Biodegradable Ca2+ Doped Mesoporous Silica Nanoparticles Promote Chemotherapy Synergism with Calcicoptosis and Activate Anti-Tumor Immunity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of CMSNs
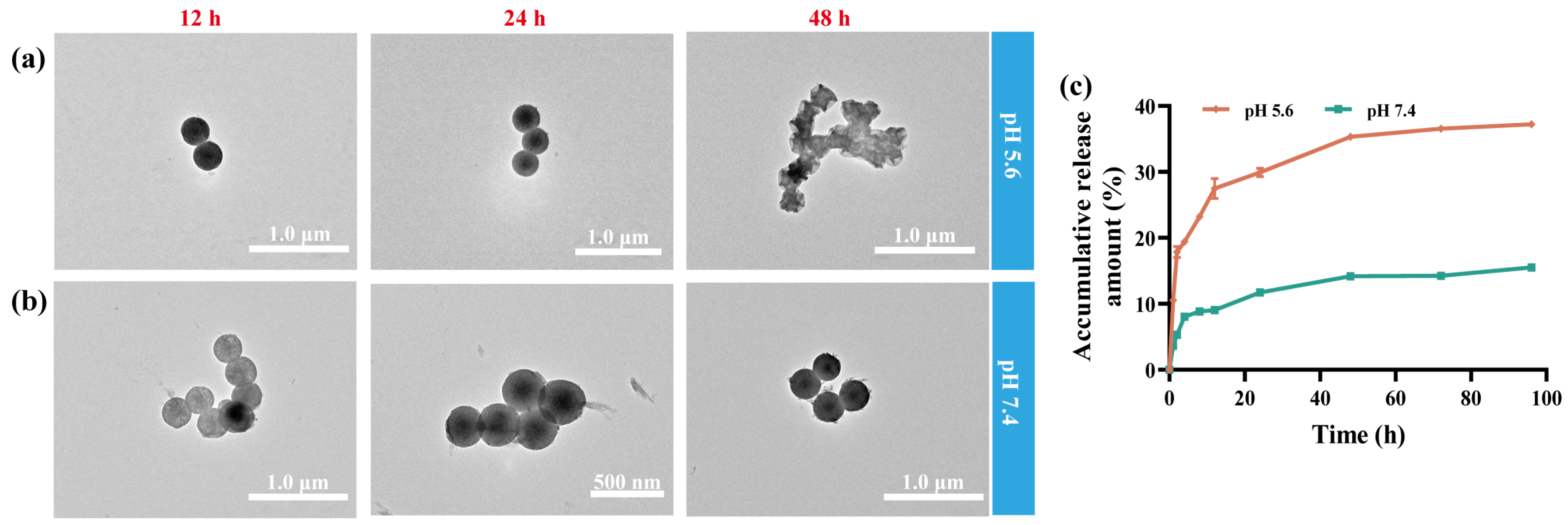
2.2. Biodegradability
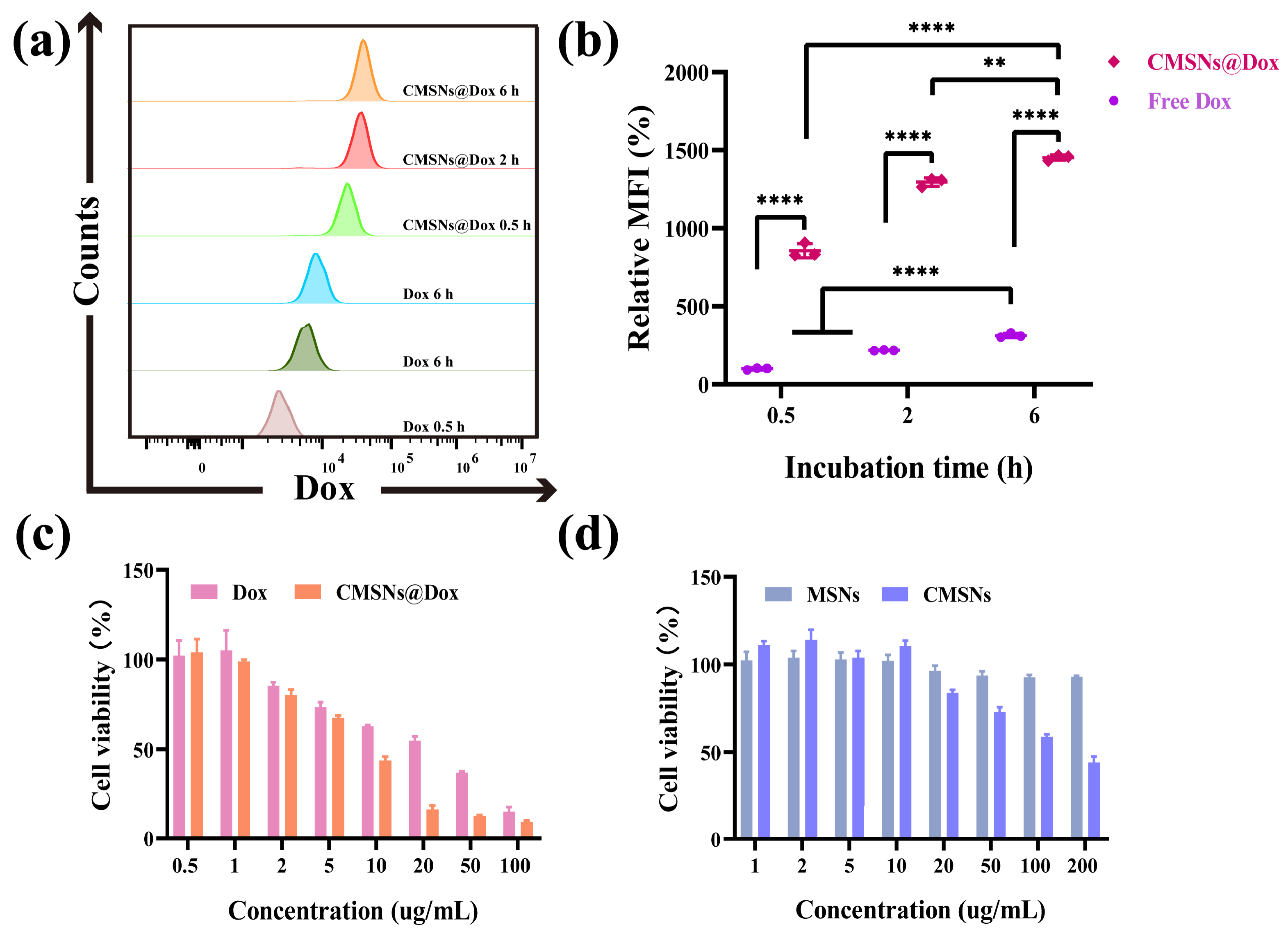
2.3. Dox Loading and pH Responsive Drug Release
2.4. Calcium Ions Dependent Mitochondrial Dysfunction
2.5. Cell Internalization
2.6. In Vitro Cytotoxicity and Anti-Tumor Efficiency
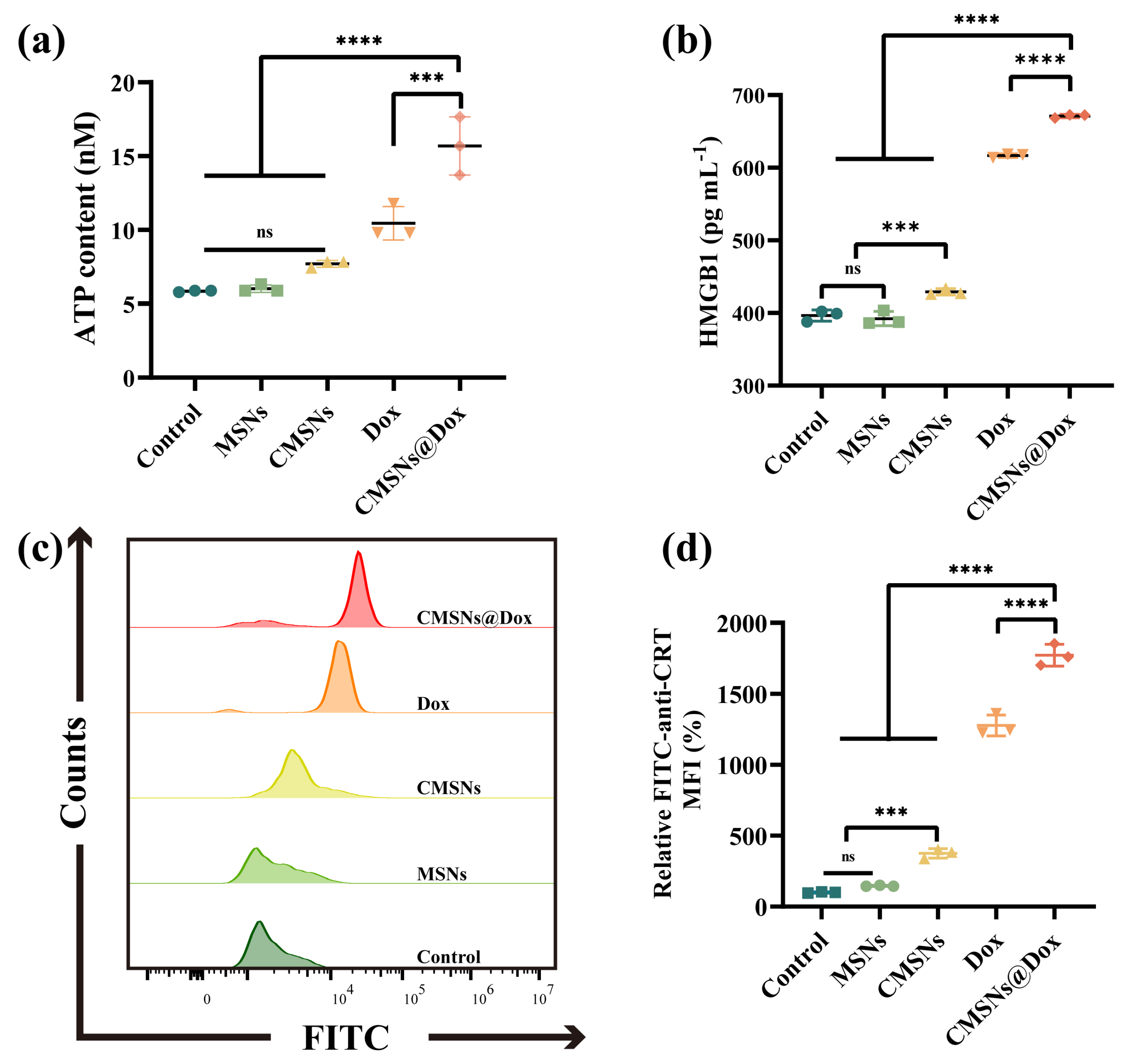
2.7. Immunogenic Cell Death and Immune Activation In Vitro
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Virous MSNs
3.3. Structural Characterizations
3.4. In Vitro Biodegradation and Stability
3.5. Drug Loading and Release
3.6. Cell Culture
3.7. Intracellular Ca2+ Detection
3.8. Cell Internalization
3.9. Mitochondrial Membrane Potential Analysis
3.10. In Vitro Cytotoxicity
3.11. Apoptosis
3.12. Immunogenic Cell Death
3.13. Dendritic Cells Maturation
3.14. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Hu, D.; Xu, H.; Patra, H.K.; Liu, X.; Zhou, Z.; Tang, J.; Slater, N.; Shen, Y. Progress and perspective of microneedle system for anti-cancer drug delivery. Biomaterials 2021, 264, 120410–120421. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Z.L.; Chen, J. ATP Binding Enables Substrate Release from Multidrug Resistance Protein 1. Cell 2018, 172, 81–89.e10. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Bigham, A.; Taheriazam, A.; Saghari, Y.; Mirzaei, S.; Hashemi, M.; Hushmandi, K.; Karimi-Maleh, H.; Nazarzadeh Zare, E.; et al. (Nano)platforms in breast cancer therapy: Drug/gene delivery, advanced nanocarriers and immunotherapy. Med. Res. Rev. 2023, 43, 2115–2176. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zou, J.; Chen, X. In Response to Precision Medicine: Current Subcellular Targeting Strategies for Cancer Therapy. Adv. Mater. 2023, 35, e2209529. [Google Scholar] [CrossRef]
