Formation of nanoparticles.
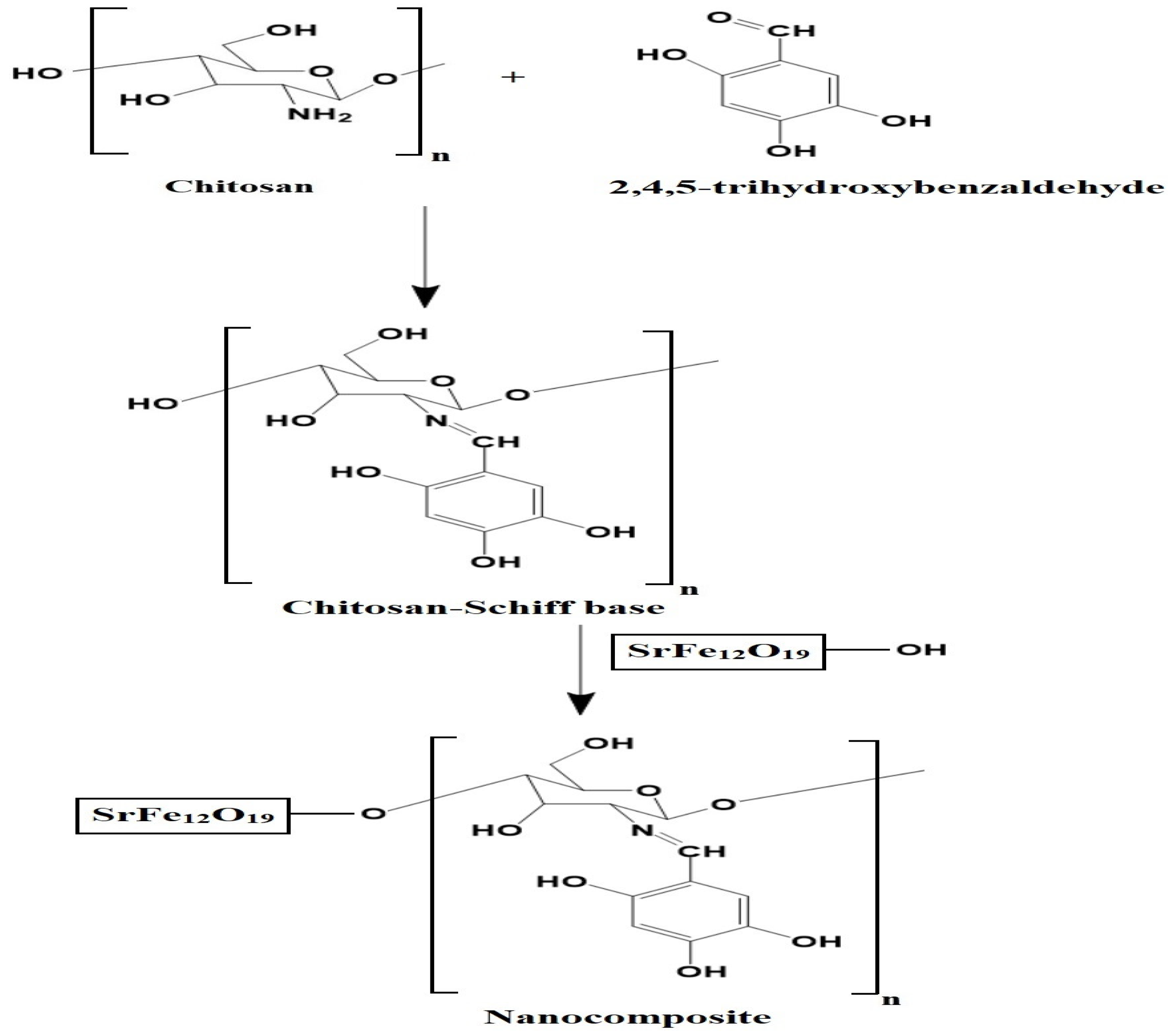
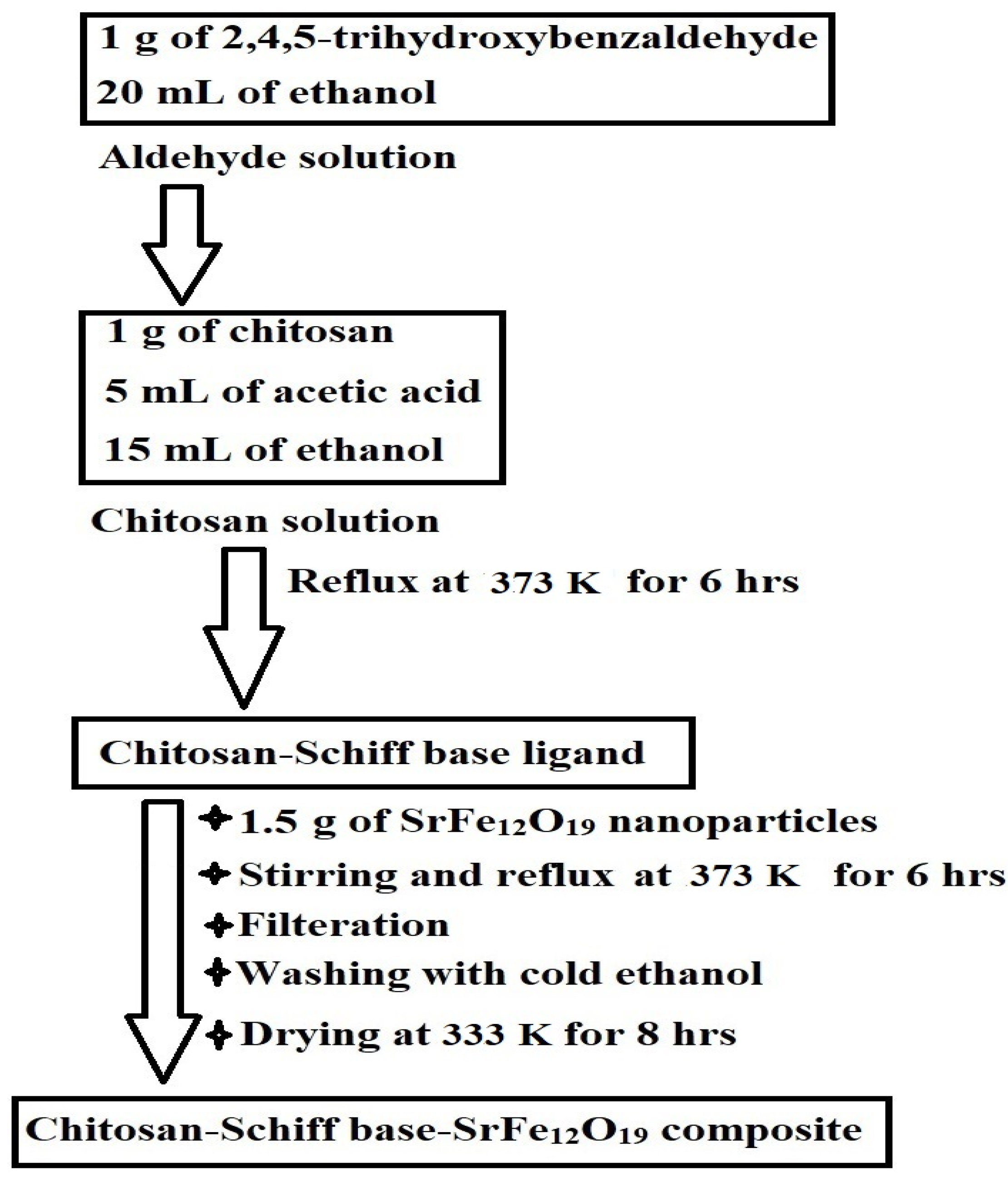
Scheme 2 depicts the synthesis of the chitosan–Schiff base–SrFe12O19 nanocomposite. Chitosan reacts with 2,4,5-trihydroxybenzaldehyde to form a novel chitosan–Schiff base ligand, which then reacts with SrFe12O19 nanoparticles to produce the nanocomposite. This mechanism is based on the known reactivity of chitosan and its derivatives with aldehydes to form Schiff bases [23,29].
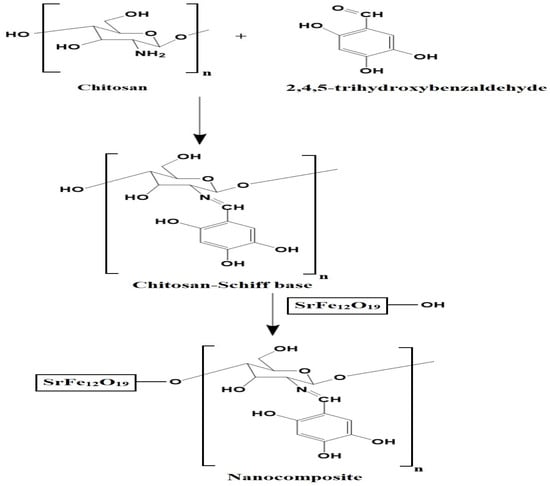
Scheme 2.
Synthesis of the chitosan–Schiff base–SrFe12O19 nanocomposite.
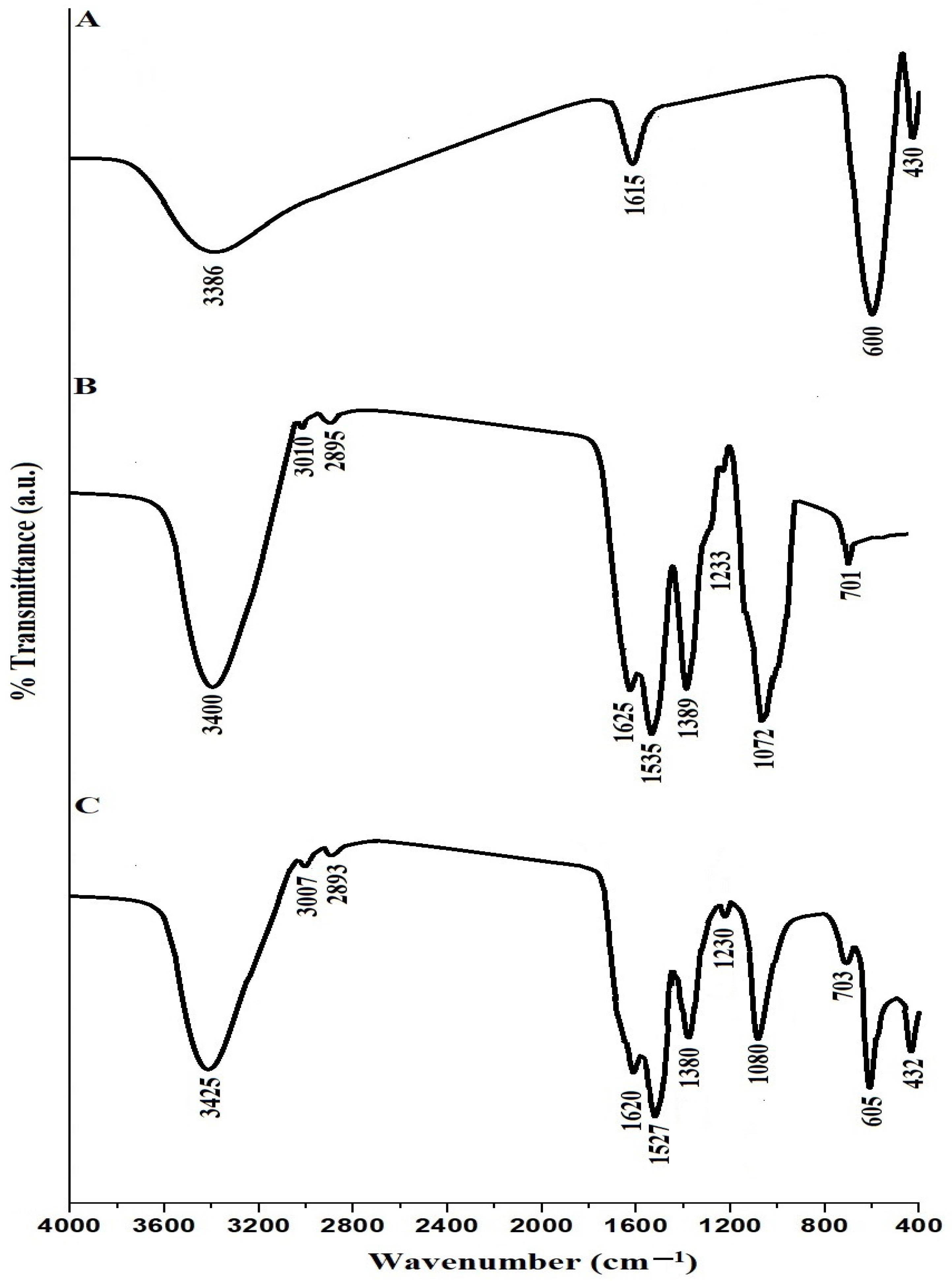
Figure 1A–C illustrate the FT-IR spectra of the SrFe12O19 nanoparticles, chitosan–Schiff base ligand, and chitosan–Schiff base–SrFe12O19 nanocomposite, respectively. In the FT-IR spectrum of SrFe12O19 nanoparticles (Figure 1A), the bands around 430 and 600 cm⁻1 correspond to the stretching vibrations of Sr-O and Fe-O, respectively [30]. These bands confirm the successful synthesis of SrFe12O19 nanoparticles. Additionally, the bands around 1615 and 3386 cm⁻1 correspond to the bending and stretching vibrations of adsorbed H-O-H or residual hydroxyl groups, indicating the presence of surface hydroxyl groups. In the FT-IR spectrum of the chitosan–Schiff base ligand (Figure 1B), the band around 701 cm⁻1 corresponds to the bending vibration of CH aromatic, and bands around 3400, 3010, 2895, 1625, 1535, 1389, 1233, and 1072 cm⁻1 correspond to the stretching vibrations of OH, CH aromatic, CH aliphatic, C=N, C=C, C-N, C-O, and C-O-C, respectively [23]. These characteristic bands confirm the successful synthesis of the chitosan–Schiff base ligand. The FT-IR spectrum of the chitosan–Schiff base–SrFe12O19 nanocomposite (Figure 1C) shows shifts in these bands, suggesting their involvement in the assembly of SrFe12O19 nanoparticles. The presence of bands around 432 and 605 cm⁻1, corresponding to the stretching vibrations of Sr-O and Fe-O, respectively, confirms the successful functionalization of SrFe12O19 nanoparticles with the chitosan–Schiff base ligand.
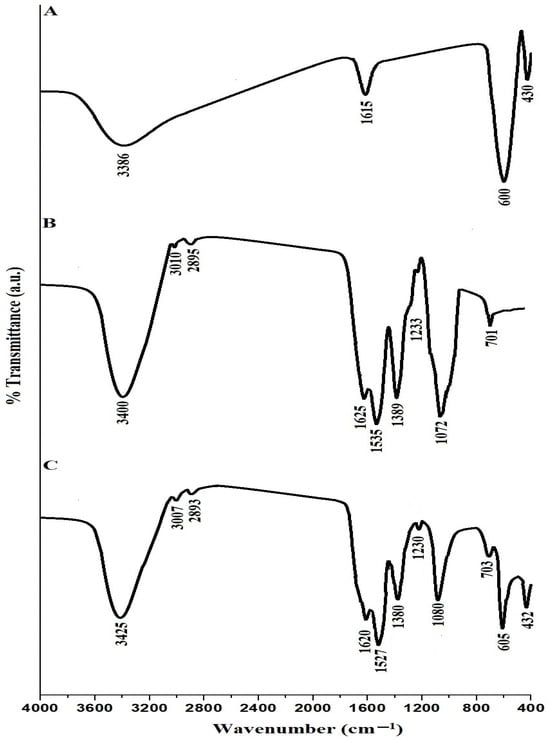
Figure 1.