- Palazzolo, S.; Bayda, S.; Hadla, M.; Caligiuri, I.; Corona, G.; Toffoli, G.; Rizzolio, F. The Clinical Translation of Organic Nanomaterials for Cancer Therapy: A Focus on Polymeric Nanoparticles, Micelles, Liposomes and Exosomes. Curr. Med. Chem. 2018, 25, 4224–4268. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Gao, D.; Shen, J.; Wang, Q. A Review of Mesoporous Silica Nanoparticle Delivery Systems in Chemo-Based Combination Cancer Therapies. Front. Chem. 2020, 8, 598722–598738. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.R.; Colilla, M.; Vallet-Regi, M. Advances in mesoporous silica-based nanocarriers for co-delivery and combination therapy against cancer. Expert. Opin. Drug Deliv. 2017, 14, 229–243. [Google Scholar] [CrossRef]
- Hadipour Moghaddam, S.P.; Yazdimamaghani, M.; Ghandehari, H. Glutathione-sensitive hollow mesoporous silica nanoparticles for controlled drug delivery. J. Control. Release 2018, 282, 62–75. [Google Scholar] [CrossRef]
- Theivendran, S.; Gu, Z.; Tang, J.; Yang, Y.; Song, H.; Yang, Y.; Zhang, M.; Cheng, D.; Yu, C. Nanostructured Organosilica Nitric Oxide Donors Intrinsically Regulate Macrophage Polarization with Antitumor Effect. ACS Nano 2022, 16, 10943–10957. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, F.; Xu, N.; Yao, Q.; Wang, R.; Xie, X.; Zhang, F.; He, Y.; Shao, D.; Dong, W.F.; et al. Red-light-triggered self-destructive mesoporous silica nanoparticles for cascade-amplifying chemo-photodynamic therapy favoring antitumor immune responses. Biomaterials 2022, 281, 121368–121377. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Feng, L.; Bian, Y.; Yuan, M.; Zhu, Y.; Yang, P.; Cheng, Z.; Lin, J. Mn2+/Fe3+/Co2+ and Tetrasulfide Bond Co-Incorporated Dendritic Mesoporous Organosilica as Multifunctional Nanocarriers: One-Step Synthesis and Applications for Cancer Therapy. Adv. Healthc. Mater. 2022, 11, e2200665. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, X.; Ito, A.; Sogo, Y.; Watanabe, Y.; Tsuji, N.M.; Ohno, T. Biodegradable Metal Ion-Doped Mesoporous Silica Nanospheres Stimulate Anticancer Th1 Immune Response in Vivo. ACS Appl. Mater. Inter. 