FT-IR spectra of the SrFe12O19 nanoparticles (A), chitosan–Schiff base ligand (B), and chitosan–Schiff base–SrFe12O19 nanocomposite (C).
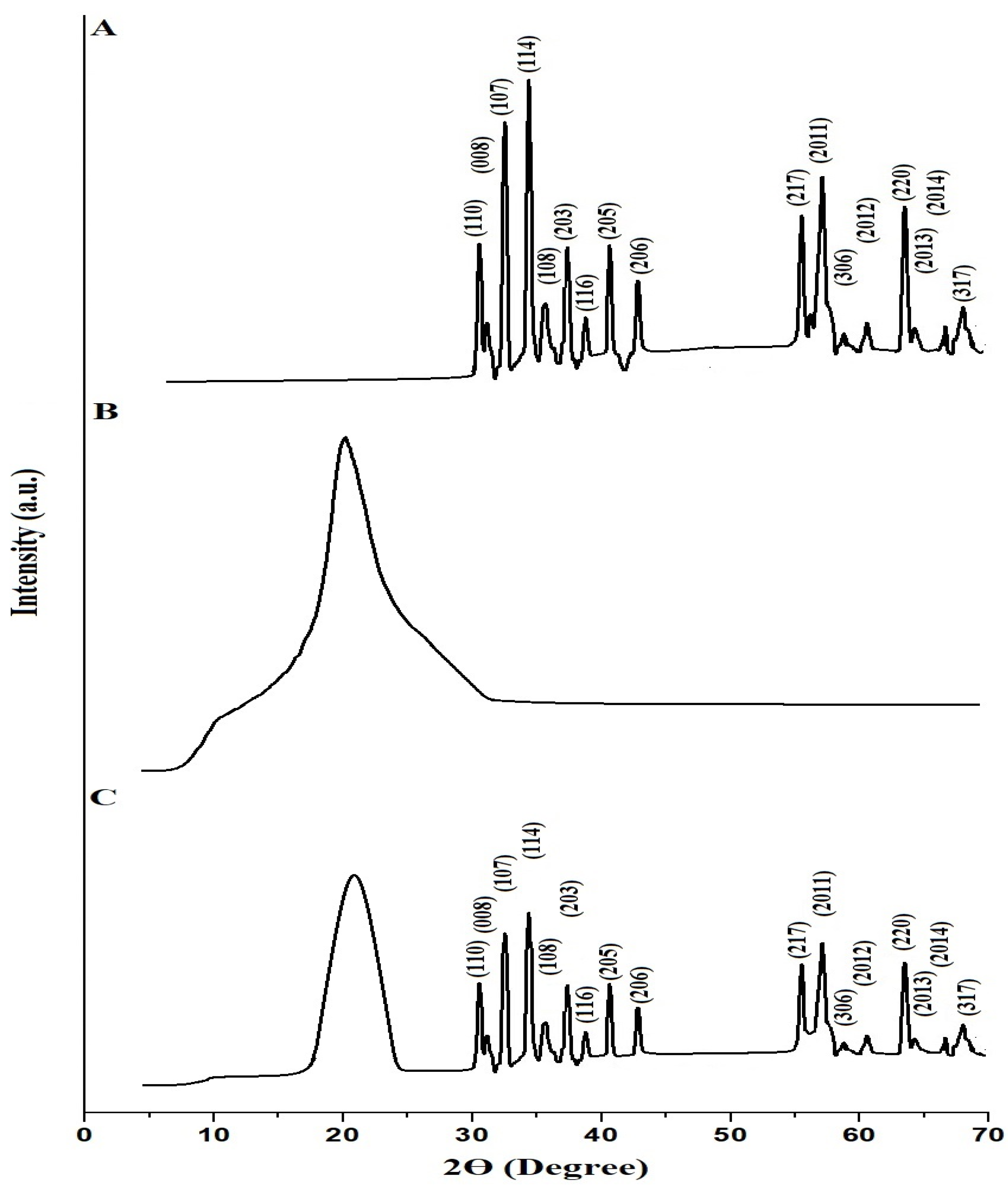
The XRD patterns of the SrFe12O19 nanoparticles, chitosan–Schiff base ligand, and chitosan–Schiff base–SrFe12O19 nanocomposite are shown in Figure 2A–C, respectively. The characteristic peaks in the XRD pattern of the SrFe12O19 nanoparticles (Figure 2A) confirm their hexagonal crystal structure, consistent with JCPDS card no. 33-1340 [31]. Peaks at 2Ɵ° (hkl) of 30.48° (110), 31.03° (008), 32.49° (107), 34.43° (114), 35.62° (108), 37.36° (203), 38.83° (116), 40.67° (205), 42.87° (206), 55.46° (217), 56.93° (2011), 58.76° (306), 60.59° (2012), 63.44° (220), 64.17° (2013), 66.57° (2014), and 67.94° (317) correspond to various Miller planes, confirming the successful synthesis of SrFe12O19 nanoparticles with an average crystallite size of 67.87 nm. The XRD pattern of the chitosan–Schiff base ligand (Figure 2B) shows two reflections at 2Ɵ = 10.5° and 20.5°, indicating its crystalline nature, as reported in a similar work by Pawariya et al. [32]. The presence of these peaks alongside SrFe12O19 peaks in the XRD pattern of the nanocomposite (Figure 2C) confirms the successful functionalization of SrFe12O19 nanoparticles with the chitosan–Schiff base ligand.
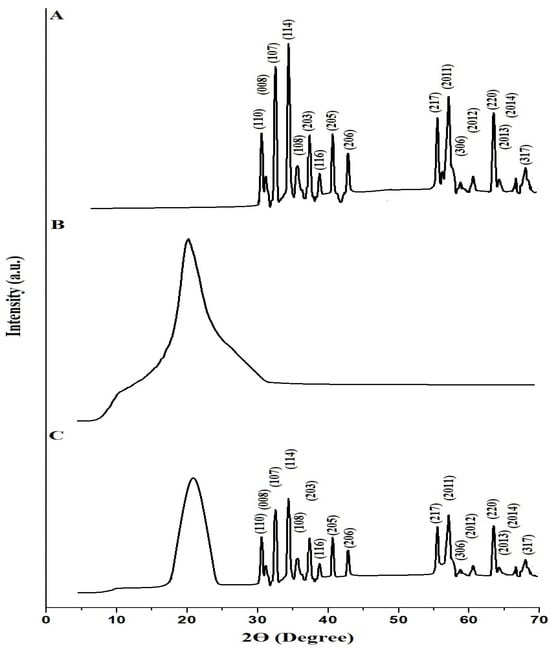
Figure 2.
XRD patterns of the SrFe12O19 nanoparticles (A), chitosan–Schiff base ligand (B), and chitosan–Schiff base–SrFe12O19 nanocomposite (C).
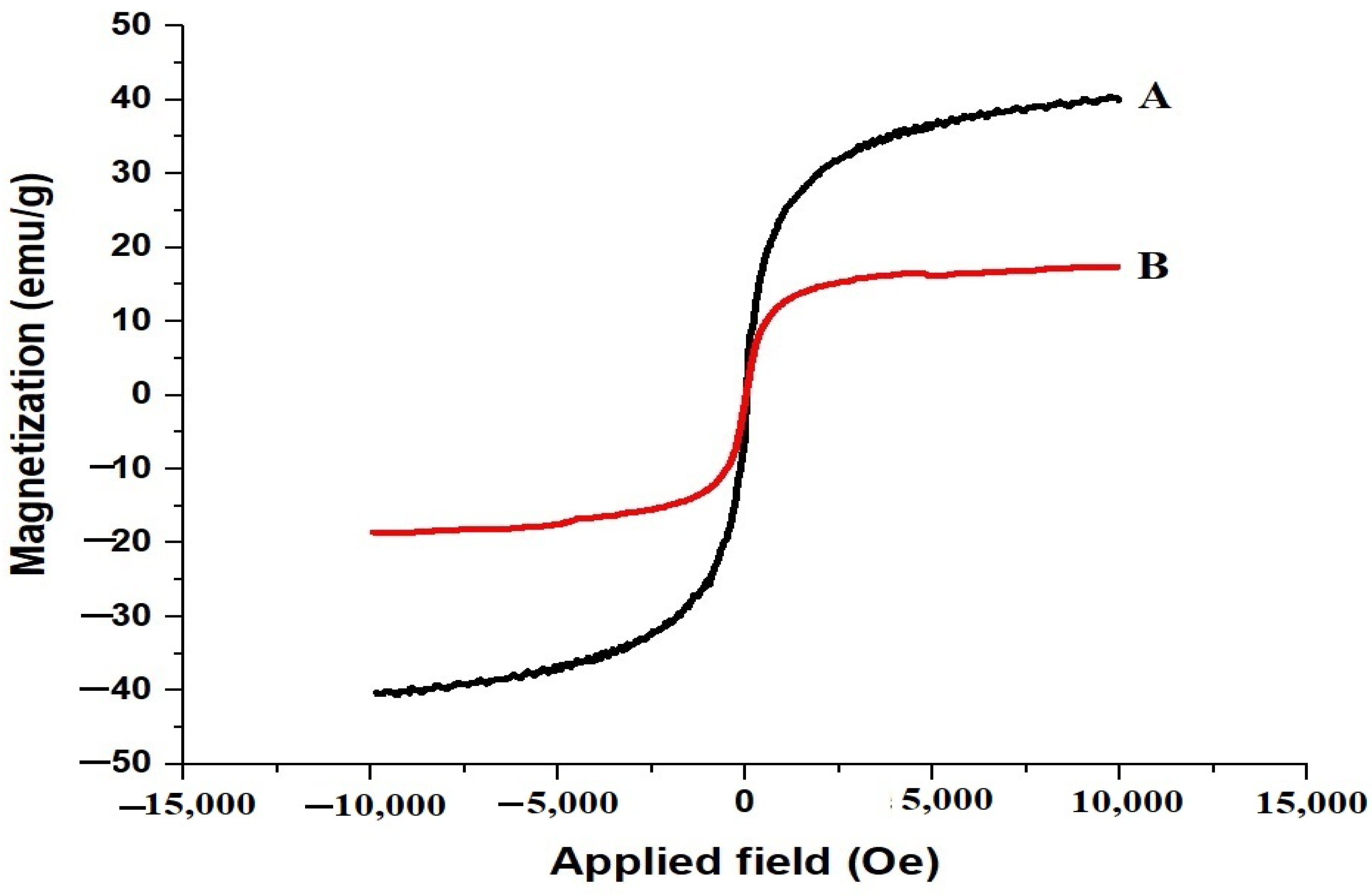
The magnetization curves of the SrFe12O19 nanoparticles and the chitosan–Schiff base-SrFe12O19 nanocomposite are shown in Figure 3A,B, respectively. The saturation magnetization values of 40.29 emu/g for the SrFe12O19 nanoparticles and 17.32 emu/g for the nanocomposite indicate a decrease in magnetization due to the presence of the non-magnetic chitosan–Schiff base ligand. Despite this reduction, the magnetization value of the nanocomposite remains sufficient for magnetic separation, facilitating the easy recovery and reuse of the adsorbent.
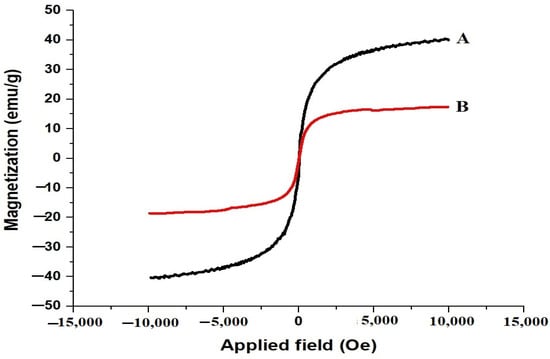
Figure 3.
Magnetization curves of the SrFe12O19 nanoparticles (A) and chitosan–Schiff base–SrFe12O19 nanocomposite (B).
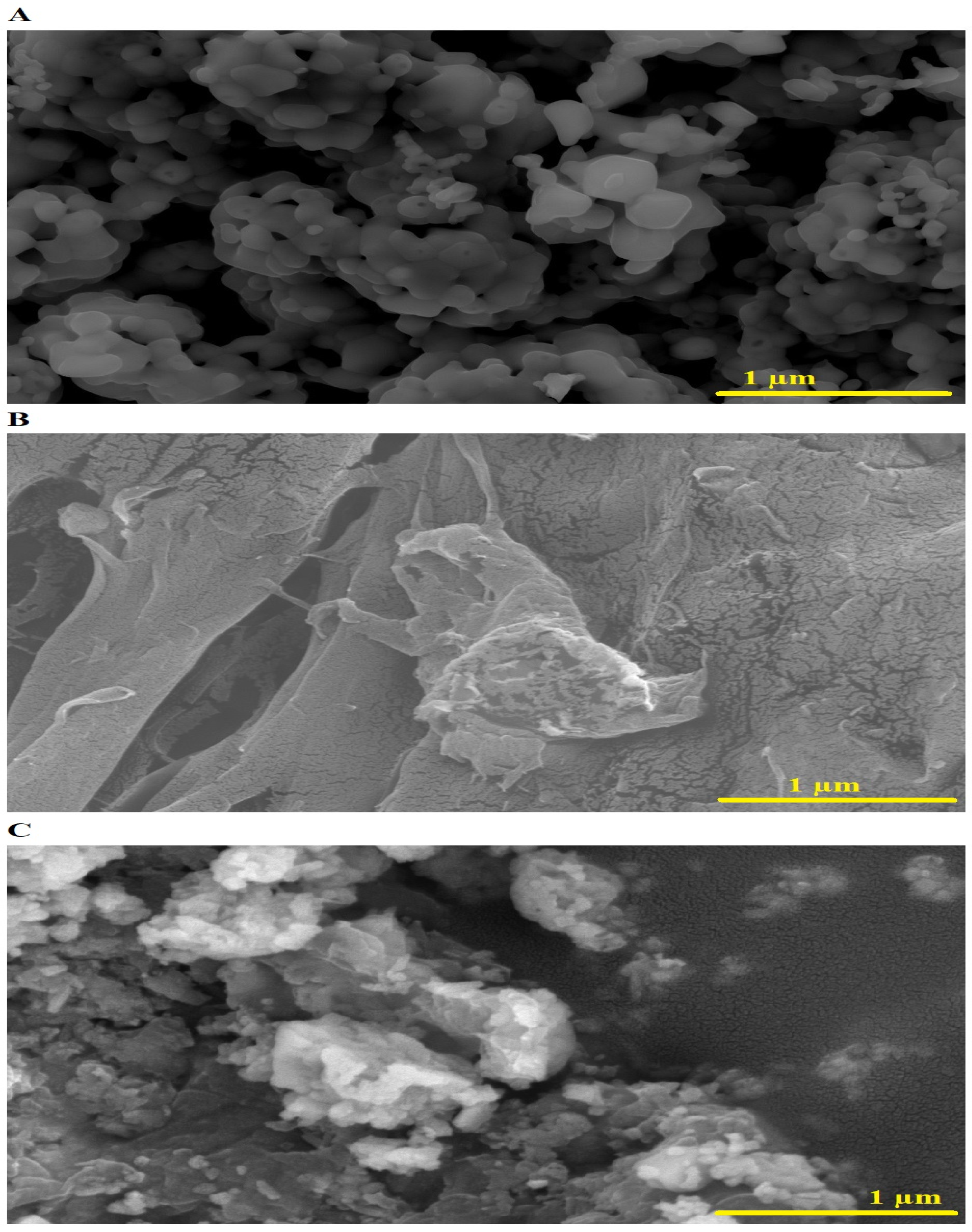
SEM images of the SrFe12O19 nanoparticles, chitosan–Schiff base ligand, and chitosan–Schiff base–SrFe12O19 nanocomposite are shown in Figure 4A–C, respectively. The SrFe12O19 nanoparticles exhibit a quasi-spherical shape with an average size of 160.65 nm (Figure 4A). The chitosan–Schiff base ligand shows a roughened surface due to the Schiff base preparation process (Figure 4B). Upon adding SrFe12O19 nanoparticles, the nanocomposite’s surface becomes even rougher, enhancing its adsorption capacity by providing more active sites for Pb(II) ions (Figure 4C).
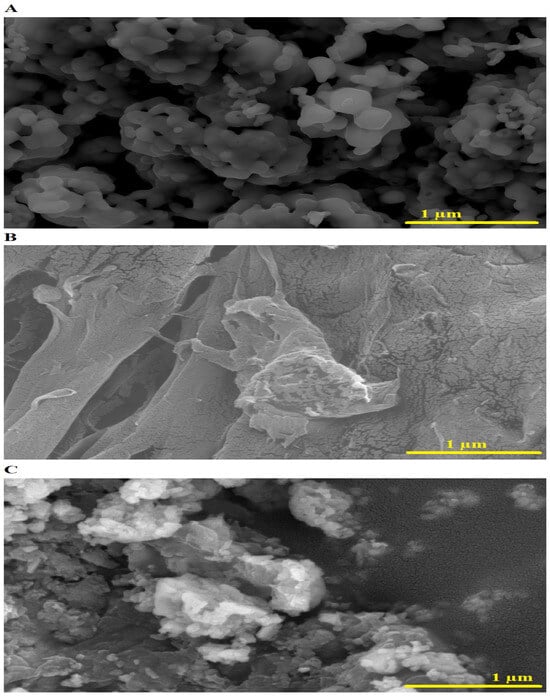
Figure 4.
SEM images of the SrFe12O19 nanoparticles (A), chitosan–Schiff base ligand (B), and chitosan–Schiff base–SrFe12O19 nanocomposite (C).
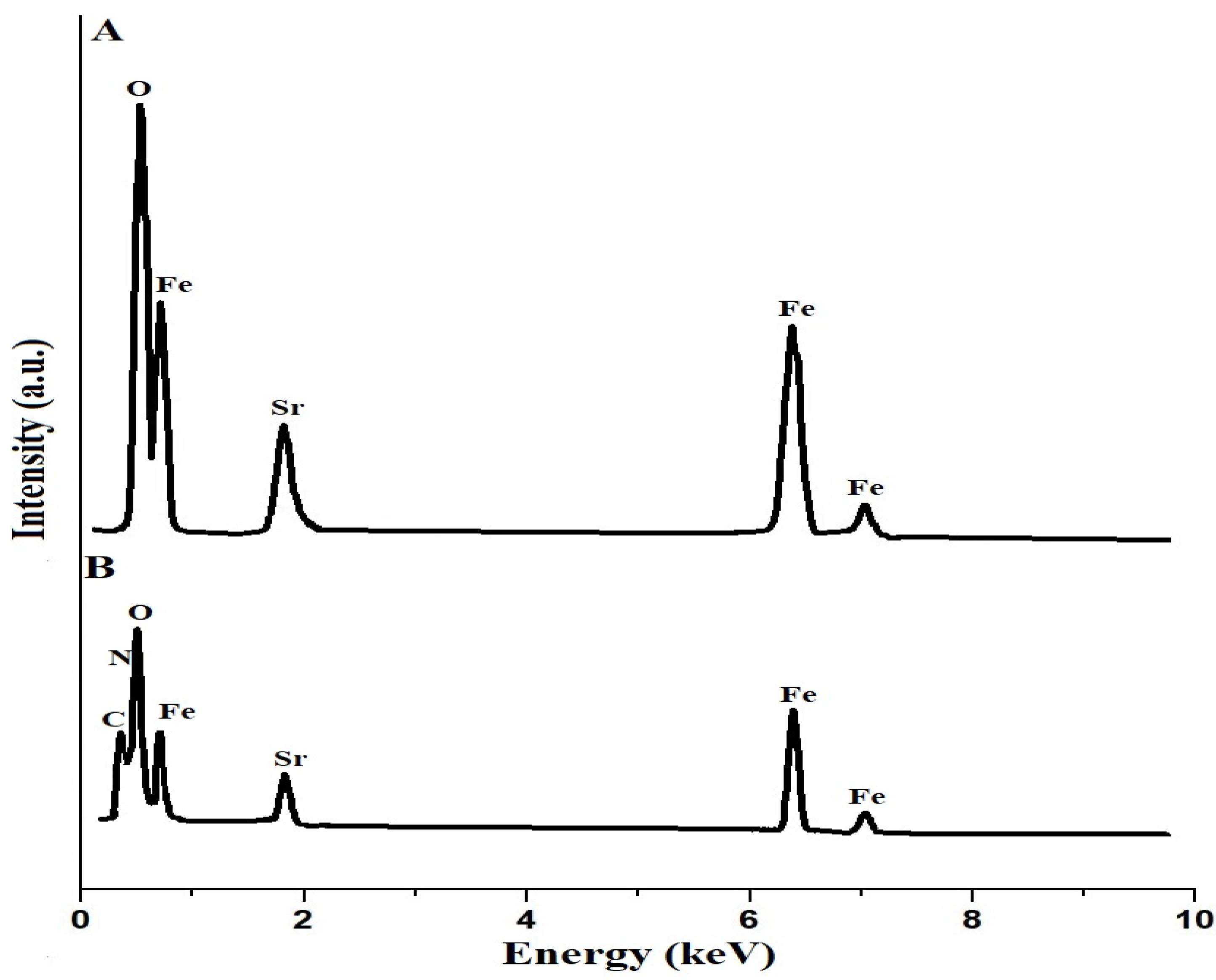
Figure 5A,B show the EDX patterns of the SrFe12O19 nanoparticles and the chitosan–Schiff base–SrFe12O19 nanocomposite, respectively. The results show that the SrFe12O19 nanoparticles consist of Fe, Sr, and O with weight percentages equal to 64.60, 8.73, and 26.67%, respectively. Also, the chitosan–Schiff base–SrFe12O19 nanocomposite consists of Fe, Sr, O, C, and N with weight percentages equal to 28.10, 5.83, 33.42, 26.94, and 5.71%, respectively. Hence, the presence of C and N atoms in the chitosan–Schiff base-SrFe12O19 nanocomposite, in addition to Fe, Sr, and O atoms, confirmed the functionalization of the SrFe12O19 nanoparticles with the chitosan–Schiff base ligand. The presence of these additional elements and their corresponding functional groups (imine and hydroxyl groups) is critical for the nanocomposite’s enhanced performance in removing Pb(II) ions.
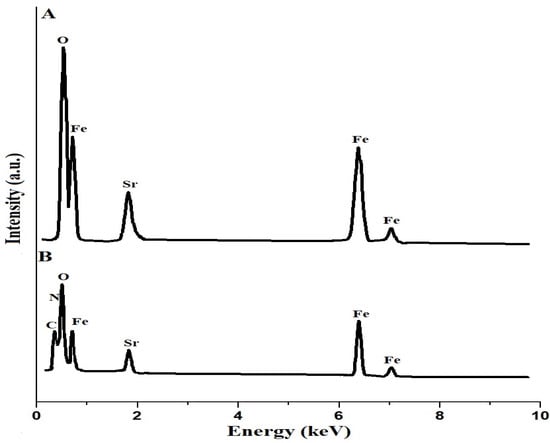
Figure 5.
EDX patterns of the SrFe12O19 nanoparticles (A) and the chitosan–Schiff base–SrFe12O19 nanocomposite (B).
2.2. Removal of Pb(II) Ions from Aqueous Media
2.2.1. Effect of pH
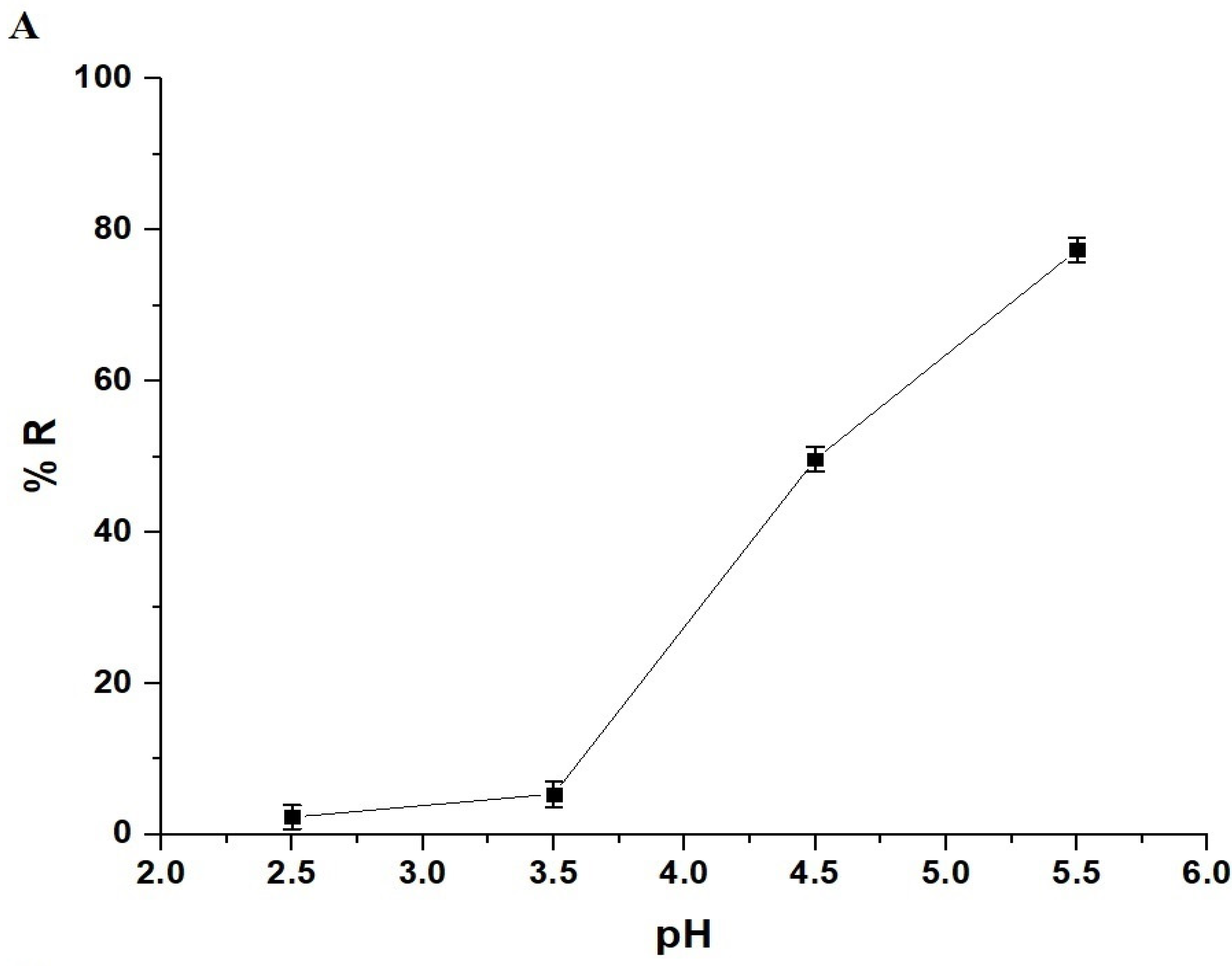
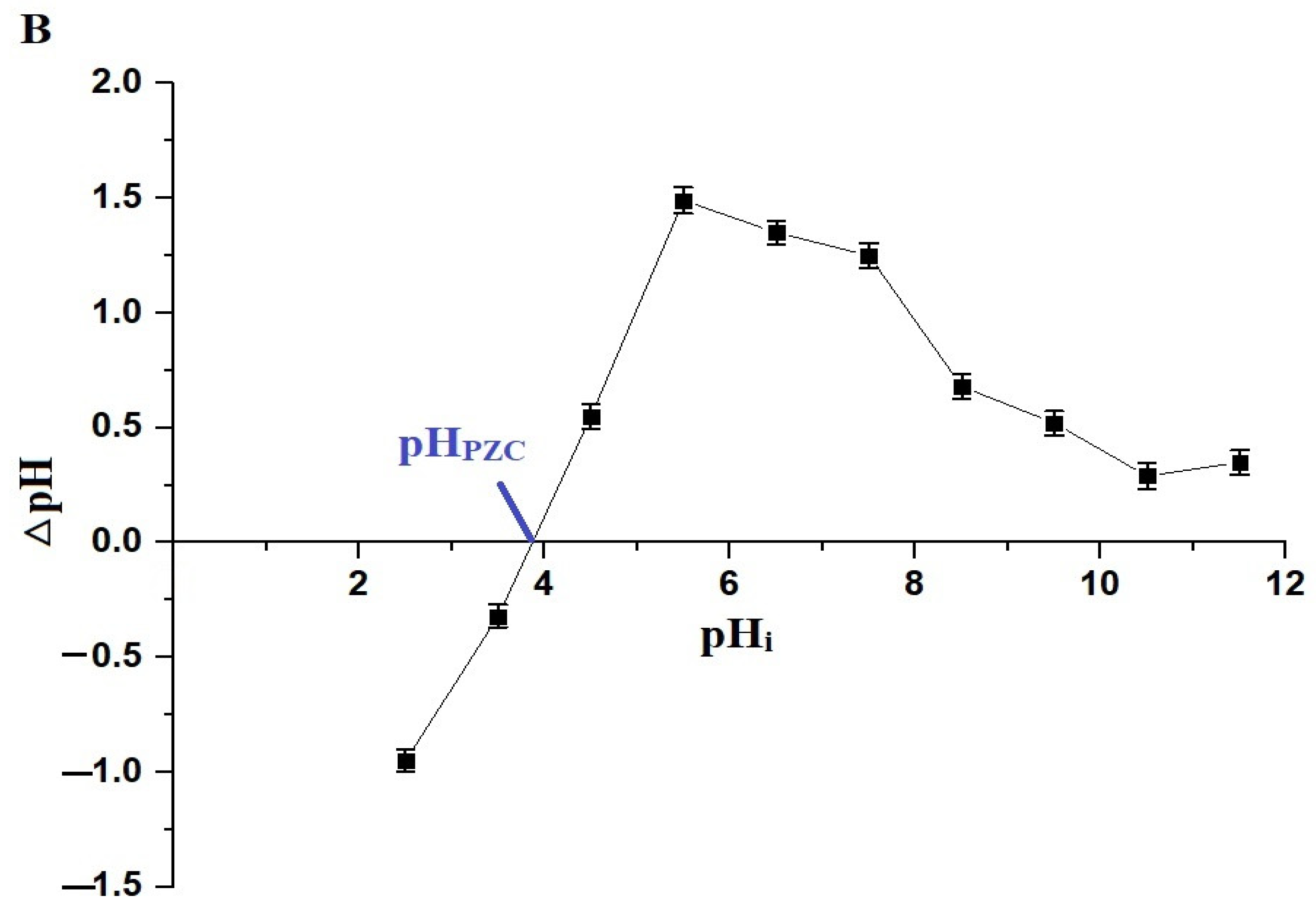
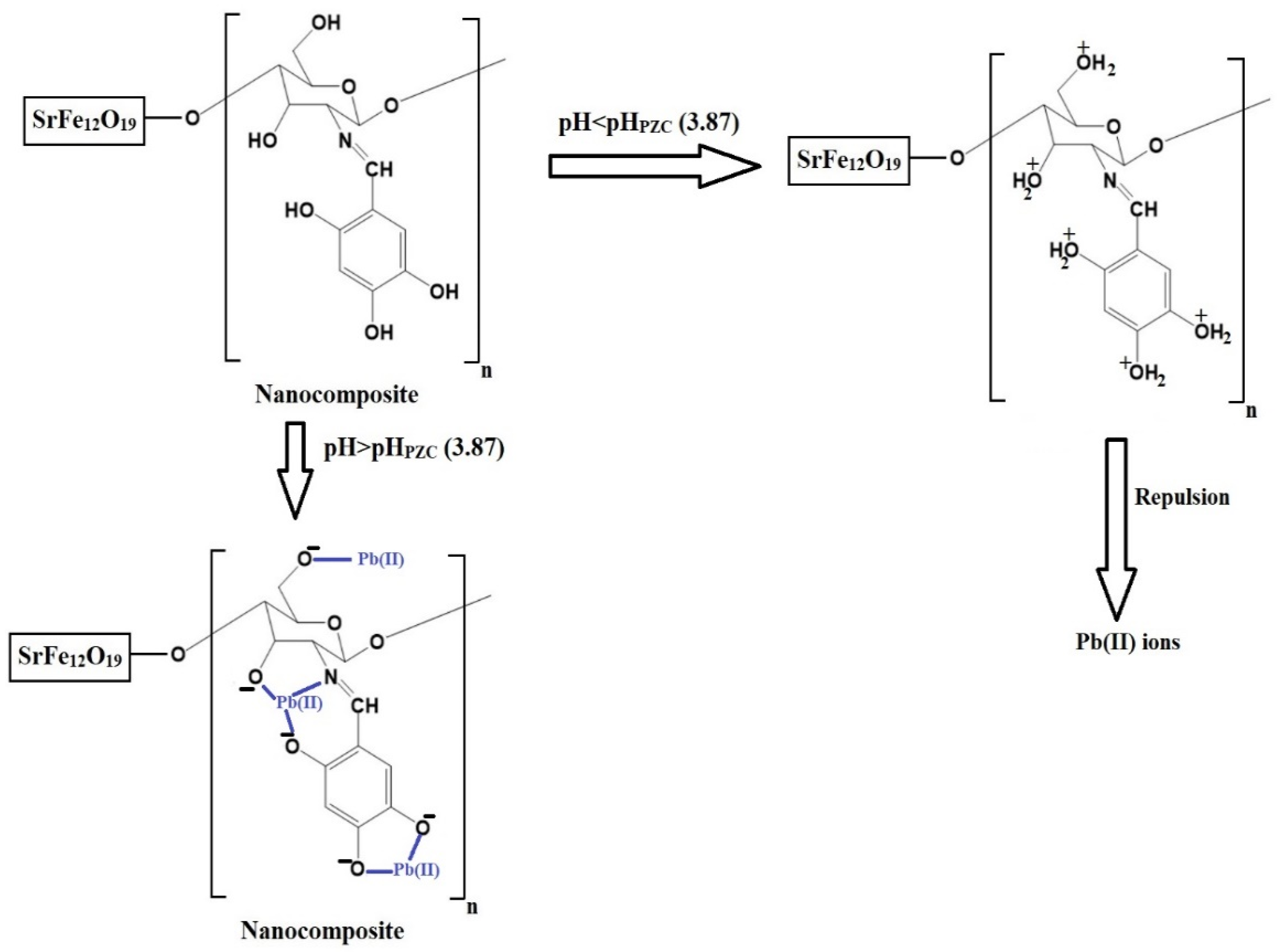
The pH of a solution affects both the adsorbates and adsorbents. In this study, a pH range of 2.5–5.5 was chosen to investigate the removal of Pb(II) ions using the chitosan–Schiff base–SrFe12O19 nanocomposite (Figure 6A). pH values higher than 5.5 were not investigated due to the precipitation of Pb(II) ions at these values. The goal of this research was to examine the removal of Pb(II) ions through adsorption using the synthesized nanocomposite, excluding any effects from precipitation. The point of zero charge (pHPZC) for the nanocomposite was 3.87 (Figure 6B). Below this pH, the nanocomposite showed a poor removal efficiency due to the protonation of the active sites and the repulsion between the protonated sites and Pb(II) ions (Scheme 3) [33,34]. However, when the solution pH increased above 3.87, the adsorption of the Pb(II) ions onto the chitosan–Schiff base–SrFe12O19 nanocomposite was primarily driven by the