2017, 9, 43538–43544. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Regi, M.; Schuth, F.; Lozano, D.; Colilla, M.; Manzano, M. Engineering mesoporous silica nanoparticles for drug delivery: Where are we after two decades? Chem. Soc. Rev. 2022, 51, 5365–5451. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-G.; Han, Y.-H.; Zhang, J.-T.; Kankala, R.K.; Wang, S.-B.; Chen, A.-Z. Rerouting engineered metal-dependent shapes of mesoporous silica nanocontainers to biodegradable Janus-type (sphero-ellipsoid) nanoreactors for chemodynamic therapy. Chem. Eng. J. 2019, 370, 1188–1199. [Google Scholar] [CrossRef]
- Yu, X.; Wang, X.; Yamazaki, A.; Li, X. Tumor microenvironment-regulated nanoplatforms for the inhibition of tumor growth and metastasis in chemo-immunotherapy. J. Mater. Chem. B 2022, 10, 3637–3647. [Google Scholar] [CrossRef] [PubMed]
- Shao, L.; Hu, T.; Fan, X.; Wu, X.; Zhou, F.; Chen, B.; Tan, S.; Xu, H.; Pan, A.; Liang, S.; et al. Intelligent Nanoplatform with Multi Therapeutic Modalities for Synergistic Cancer Therapy. ACS Appl. Mater. Inter. 2022, 14, 13122–13135. [Google Scholar] [CrossRef]
- Qin, G.; Xie, W.; Luo, X.; Zou, G.; Mo, Q.; Zhong, W. Manganese-doped stellate mesoporous silica nanoparticles: A bifunctional nanoplatform for enhanced chemodynamic therapy and tumor imaging. Microporous Mesoporous Mater. 2024, 370, 113012. [Google Scholar] [CrossRef]
- Lei, G.; Zhuang, L.; Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef]
- Liu, T.; Zhou, Z.; Zhang, M.; Lang, P.; Li, J.; Liu, Z.; Zhang, Z.; Li, L.; Zhang, L. Cuproptosis-immunotherapy using PD-1 overexpressing T cell membrane-coated nanosheets efficiently treats tumor. J. Control. Release 2023, 362, 502–512. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D.; et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Lv, M.; Chen, M.; Zhang, R.; Zhang, W.; Wang, C.; Zhang, Y.; Wei, X.; Guan, Y.; Liu, J.; Feng, K.; et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020, 30, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, H.; Xu, X.; Yao, S.; Yu, W.; Guo, Z. Zinc Ion-Induced Immune Responses in Antitumor Immunotherapy. CCS Chem. 2024, 1–20. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Onuma, K.; Sogo, Y.; Ohno, T.; Ito, A. Zn- and Mg-containing tricalcium phosphates-based adjuvants for cancer immunotherapy. Sci. Rep. 2013, 3, 2203. [Google Scholar] [CrossRef]
- An, J.; Zhang, K.; Wang, B.; Wu, S.; Wang, Y.; Zhang, H.; Zhang, Z.; Liu, J.; Shi, J. Nanoenabled Disruption of Multiple Barriers in Antigen Cross-Presentation of Dendritic Cells via Calcium Interference for Enhanced Chemo-Immunotherapy. ACS Nano 2020, 14, 7639–7650. [Google Scholar] [CrossRef]
- Awad, H.H.; El-Derany, M.O.; Mantawy, E.M.; Michel, H.E.; El-Naa, M.M.; Salah El-Din, R.A.; El-Brairy, A.I.; El-Demerdash, E. Comparative study on beneficial effects of vitamins B and D in attenuating doxorubicin induced cardiotoxicity in rats: Emphasis on calcium homeostasis. Biomed. Pharmacother. 2021, 140, 111679–111693. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, B.; Liang, S.; Long, M.; Xu, H. Synthesis of pH-Responsive Biodegradable Mesoporous Silica-Calcium Phosphate Hybrid Nanoparticles as a High Potential Drug Carrier. ACS Appl. Mater. Inter. 2017, 9, 44402–44409. [Google Scholar] [CrossRef]
- Choi, E.; Lim, D.-K.; Kim, S. Calcium-doped mesoporous silica nanoparticles as a lysosomolytic nanocarrier for amine-free loading and cytosolic delivery of siRNA. J. Ind. Eng. Chem. 2020, 81, 71–80. [Google Scholar] [CrossRef]
- Chen, M.; Hu, J.; Bian, C.; Zhu, C.; Chen, C.; Guo, Z.; Zhang, Z.; Agyekum, G.A.; Zhang, Z.; Cao, X. pH-Responsive and Biodegradable ZnO-Capped Mesoporous Silica Composite Nanoparticles for Drug Delivery. Materials 2020, 13, 3950. [Google Scholar] [CrossRef]
- Chang, L.; Yan, H.; Chang, J.; Gautrot, J.E. Cationic polymer brush-coated bioglass nanoparticles for the design of bioresorbable RNA delivery vectors. Eur. Polym. J. 2021, 156, 110593–110601. [Google Scholar] [CrossRef]
- Gao, S.; Liu, Y.; Liu, M.; Yang, D.; Zhang, M.; Shi, K. Biodegradable mesoporous nanocomposites with dual-targeting function for enhanced anti-tumor therapy. J. Control. Release 2022, 341, 383–398. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Li, Z.; Feng, J.; Xiong, W.; Yang, J.; Lu, X.; Yang, S.; Xu, Y.; Wu, A.; Shen, Z. Cycloacceleration of ferroptosis and calcicoptosis for magnetic resonance imaging-guided colorectal cancer therapy. Nano Today 2022, 47, 101663–101677. [Google Scholar] [CrossRef]
- Gong, F.; Xu, J.; Liu, B.; Yang, N.; Cheng, L.; Huang, P.; Wang, C.; Chen, Q.; Ni, C.; Liu, Z. Nanoscale CaH2 materials for synergistic hydrogen-immune cancer therapy. Chem 2022, 8, 268–286. [Google Scholar] [CrossRef]
- de Barros, M.R.; Bittencourt, O.R.; Crocomo, P.Z.; Mafra, G.; Carasek, E.; Magosso, H.A.; Jost, C.L.; Winiarski, J.P. Adsorption of hazardous and noxious 4-nitrophenol by a silsesquioxane organic-inorganic hybrid material. J. Sol-Gel Sci. Technol. 2021, 99, 402–412. [Google Scholar] [CrossRef]
- Li, Z.; Hu, M.; Shen, K.; Zhou, F.; Chen, Z.; Cheng, X.; Liu, Q.; Wu, X. Enhancing thermal safety of hydrophobic silica aerogels by incorporating sodium dodecyl sulfate intercalated layered double hydroxides. J. Sol-Gel Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Narayan, R.; Nayak, U.Y.; Raichur, A.M.; Garg, S. Mesoporous Silica Nanoparticles: A Comprehensive Review on Synthesis and Recent Advances. Pharmaceutics 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, C.; Chang, J. Ca-Doped mesoporous SiO2/dental resin composites with enhanced mechanical properties, bioactivity and antibacterial properties. J. Mater. Chem. B 2018, 6, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Dong, X.; Liang, J.; Shi, J. Preparation of mesoporous calcium doped silica spheres with narrow size dispersion and their drug loading and degradation behavior. Microporous Mesoporous Mater. 2007, 102, 151–158. [Google Scholar] [CrossRef]