coordination chemistry of the functional groups present in the chitosan–Schiff base ligand (Scheme 3). This coordination mechanism is supported by other works by Yuvaraja et al. and Shahraki et al., which highlight similar interactions in related systems [23,35,36]. The removal efficiency of Pb(II) ions using the chitosan–Schiff base–SrFe12O19 nanocomposite reached its maximum value (77.34%) at a pH of 5.5. Under the same conditions (pH 5.5), it was found that the percentage of Pb(II) ions removed using strontium ferrite alone was about 19.24%. This finding underscores the significant enhancement in performance attributed to the functionalization of strontium ferrite nanoparticles with the chitosan–Schiff base ligand. The dramatic increase in the removal efficiency with strontium ferrite alone from 19.24% to 77.34% with the nanocomposite highlights the critical role of the active functional groups (-OH and -C=N) in the chitosan–Schiff base ligand. These groups facilitate the better complexation and adsorption of Pb(II) ions, demonstrating the efficacy of the nanocomposite in environmental remediation applications.
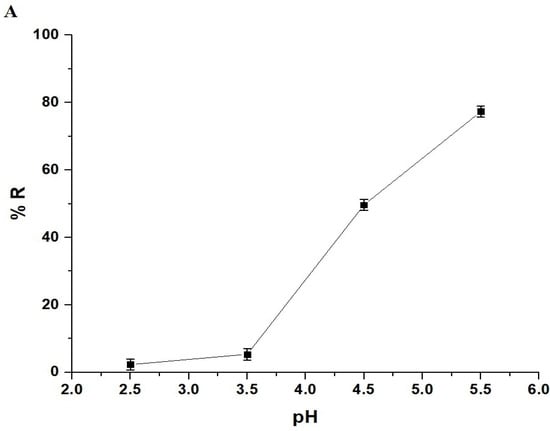
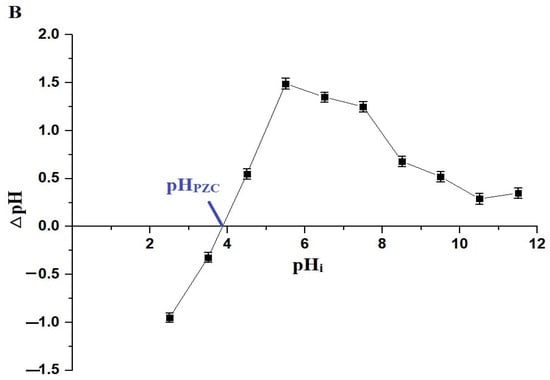
Figure 6.
Effect of pH on the removal percentage of Pb(II) ions by the chitosan–Schiff base–SrFe12O19 nanocomposite (A). The point of zero charge (pHPZC) of the chitosan–Schiff base–SrFe12O19 nanocomposite (B).
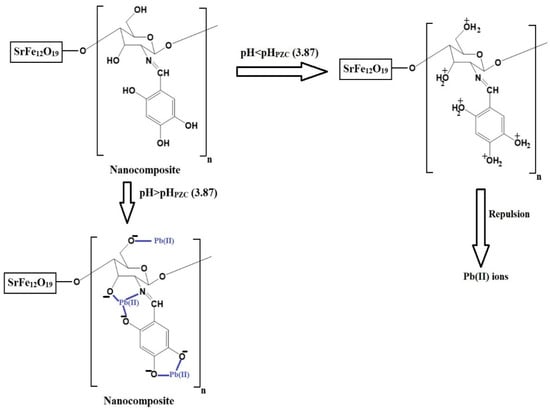
Scheme 3.
Proposed adsorption mechanism of Pb(II) ions on the chitosan–Schiff base–SrFe12O19 nanocomposite.
2.2.2. Effect of Contact Time
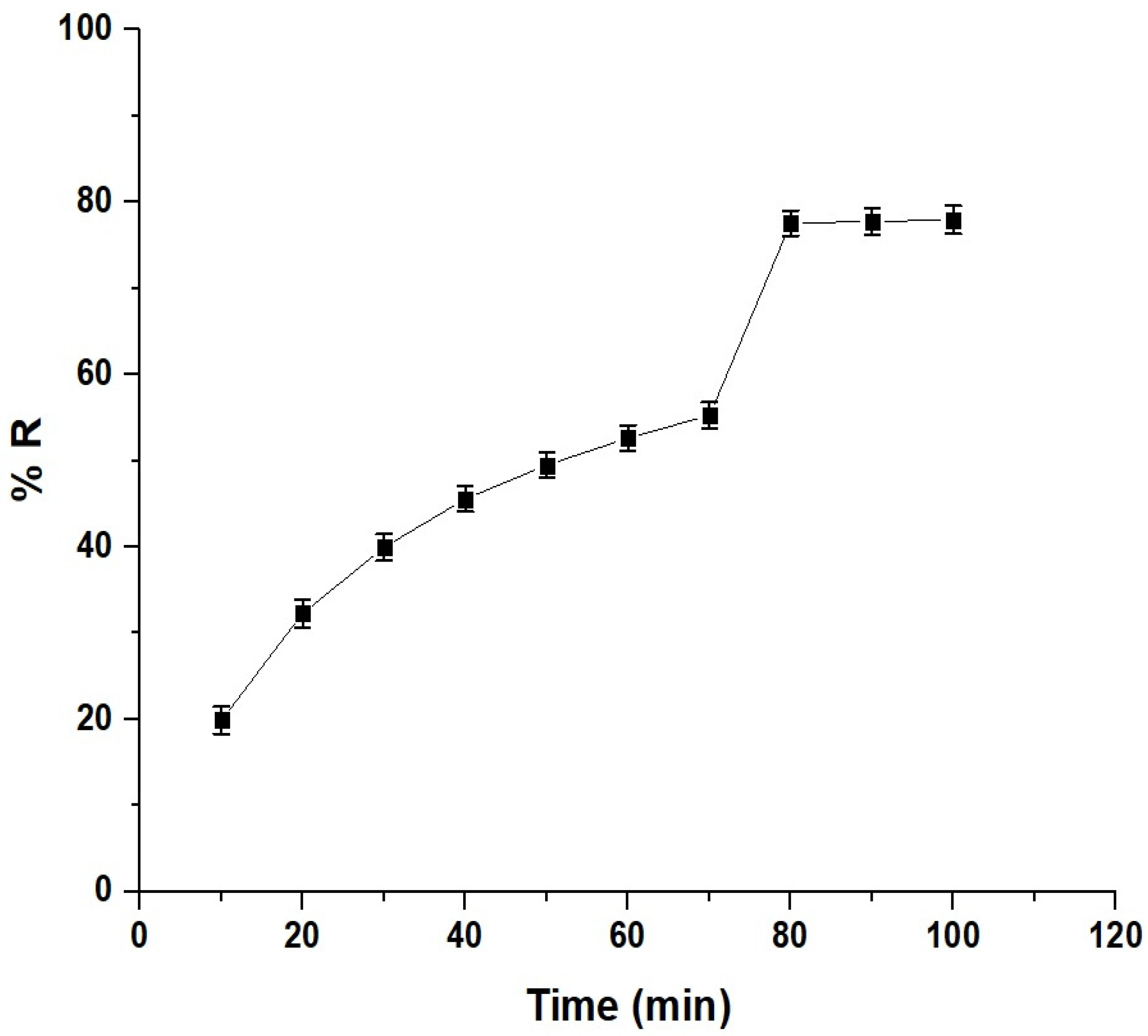
The contact time in adsorption refers to the duration that the adsorbent is in contact with the solution containing the adsorbate. It influences the efficiency and kinetics of the adsorption. In this study, a time range of 10–100 min was chosen to investigate the Pb(II) ion removal using the chitosan–Schiff base–SrFe12O19 nanocomposite (Figure 7). The removal efficiency increased with the contact time, reaching a maximum of 77.56% at 80 min. Beyond 80 min, the efficiency stabilized due to the saturation of the active sites on the nanocomposite’s surface [33,34].
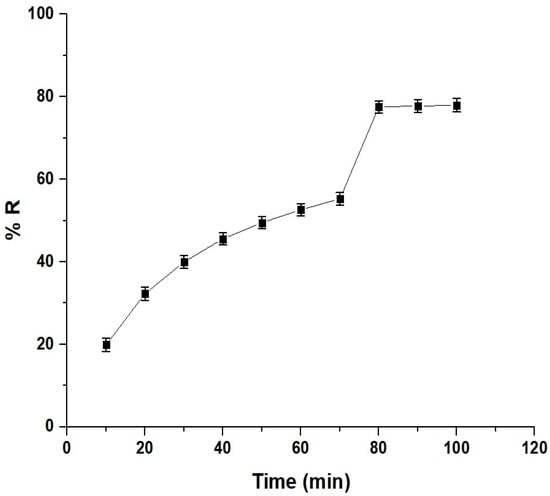
Figure 7.
Effect of the contact time on the removal percentage of Pb(II) ions by the chitosan–Schiff base–SrFe12O19 nanocomposite.
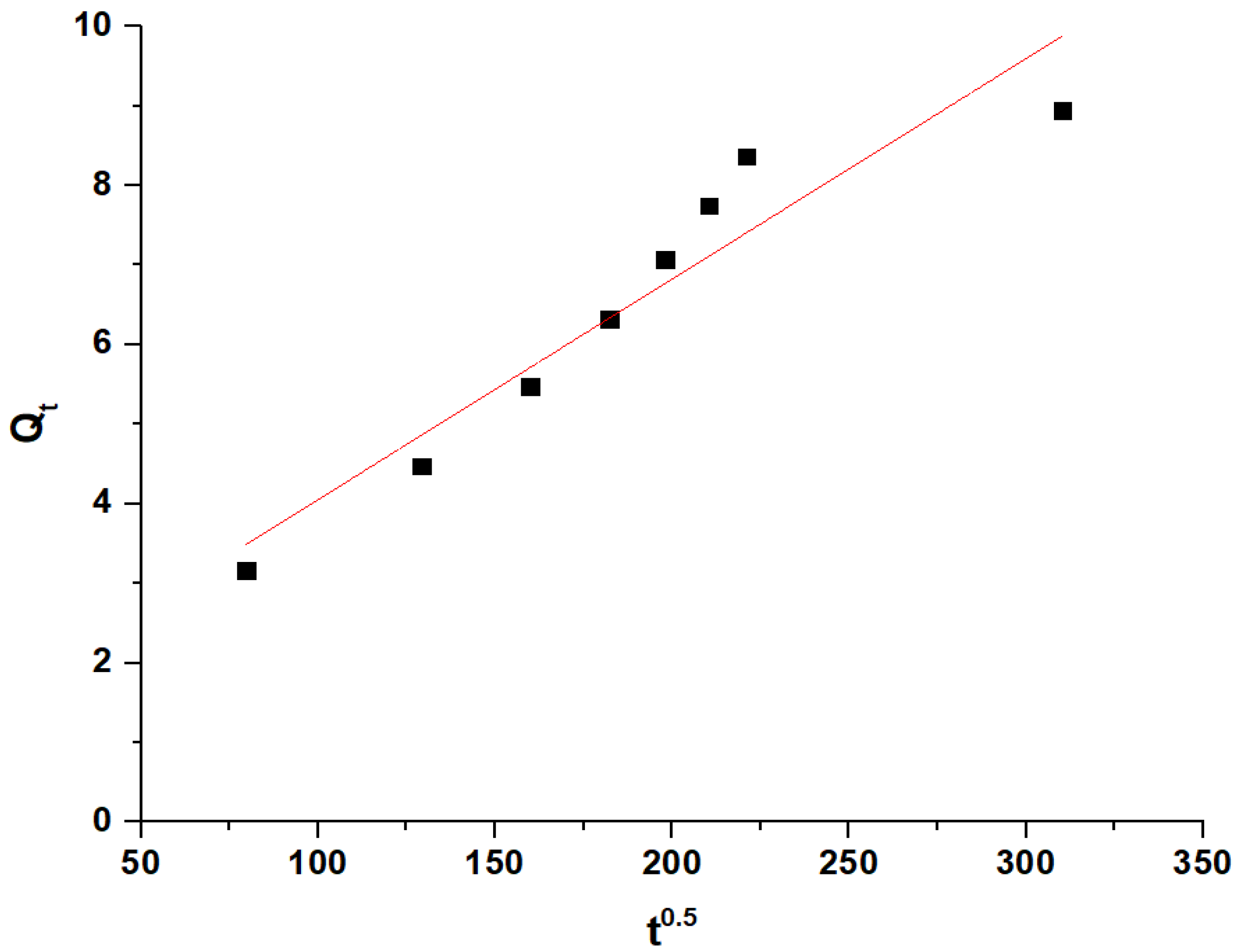
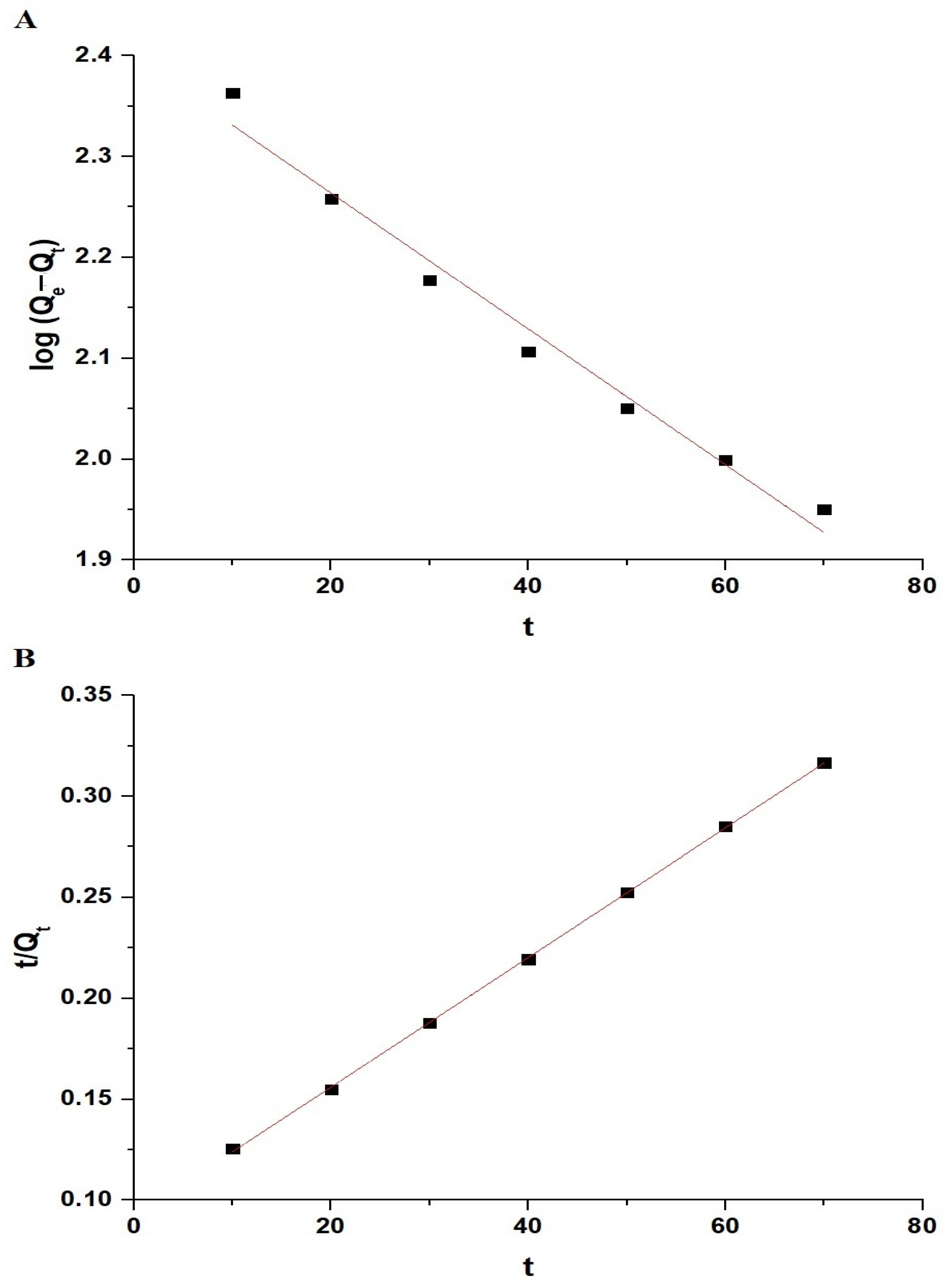
The noticeable increase in the removal efficiency of Pb(II) ions between 70 and 80 min is due to the transition from the surface adsorption to the intraparticle diffusion. During this period, the Pb(II) ions begin to penetrate and diffuse into the internal pores of the adsorbent, significantly enhancing the adsorption capacity as these newly accessible internal sites become occupied. This observation is supported by the intraparticle diffusion model (Equation (1)) [37]:
where Qt is the amount of adsorbate adsorbed at time (t) (mg/g), KID is the intraparticle diffusion rate constant (mg/g min0.5), and C represents the boundary layer constant (mg/g). The linear portion of the intraparticle diffusion plot (Figure 8) indicates that intraparticle diffusion is the rate-limiting step during this period. The values of KID and C are 0.0277 (mg/g min0.5) and 1.2849 mg/g, respectively.
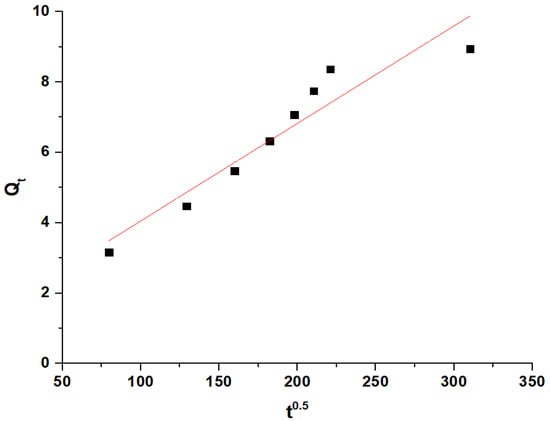
Figure 8.
Intraparticle diffusion model.
To examine the kinetics of the adsorption process, we employed two models: the pseudo-first-order and pseudo-second-order kinetic models, represented by Equations (2) and (3), respectively [33,34]:
where Qt represents the amount of Pb(II) ions adsorbed at a given time (t) (mg/g), Qe represents the amount of Pb(II) ions adsorbed at equilibrium (mg/g), K1 represents the pseudo-first-order rate constant (1/min), and K2 represents the pseudo-second-order rate constant (g/mg.min). Figure 9A,B display the fitting of the experimental adsorption data to the pseudo-first-order and pseudo-second-order models, respectively. Table 1 displays the constant values for both kinetic models. The data in Table 1 indicate that the R2 value for the pseudo-second-order model exceeds that of the pseudo-first-order model. Furthermore, the calculated sorption capacity in the pseudo-second-order model closely matches the experimental sorption capacity (QExp) in comparison to the pseudo-first-order model. Consequently, the sorption of Pb(II) ions on the chitosan–Schiff base–SrFe12O19 nanocomposite is more accurately depicted by the pseudo-second-order kinetic model.
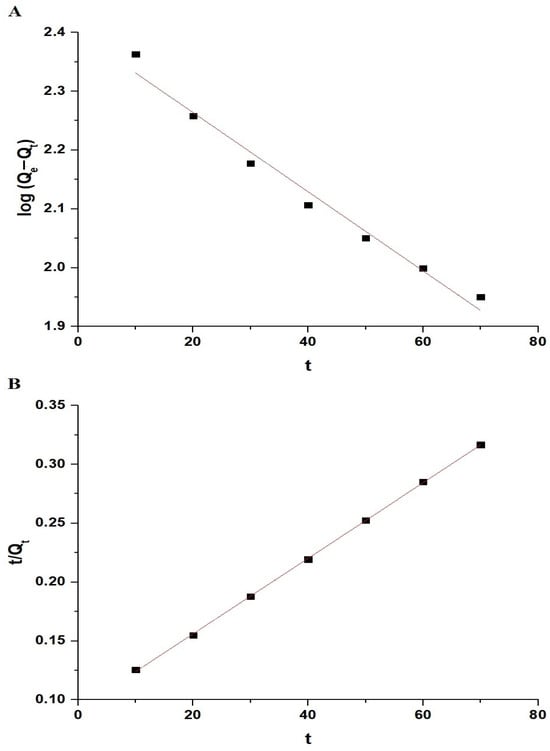
Figure 9.
The fitting of the experimental sorption data to the pseudo-first-order (A) and pseudo-second-order (B) models.

Table 1.
Kinetic constants for the sorption of Pb(II) ions by the chitosan–Schiff base–SrFe12O19 nanocomposite.
2.2.3. Effect of Temperature
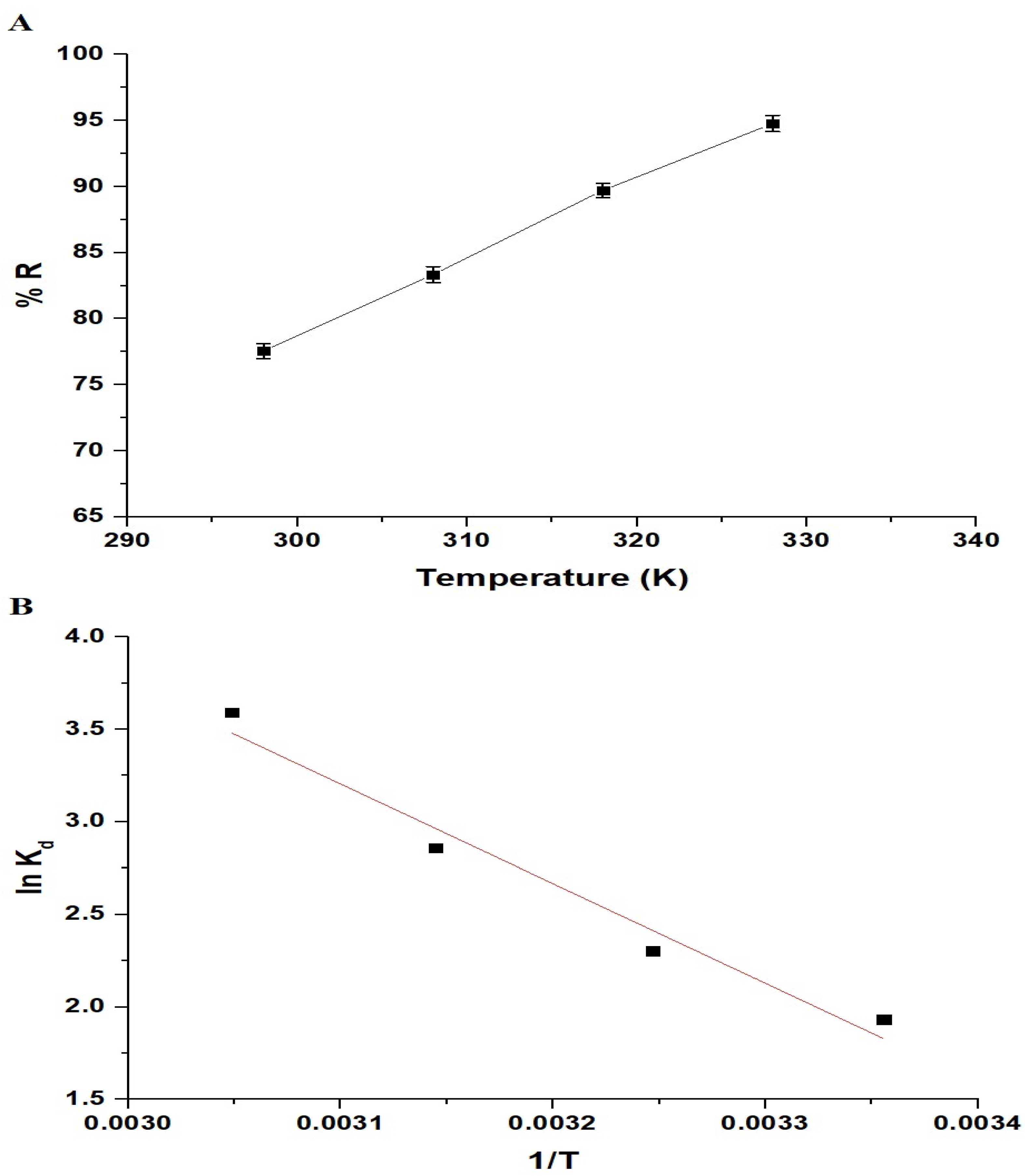
As shown in Figure 10A, the percentage of Pb(II) ion removal increased from 77.56% to 94.78% as the temperature rose from 298 K to 328 K. Hence, temperature influences the sorption of Pb(II) ions. Thermodynamic parameters such as the enthalpy change (ΔHo), Gibbs free energy change (ΔGo), and entropy change (ΔSo) were used to assess the sorption process. These constants were calculated using Equations (4)–(6) [33,34]:
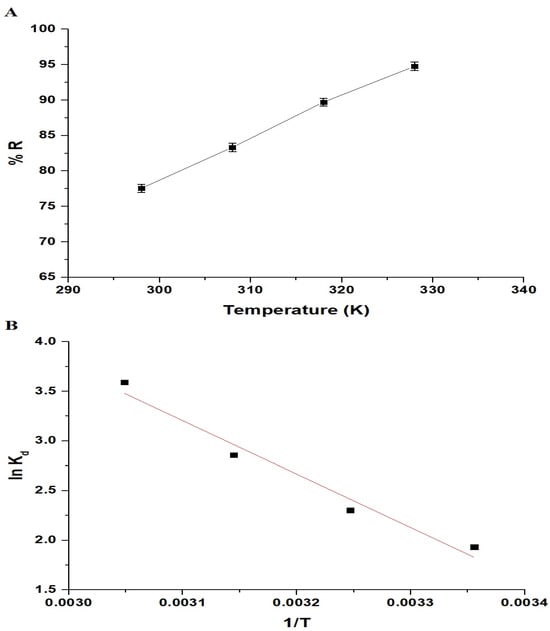
Figure 10.