- dos Santos, G.A.; Abreu e Lima, R.S.; Pestana, C.R.; Lima, A.S.; Scheucher, P.S.; Thome, C.H.; Gimenes-Teixeira, H.L.; Santana-Lemos, B.A.; Lucena-Araujo, A.R.; Rodrigues, F.P.; et al. (+)alpha-Tocopheryl succinate inhibits the mitochondrial respiratory chain complex I and is as effective as arsenic trioxide or ATRA against acute promyelocytic leukemia in vivo. Leukemia 2012, 26, 451–460. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Galassi, C.; Zitvogel, L.; Galluzzi, L. Immunogenic cell stress and death. Nat. Immunol. 2022, 23, 487–500. [Google Scholar] [CrossRef]
- Galluzzi, L.; Humeau, J.; Buque, A.; Zitvogel, L.; Kroemer, G. Immunostimulation with chemotherapy in the era of immune checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2020, 17, 725–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Montesdeoca, N.; Karges, J.; Xiao, H. Immunogenic Cell Death Inducing Metal Complexes for Cancer Therapy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202300662. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, M.; Pan, L.; Shi, J. Tumor vascular-targeted co-delivery of anti-angiogenesis and chemotherapeutic agents by mesoporous silica nanoparticle-based drug delivery system for synergetic therapy of tumor. Int. J. Nanomed. 2016, 11, 93–105. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Tang, X.; Huang, G. Biodegradable Ca2+ Doped Mesoporous Silica Nanoparticles Promote Chemotherapy Synergism with Calcicoptosis and Activate Anti-Tumor Immunity. Inorganics 2024, 12, 152. https://doi.org/10.3390/inorganics12060152
Liu C, Tang X, Huang G. Biodegradable Ca2+ Doped Mesoporous Silica Nanoparticles Promote Chemotherapy Synergism with Calcicoptosis and Activate Anti-Tumor Immunity. Inorganics. 2024; 12(6):152. https://doi.org/10.3390/inorganics12060152
Chicago/Turabian StyleLiu, Chao, Xiaohui Tang, and Gaofei Huang. 2024. "Biodegradable Ca2+ Doped Mesoporous Silica Nanoparticles Promote Chemotherapy Synergism with Calcicoptosis and Activate Anti-Tumor Immunity" Inorganics 12, no. 6: 152. https://doi.org/10.3390/inorganics12060152