Effect of temperature on the removal percentage of Pb(II) ions by the chitosan–Schiff base–SrFe12O19 nanocomposite (A). The plot of lnKd versus 1/T (B).
R signifies the universal gas constant (KJ/molK), T represents the temperature of the sorption (K), and Kd signifies the distribution coefficient (L/g). Figure 10B illustrates the relationship between the lnKd and 1/T, while the thermodynamic constants are provided in Table 2. Upon reviewing Table 2, it becomes apparent that the negative values of ΔGo indicate the spontaneity of the Pb(II) sorption onto the chitosan–Schiff base–SrFe12O19 nanocomposite. Additionally, the magnitude and positive sign of the ΔHo indicate an endothermic and chemisorptive sorption process. The positive value of ΔSo suggests an increased level of randomness at the interface between the adsorbent and the solution.

Table 2.
Thermodynamic constants for the sorption of Pb(II) ions by the chitosan–Schiff base–SrFe12O19 nanocomposite.
2.2.4. Effect of Concentration
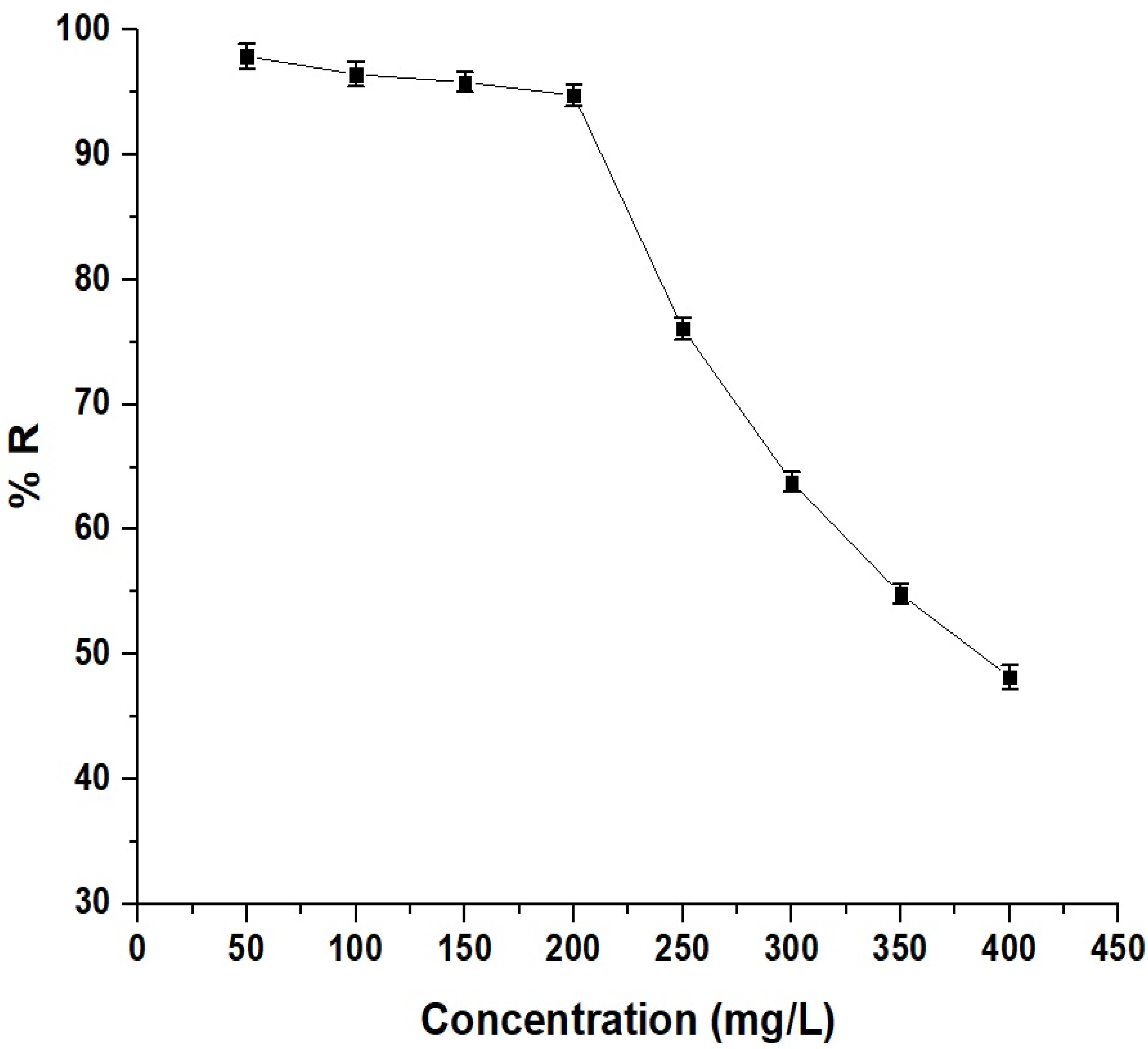
As shown in Figure 11, the percentage of Pb(II) ion removal decreased from 97.90% to 63.82% as the concentration increased from 50 to 300 mg/L. This decrease is due to the saturation of available adsorption sites on the adsorbent’s surface. Initially, at lower concentrations, there are many vacant sites for adsorbate molecules. As the concentration increases, more sites become occupied, leaving fewer sites available for additional adsorption, resulting in a lower adsorption percentage [33,34].
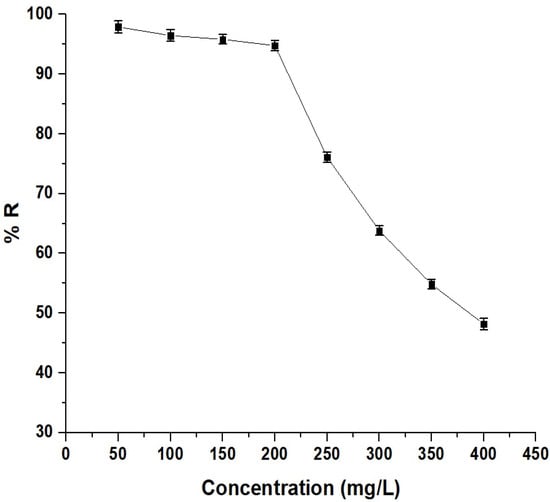
Figure 11.
Effect of concentration on the removal percentage of Pb(II) ions by the chitosan–Schiff base–SrFe12O19 nanocomposite.
To examine the mechanism of the adsorption process, we employed two isotherms: the Langmuir and Freundlich isotherms, represented by Equations (7) and (8), respectively [33,34]:
where 1/n represents the heterogeneity constant, KL represents the Langmuir equilibrium constant (L/mg), KF represents the Freundlich equilibrium constant (mg/g)(L/mg)1/n, and Qmax represents the Langmuir maximum adsorption capacity (mg/g). Equation (9) can be employed to determine the Qmax by applying the Freundlich equilibrium isotherm [33,34]:
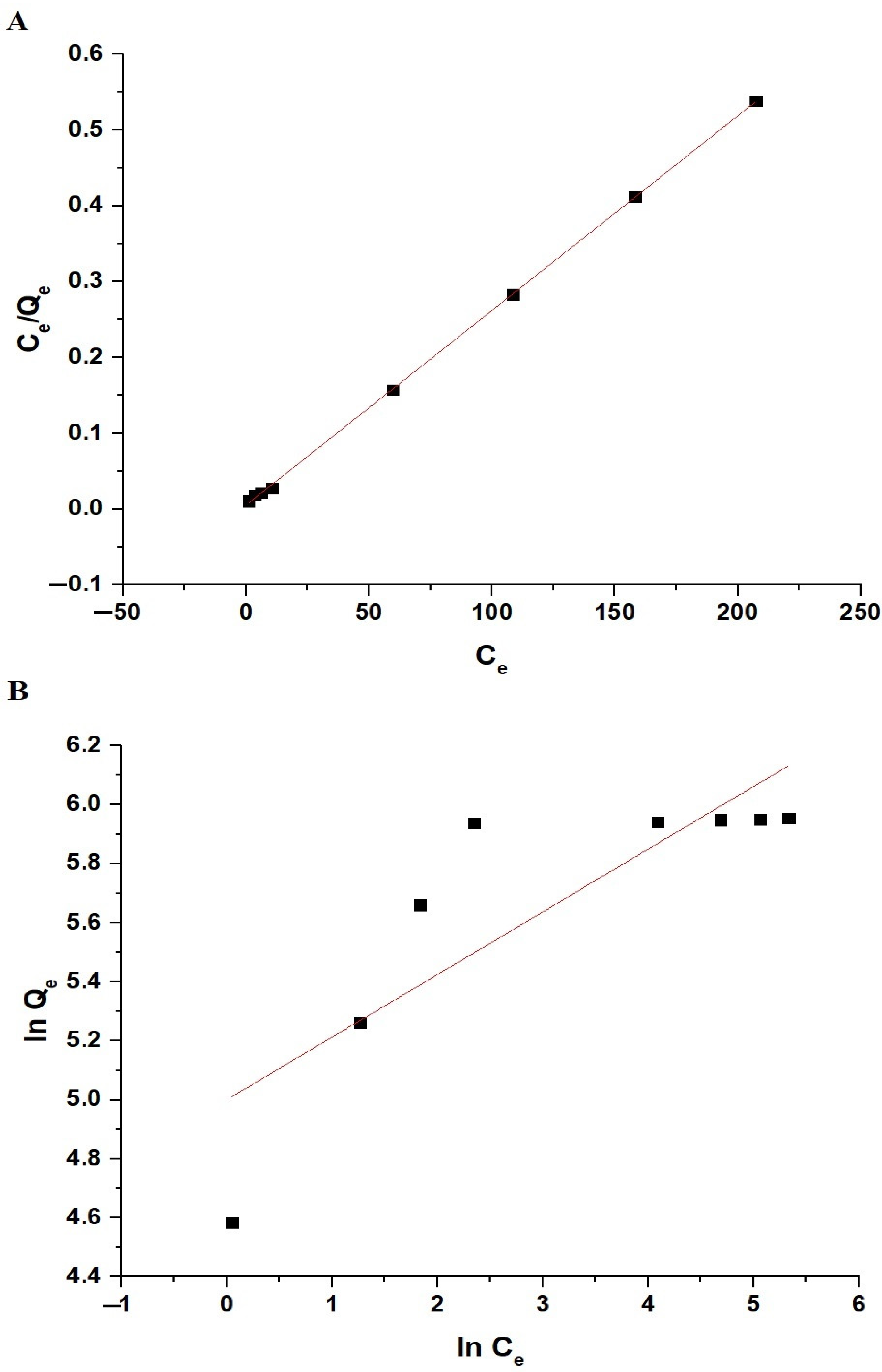
Figure 12A,B illustrate the fitting of the experimental adsorption data to the Langmuir and Freundlich isotherms, respectively. Table 3 displays the constant values for both equilibrium isotherms. The data in Table 3 indicate that the R2 value for the Langmuir isotherm exceeds that of the Freundlich isotherm. Consequently, the sorption of Pb(II) ions on the chitosan–Schiff base–SrFe12O19 nanocomposite is more accurately depicted by the Langmuir isotherm. This implies that the adsorption process occurs on a homogenous surface with uniform adsorption sites, forming a monolayer of adsorbate molecules.
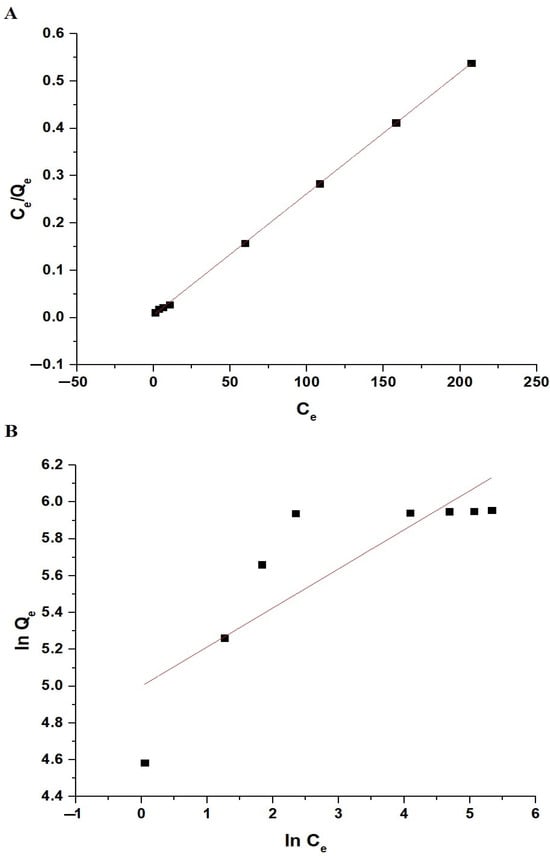
Figure 12.
The fitting of the experimental sorption data to the Langmuir (A) and Freundlich (B) isotherms.

Table 3.
Equilibrium constants for the sorption of Pb(II) ions by the chitosan–Schiff base–SrFe12O19 nanocomposite.
In this study, the sorption capacity of the chitosan–Schiff base–SrFe12O19 nanocomposite was compared with the values listed in Table 4 [38,39,40,41,42,43,44,45] from previous literature. Remarkably, the sorption capacity of the chitosan–Schiff base–SrFe12O19 nanocomposite exceeds those of most of the adsorbents cited in the literature. The chitosan–Schiff base–SrFe12O19 nanocomposite has a higher sorption capacity due to the introduction of functional groups like hydroxyl and imine, which have a high affinity for Pb(II) ions. Additionally, its high surface area, porous structure, and the synergistic effect of its components enhance its overall adsorption performance.

Table 4.
Comparative analysis of Pb(II) ion adsorption capacities across different adsorbents.
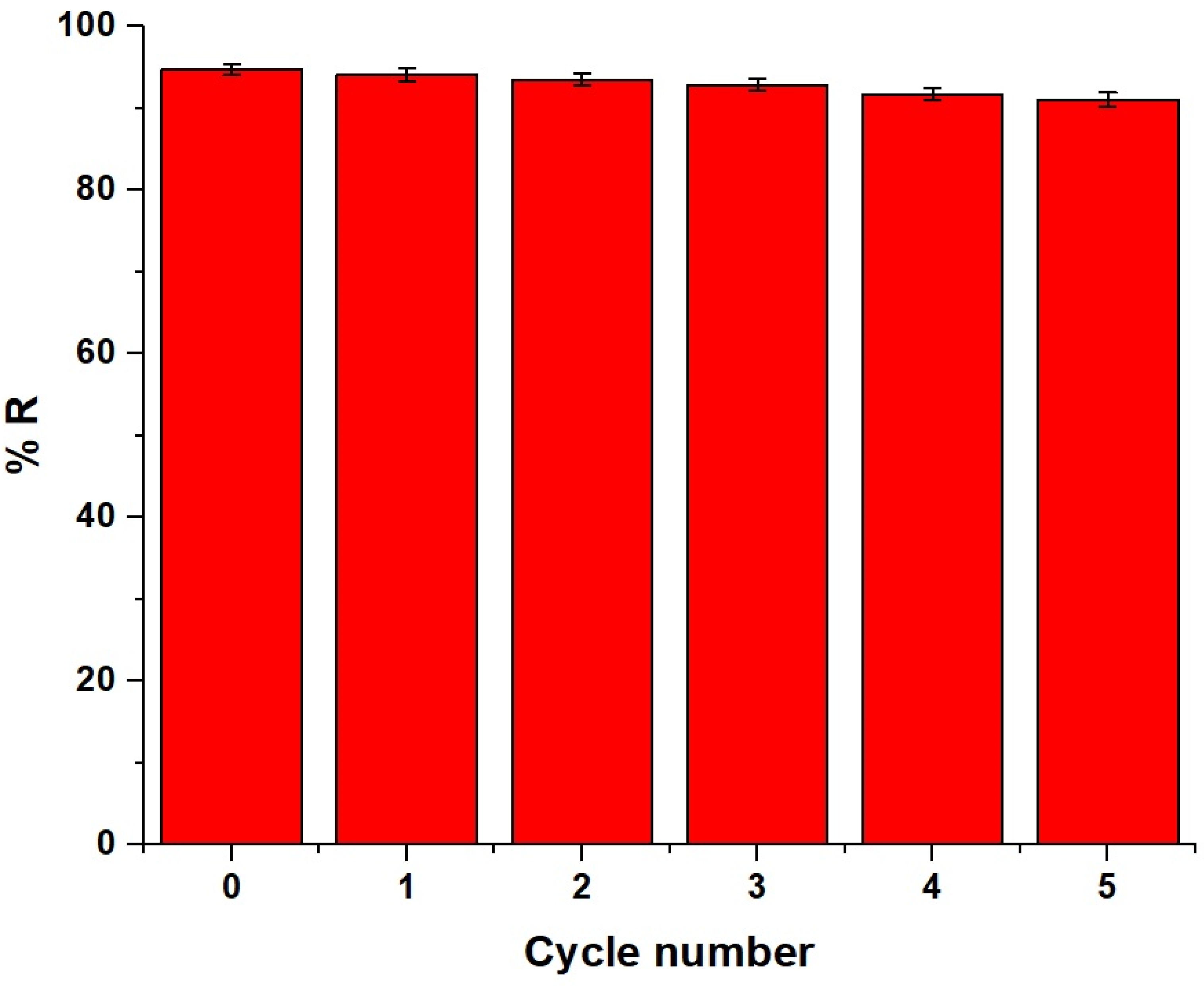
2.2.5. Reusability
The adsorbent material loaded with Pb(II) ions was regenerated by stirring it in 50 mL of 1 M of ethylenediaminetetraacetic acid disodium salt dihydrate, where the regeneration efficiency equals 99.87%. EDTA is highly effective in desorbing Pb(II) ions due to its strong chelating properties, which form stable complexes with heavy-metal ions, facilitating their removal from the adsorbent surface. After that, the reusability of the adsorbent was studied for five cycles under optimum conditions, as described in the Experiment Section, and the results are displayed in Figure 13. After five cycles, the removal percentage of Pb(II) ions decreased slightly, confirming that the synthesized adsorbent could be used repeatedly. The incomplete desorption of Pb(II) ions was the cause of the slight decrease in the removal efficiency after multiple cycles.
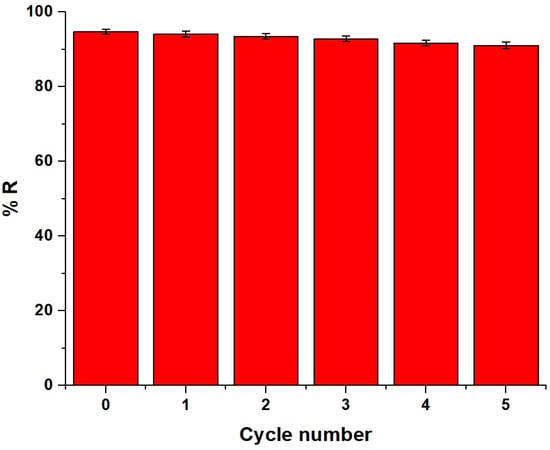
Figure 13.
Reusability of the chitosan–Schiff base–SrFe12O19 nanocomposite for the adsorption of Pb(II) ions.
3. Experiment
3.1. Chemicals
Strontium(II) nitrate tetrahydrate (Sr(NO3)2·4H2O, CAS No. 10042-76-9), propylene glycol (C3H8O2, CAS No. 57-55-6), iron(III) nitrate nonahydrate (Fe(NO3)3·9H2O, CAS No. 7782-61-8), sodium hydroxide (NaOH, CAS No. 1310-73-2), tartaric acid (C4H6O6, CAS No. 87-69-4), acetic acid (CH3COOH, CAS No. 64-19-7), chitosan ((C6H11NO4)n, CAS No. 9012-76-4), hydrochloric acid (HCl, CAS No. 7647-01-0), ethanol (C2H5OH, CAS No. 64-17-5), 2,4,5-trihydroxybenzaldehyde (C7H6O4, CAS No. 35094-87-2), lead nitrate (Pb(NO3)2, CAS No. 10099-74-8), and ethylenediaminetetraacetic acid disodium salt dihydrate (C10H16N2Na4O10, CAS No. 6381-92-6) were acquired from Sigma-Aldrich and utilized as received, without undergoing further purification.
3.2. Synthesis of Strontium Ferrite (SrFe12O19) Nanoparticles
A Fe(III) solution was created by dissolving 6.50 g of iron(III) nitrate nonahydrate in 50 mL of distilled water. Subsequently, a composite of iron tartrate and propylene glycol was formed by introducing a solution of tartaric acid, made by dissolving 3.63 g of tartaric acid in 50 mL of distilled water, into the Fe(III) solution. This mixture was then agitated at ambient temperature for 10 min before 5 mL of propylene glycol was added and the agitation was continued for an additional 10 min. A Sr(II) solution was prepared by dissolving 0.38 g of strontium(II) nitrate tetrahydrate in 50 mL of distilled water. Following this, a composite of strontium tartrate and propylene glycol was formed by adding a tartaric acid solution, which was concocted by dissolving 0.21 g of tartaric acid in 50 mL of distilled water, into the Sr(II) solution. This mixture was stirred at ambient temperature for 10 min, after which 5 mL of propylene glycol was added, and stirring persisted for another 10 min. The strontium tartrate/propylene glycol composite was then combined with the iron tartrate/propylene glycol composite, stirring continuously at 413 K until the solution completely evaporated. The residual powder was subsequently calcined at 1273 K for 2 h to yield strontium ferrite (SrFe12O19) nanoparticles. These experimental steps are concisely depicted in Scheme 4.
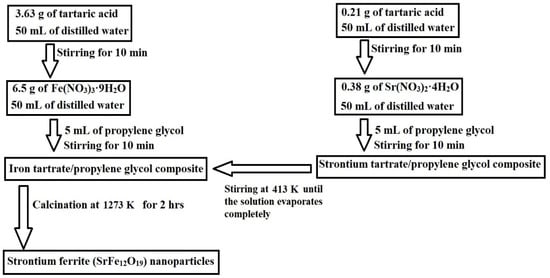
Scheme 4.
The practical steps for the synthesis of strontium ferrite (SrFe12O19) nanoparticles.
3.3. Functionalization of Strontium Ferrite Nanoparticles with Novel Chitosan–Schiff Base Ligand
A chitosan solution was prepared by dissolving 1 g of chitosan in a mixture of 5 mL of acetic acid and 15 mL of ethanol. An aldehyde solution of 1 g of 2,4,5-trihydroxybenzaldehyde in 20 mL of ethanol was slowly added to the chitosan solution. The resulting mixture was refluxed at 373 K for 6 h to obtain the chitosan–Schiff base ligand. After that, approximately 1.5 g of SrFe12O19 nanoparticles was added to the mixture under vigorous stirring and reflux for another 6 h. The formed chitosan–Schiff base–SrFe12O19 nanocomposite was subsequently filtered, repeatedly washed with cold ethanol, and oven-dried at 333 K for 8 h. These experimental steps are concisely depicted in Scheme 5.
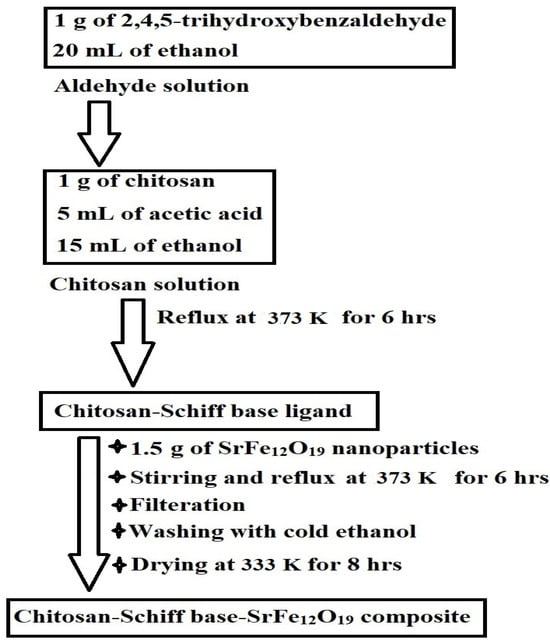
Scheme 5.
The practical steps for the functionalization of strontium ferrite nanoparticles with the novel chitosan–Schiff base ligand.
3.4. Instrumentation
Fourier transform infrared (FT-IR) spectra of the synthesized samples were obtained utilizing a Nicolet FT-IR spectrometer, employing KBr pellets, within the range of 400–4000 cm−1. X-ray diffraction (XRD) analysis of the synthesized samples was conducted using a Bruker D8 Advance X-ray diffractometer equipped with Cu Kα radiation (λ= 1.54 Å), with the scanning range set from 2Ɵ = 10° to 2Ɵ = 70°. The magnetic characteristics were examined utilizing an MPMS-XL-7 vibrating-sample magnetometer (VSM). The surface images and elemental composition of the synthesized samples were recorded by a JSM-IT800 Schottky field-emission scanning electron microscope (FE-SEM)/energy-dispersive X-ray spectroscope (EDX).
3.5. Removal of Pb(II) Ions from Aqueous Media
Adsorption experiments of Pb(II) ions regarding the effect of the pH (2.5–5.5) were performed in a series of 250 mL conical flasks holding 100 mL of Pb(II) solution (200 mg/L) with 0.05 g of the chitosan–Schiff base–SrFe12O19 nanocomposite as an adsorbent at 298 K. Then, the mixture was magnetically stirred for 180 min. Adsorption experiments of the Pb(II) ions regarding the effect of the contact time (10–100 min) were performed in a series of 250 mL conical flasks holding 100 mL of Pb(II) solution (200 mg/L) with 0.05 g of chitosan–Schiff base–SrFe12O19 nanocomposite as an adsorbent at 298 K and a pH of 5.5. Adsorption experiments of the Pb(II) ions regarding the effect of temperature (298–328 K) were performed in a series of 250 mL conical flasks holding 100 mL of Pb(II) solution (200 mg/L) with 0.05 g of chitosan–Schiff base–SrFe12O19 nanocomposite as an adsorbent at a pH of 5.5 and a contact time of 80 min. Adsorption experiments of the Pb(II) ions regarding the effect of the concentration (50–400 mg/L) were performed in a series of 250 mL conical flasks holding 100 mL of Pb(II) solution with 0.05 g of chitosan–Schiff base–SrFe12O19 nanocomposite as an adsorbent at a pH of 5.5, 298 K, and a contact time of 80 min.
The amount of Pb(II) ions adsorbed by the chitosan–Schiff base–SrFe12O19 nanocomposite (Q, mg/g) was calculated using Equation (10) [33,34]. Also, the removal percentage of Pb(II) ions using the chitosan–Schiff base–SrFe12O19 nanocomposite (% R) was calculated using Equation (11) [33,34]:
where V (L) represents the volume of the Pb(II) solution; M (g) represents the amount of the chitosan–Schiff base–SrFe12O19 nanocomposite; Ci (mg/L) represents the initial concentration of Pb(II) ions; Ce (mg/L) represents the equilibrium concentration of Pb(II) ions.
The analytical method used for the determination of Pb(II) ions involved the use of atomic absorption spectroscopy (AAS). A 210-Buck Scientific atomic absorption spectrophotometer measured the concentration of Pb(II) ions before and after adsorption. The key analytical parameters included a wavelength of 283.3 nm, a slit width of 0.7 nm, an air–acetylene flame, and a lamp current of 10 mA. Calibration curves were prepared using standard solutions of Pb(II) ions to ensure accurate quantification.
3.6. Point of Zero Charge (pHPZC)
The point of zero charge (pHPZC) of the chitosan–Schiff base–SrFe12O19 nanocomposite was determined by the salt addition procedure [33,34]. A collection of 150 mL Erlenmeyer flasks were set up, each containing 25 mL of a 0.01 M KNO3 solution, with the initial pH (pHi) values adjusted between 2.50 and 11.50. The pH levels of these solutions were modified using 0.1 M HCl or NaOH. Subsequently, 0.05 g of the chitosan–Schiff base–SrFe12O19 nanocomposite was added to each flask. The mixtures were continuously stirred for 6 h. Following the separation of the solutions, the final pH (pHf) of the supernatants was recorded. The point of zero charge (pHPZC) was identified by plotting the change in the pH (ΔpH) against the pHi, locating the pHPZC at the point where the curve crosses the pHi axis at zero [46].
4. Conclusions
Strontium ferrite (SrFe12O19) nanoparticles were facilely synthesized using the Pechini sol–gel technique and subsequently functionalized with a newly developed chitosan–Schiff base ligand, resulting in the creation of a novel nanocomposite. The chitosan–Schiff base ligand was synthesized through the reaction of chitosan with 2,4,5-trihydroxybenzaldehyde. The functionalization of the SrFe12O19 nanoparticles with the chitosan–Schiff base ligand was confirmed by various characterization techniques, including XRD, FT-IR, SEM, EDX, and VSM. XRD analysis showed characteristic peaks indicating successful synthesis and functionalization. FT-IR spectroscopy revealed the presence of functional groups corresponding to both SrFe12O19 and the chitosan–Schiff base ligand, confirming their integration. SEM images depicted the morphological changes and the successful coating of the nanoparticles. EDX analysis demonstrated the elemental composition, confirming the presence of C and N atoms in addition to Fe, Sr, and O, which further validated the functionalization. VSM measurements indicated a significant decrease in the saturation magnetization value, corroborating the successful functionalization of the SrFe₁₂O₁₉ nanoparticles with the chitosan–Schiff base ligand. The fabricated nanocomposite effectively adsorbed Pb(II) ions from aqueous solutions through complexation with hydroxyl (-OH) and imine (-C=N) groups. The maximum adsorption capacity of the synthesized nanocomposite for Pb(II) ions was found to be 390.63 mg/g, which is significantly higher than those of many other adsorbents reported in the literature. The adsorption of Pb(II) ions on the synthesized nanocomposite is endothermic, spontaneous, and chemical in nature, following the Langmuir equilibrium isotherm and the pseudo-second-order kinetic model. After five adsorption–desorption cycles, the nanocomposite retained a high removal efficiency, confirming its potential for repeated use without a significant loss in performance.
Author Contributions
A.S.A.-W., writing—review and editing, funding acquisition, methodology, resources; E.A.A., methodology, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number PNURSP2024R35, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors are grateful to Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia, for funding this work through Researchers Supporting Project number PNURSP2024R35.
Conflicts of Interest
The authors confirm that there are no conflicts of interest for this paper.
References
- Järup, L. Hazards of Heavy Metal Contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.C.; Joshi, A.; Mitra, D.; Gururani, P.; Kumar, N.; Joshi, H.K. Removal of Heavy Metals Using Cellulose-Based Materials: A Mini-Review. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100942. [Google Scholar] [CrossRef]
- Singh, A.; Shah, S.S.; Sharma, C.; Gupta, V.; Sundramoorthy, A.K.; Kumar, P.; Arya, S. An Approach towards Different Techniques for Detection of Heavy Metal Ions and Their Removal from Waste Water. J. Environ. Chem. Eng. 2024, 12, 113032. [Google Scholar] [CrossRef]
- Phouthavong, V.; Hagio, T.; Park, J.H.; Nijpanich, S.; Duangkhai, K.; Rujiravanit, R.; Thaveemas, P.; Chounlamany, V.; Kong, L.; Li, L.; et al. Removal of Heavy Metals by BEA Zeolite/Fe3O4 Composite Prepared via Dry-Gel Conversion Method Using Agrowaste-Derived Raw Material. Solid State Sci. 2024, 149, 107473. [Google Scholar] [CrossRef]
- Vesali-Naseh, M.; Vesali Naseh, M.R.; Ameri, P. Adsorption of Pb (II) Ions from Aqueous Solutions Using Carbon Nanotubes: A Systematic Review. J. Clean. Prod. 2021, 291, 125917. [Google Scholar] [CrossRef]
- Hussin, F.; Aroua, M.K.; Szlachta, M. Biochar Derived from Fruit By-Products Using Pyrolysis Process for the Elimination of Pb (II) Ion: An Updated Review. Chemosphere 2022, 287, 132250. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, Y.; Li, X.; Lu, J.; Zhou, J.; Huang, Z. Comparison of Heavy Metal Removals from Aqueous Solutions by Chemical Precipitation and Characteristics of Precipitates. J. Water Process Eng. 2018, 26, 289–300. [Google Scholar] [CrossRef]
- Al-Shannag, M.; Al-Qodah, Z.; Bani-Melhem, K.; Qtaishat, M.R.; Alkasrawi, M. Heavy Metal Ions Removal from Metal Plating Wastewater Using Electrocoagulation: Kinetic Study and Process Performance. Chem. Eng. J. 2015, 260, 749–756. [Google Scholar] [CrossRef]
- Xiang, H.; Min, X.; Tang, C.J.; Sillanpää, M.; Zhao, F. Recent Advances in Membrane Filtration for Heavy Metal Removal from Wastewater: A Mini Review. J. Water Process Eng. 2022, 49, 103023. [Google Scholar] [CrossRef]
- Huang, H.; Li, Z.; Wang, H.; Xia, C.; Yan, P.; Zhang, Q.; Meng, Z. Adsorption Performance of Layered Double Hydroxides for Heavy Metals Removal in Soil with the Presence of Microplastics. J. Environ. Chem. Eng. 2022, 10, 108733. [Google Scholar] [CrossRef]
- Zhang, Y.; Luo, J.; Zhang, H.; Li, T.; Xu, H.; Sun, Y.; Gu, X.; Hu, X.; Gao, B. Synthesis and Adsorption Performance of Three-Dimensional Gels Assembled by Carbon Nanomaterials for Heavy Metal Removal from Water: A Review. Sci. Total Environ. 2022, 852, 158201. [Google Scholar] [CrossRef] [PubMed]
- Mamah, S.C.; Goh, P.S.; Ng, B.C.; Abdullah, M.S.; Ismail, A.F.; Samavati, Z.; Ahmad, N.A.; Raji, Y.O. The Utilization of Chitin and Chitosan as Green Modifiers in Nanocomposite Membrane for Water Treatment. J. Water Process Eng. 2024, 62, 105394. [Google Scholar] [CrossRef]
- Rostami, M.S.; Khodaei, M.M. Recent Advances in Chitosan-Based Nanocomposites for Adsorption and Removal of Heavy Metal Ions. Int. J. Biol. Macromol. 2024, 270, 132386. [Google Scholar] [CrossRef] [PubMed]
- Doyo, A.N.; Kumar, R.; Barakat, M.A. Recent Advances in Cellulose, Chitosan, and Alginate Based Biopolymeric Composites for Adsorption of Heavy Metals from Wastewater. J. Taiwan Inst. Chem. Eng. 2023, 151, 105095. [Google Scholar] [CrossRef]
- Al-Hazmi, G.A.A.M.; Alayyafi, A.A.A.; El-Desouky, M.G.; El-Bindary, A.A. Chitosan-Nano CuO Composite for Removal of Mercury (II): Box-Behnken Design Optimization and Adsorption Mechanism. Int. J. Biol. Macromol. 2024, 261, 129769. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhou, J.; Hou, H.; Wu, X.; Li, Y. Recyclable Mg-MOF-74@cellulose Aerogel Composites for Efficient Removal of Heavy Metals from Wastewater. J. Solid State Chem. 2023, 323, 124059. [Google Scholar] [CrossRef]
- Sun, R.; Gao, S.; Zhang, K.; Cheng, W.T.; Hu, G. Recent Advances in Alginate-Based Composite Gel Spheres for Removal of Heavy Metals. Int. J. Biol. Macromol. 2024, 268, 131853. [Google Scholar] [CrossRef]
- Umpuch, C.; Fakthaisongdechakul, T. Application of Response Surface Methodology for Optimization of Reactive Black 5 Removal by Gelatin Beads Containing TTAB Modified Montmorillonite Clay. Case Stud. Chem. Environ. Eng. 2024, 9, 100758. [Google Scholar] [CrossRef]
- Begum, S.; Yuliana, N.; Saleh, N. Review of Chitosan Composite as a Heavy Metal Adsorbent: Material Preparation and Properties. Carbohydr. Polym. 2021, 259, 117613. [Google Scholar] [CrossRef]
- Upadhyay, U.; Sreedhar, I.; Singh, S.A.; Patel, C.M.; Anitha, K.L. Recent Advances in Heavy Metal Removal by Chitosan Based Adsorbents. Carbohydr. Polym. 2021, 251, 117000. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Cheng, Q.; Wang, C.; Li, H.; Han, X.; Fan, Z.; Su, G.; Pan, D.; Li, Z. Chemosphere Research Progress of Adsorption and Removal of Heavy Metals by Chitosan and Its Derivatives: A Review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Pang, Y.; Chen, D.; Kong, L.; Mehmood, S.; Wen, J.; Mallikarjuna, G. International Journal of Biological Macromolecules Modi Fi Cation of Chitosan Macromolecule and Its Mechanism for the Removal of Pb (II) Ions from Aqueous Environment. Int. J. Biol. Macromol. 2019, 136, 177–188. [Google Scholar] [CrossRef]
- Yan, Y.; Yuvaraja, G.; Liu, C.; Kong, L.; Guo, K.; Reddy, G.M.; Zyryanov, G.V. Removal of Pb (II) Ions from Aqueous Media Using Epichlorohydrin Crosslinked Chitosan Schiff’ s Base @ Fe3O4 (ECCSB @ Fe3O4). Int. J. Biol. Macromol. 2018, 117, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Oliveros, H.B.; Ouerfelli, N.; Cruz-Gonzalez, D.; Avila-Pérez, P.; Bulgariu, L.; Flaifel, M.H.; Abouzeid, F.M. Modeling of the Relationship between the Thermodynamic Parameters ΔH° and ΔS° with Temperature in the Removal of Pb Ions in Aqueous Medium: Case Study. Chem. Phys. Lett. 2023, 814, 140329. [Google Scholar] [CrossRef]
- Baykal, A. Solvothermal Synthesis of Pure SrFe12O19 Hexaferrite Nanoplatelets. J. Supercond. Nov. Magn. 2014, 27, 877–880. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Konar, M.; Rath, J.; Kumar, D. Hexagonal Strontium Ferrite: Cationic Dye Adsorption and Antibacterial Activity. Sep. Sci. Technol. 2019, 55, 415–430. [Google Scholar] [CrossRef]
- Al-wasidi, A.S.; Abdelrahman, E.A. Simple Synthesis and Characterization of Cobalt Ferrite Nanoparticles for the Successful Adsorption of Indigo Carmine Dye from Aqueous Media. Inorganic 2023, 11, 453. [Google Scholar] [CrossRef]
- Alhalili, Z.; Abdelrahman, E.A. Facile Synthesis and Characterization of Manganese Ferrite Nanoparticles for the Successful Removal of Safranine T Dye from Aqueous Solutions. Inorganic 2024, 12, 30. [Google Scholar] [CrossRef]
- Weijiang, Z.; Yace, Z.; Yuvaraja, G.; Jiao, X. Adsorption of Pb (II) Ions from Aqueous Environment Using Eco-Friendly Chitosan Schiff’s Base@Fe3O4 (CSB@Fe3O4) as an Adsorbent; Kinetics, Isotherm and Thermodynamic Studies. Int. J. Biol. Macromol. 2017, 105, 422–430. [Google Scholar] [CrossRef]
- Roohani, E.; Arabi, H.; Sarhaddi, R.; Sudkhah, S.; Shabani, A. Effect of Annealing Temperature on Structural and Magnetic Properties of Strontium Hexaferrite Nanoparticles Synthesized by Sol Gel Auto-Combustion Method. Int. J. Mod. Phys. B 2015, 29, 1550190. [Google Scholar] [CrossRef]
- Lior, P.S.; Yosef, S.; Avner, Y.; Aharon, S. Facile Sonochemical Preparation and Magnetic Properties of Strontium Hexaferrite (SrFe12O19) Nanoparticles. J. Mater. Sci. Mater. Electron. 2016, 27, 5707–5714. [Google Scholar] [CrossRef]
- Pawariya, V.; De, S.; Dutta, J. Synthesis and Characterization of Citric Acid-Modified Chitosan Schiff Base with Enhanced Antibacterial Properties for the Elimination of Bismarck Brown R and Rhodamine B Dyes from Wastewater. Int. J. Biol. Macromol. 2024, 264, 130664. [Google Scholar] [CrossRef] [PubMed]
- El, N.; Abdalla, S.; Ikhlas, M.K.; Ehab, A.M. Facile Synthesis of Sodium Iron Silicate/Sodium Iron Oxide Silicate Nanostructures from Canned Beans and Rice Husk Wastes for Efficient Removal of Cd (II) Ions from Aqueous Media. Silicon 2024, 16, 2955–2970. [Google Scholar] [CrossRef]
- Al-Wasidi, A.S.; Basha, M.T.; Alghanmi, R.M.; Al-Farraj, E.S.; Abdelrahman, E.A. Functionalization of Sodium Magnesium Silicate Hydroxide/Sodium Magnesium Silicate Hydrate Nanostructures Using 2,3-Dihydroxybenzaldehyde as a Novel Nanocomposite for the Efficient Removal of Cd (II) and Cu (II) Ions from Aqueous Media. Separations 2023, 10, 88. [Google Scholar] [CrossRef]
- Shahraki, S.; Delarami, H.S.; Khosravi, F. Synthesis and Characterization of an Adsorptive Schiff Base-Chitosan Nanocomposite for Removal of Pb (II) Ion from Aqueous Media. Int. J. Biol. Macromol. 2019, 139, 577–586. [Google Scholar] [CrossRef] [PubMed]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial Properties of Chitosan from Different Developmental Stages of the Bioconverter Insect Hermetia illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, E.A.; Hegazey, R.M.; El-Azabawy, R.E. Efficient Removal of Methylene Blue Dye from Aqueous Media Using Fe/Si, Cr/Si, Ni/Si, and Zn/Si Amorphous Novel Adsorbents. J. Mater. Res. Technol. 2019, 8, 5301–5313. [Google Scholar] [CrossRef]
- Chen, A.H.; Liu, S.C.; Chen, C.Y.; Chen, C.Y. Comparative Adsorption of Cu(II), Zn(II), and Pb (II) Ions in Aqueous Solution on the Crosslinked Chitosan with Epichlorohydrin. J. Hazard. Mater. 2008, 154, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Joseph, I.V.; Tosheva, L.; Doyle, A.M. Journal of Environmental Chemical Engineering Simultaneous Removal of Cd (II), Co (II), Cu (II), Pb (II), and Zn (II) Ions from Aqueous Solutions via Adsorption on FAU-Type Zeolites Prepared from Coal Fl y Ash. J. Environ. Chem. Eng. 2020, 8, 103895. [Google Scholar] [CrossRef]
- Roy, A.; Bhattacharya, J. Removal of Cu (II), Zn (II) and Pb (II) from Water Using Microwave-Assisted Synthesized Maghemite Nanotubes. Chem. Eng. J. 2012, 211–212, 493–500. [Google Scholar] [CrossRef]
- Kenawy, I.M.; Hafez, M.A.H.; Ismail, M.A.; Hashem, M.A. Adsorption of Cu (II), Cd (II), Hg (II), Pb (II) and Zn (II) from Aqueous Single Metal Solutions by Guanyl-Modified Cellulose. Int. J. Biol. Macromol. 2018, 107, 1538–1549. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Basavaraju, U.; Kumar, T.S.S.; Kumar, S.; Khan, N.A.; Singh, J.; Singh, L.; Ramamurthy, P.C. Graphene Oxide-Based Novel MOF Nanohybrid for Synergic Removal of Pb (II) Ions from Aqueous Solutions: Simulation and Adsorption Studies. Environ. Res. 2023, 216, 114750. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, M.; Shokuhi, A.; Ardjmand, M.; Mirabi, A. Preparation of Magnetic Nanocomposite Based on Polyaniline/Fe3O4 towards Removal of Lead (II) Ions from Real Samples. Synth. Met. 2018, 245, 1–9. [Google Scholar] [CrossRef]
- Bagdat, S.; Tokay, F.; Demirci, S.; Yilmaz, S.; Sahiner, N. Removal of Cd (II), Co (II), Cr (III), Ni (II), Pb (II) and Zn (II) Ions from Wastewater Using Polyethyleneimine (PEI) Cryogels. J. Environ. Manag. 2023, 329, 117002. [Google Scholar] [CrossRef] [PubMed]
- Waly, S.M.; El-wakil, A.M.; El-maaty, W.M.A.; Awad, F.S. Efficient Removal of Pb (II) and Hg (II) Ions from Aqueous Solution by Amine and Thiol Modified Activated Carbon. J. Saudi Chem. Soc. 2021, 25, 101296. [Google Scholar] [CrossRef]
- Alghanmi, R.M.; Abdelrahman, E.A. Simple Production and Characterization of ZnO/MgO Nanocomposite as a Highly Effective Adsorbent for Eliminating Congo Red Dye from Water-Based Solutions. Inorg. Chem. Commun. 2024, 161, 112137. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).