Physicochemical and Toxicological Screening of Silver Nanoparticle Biosynthesis from Punica granatum Peel Extract
Abstract
1. Introduction
2. Results
2.1. Physicochemical Characterization of Green Synthesized Pg-AgNPs
2.1.1. UV–Visible Spectroscopy
2.1.2. Fourier Transform Infrared (FT-IR) Spectroscopy
2.1.3. Thermal Behavior
2.1.4. Electron Microscopy Analysis
2.2. In Vitro Toxicological Screening of Green Synthesized Pg-AgNPs
2.2.1. Antibacterial Assay
2.2.2. Cellular Viability Assessment
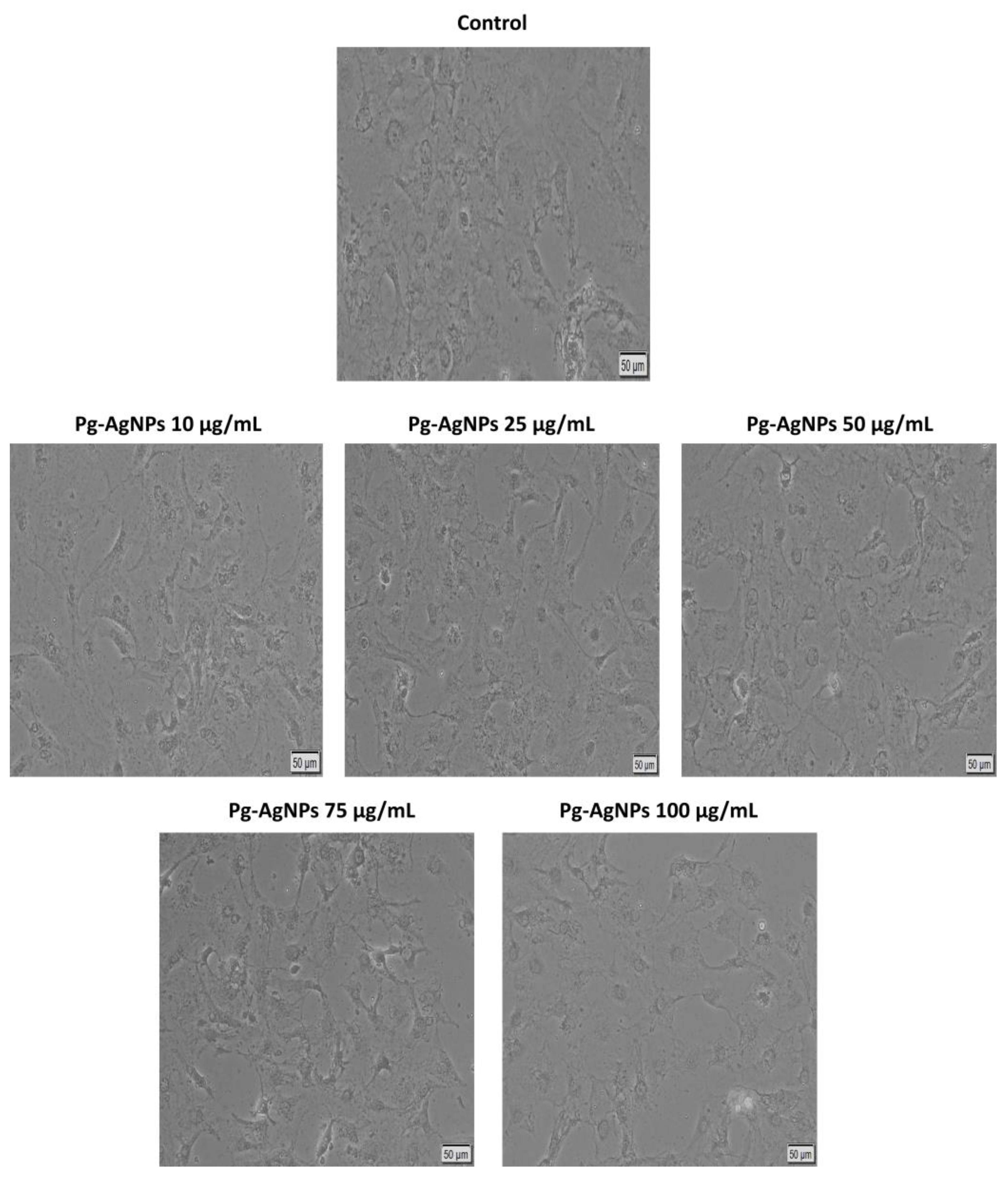
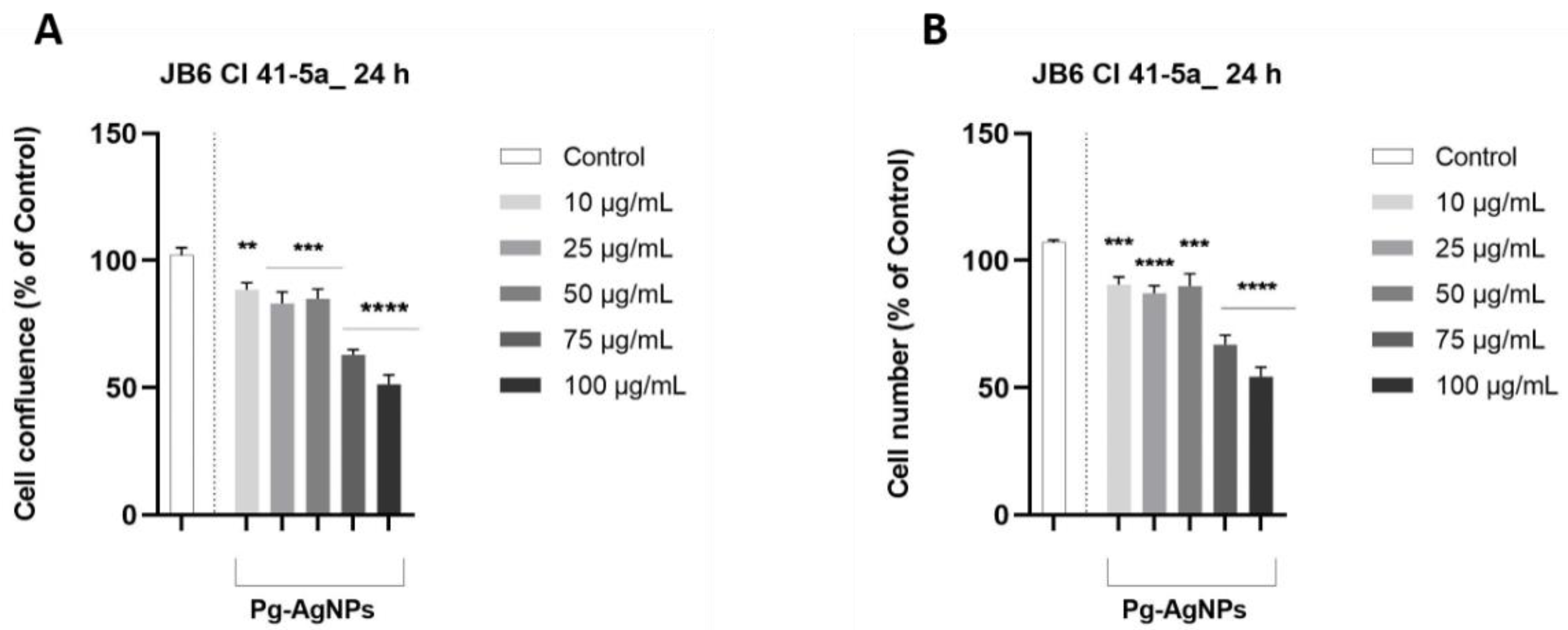
2.2.3. Evaluation of Cellular Morphology, Confluence, and Cell Number
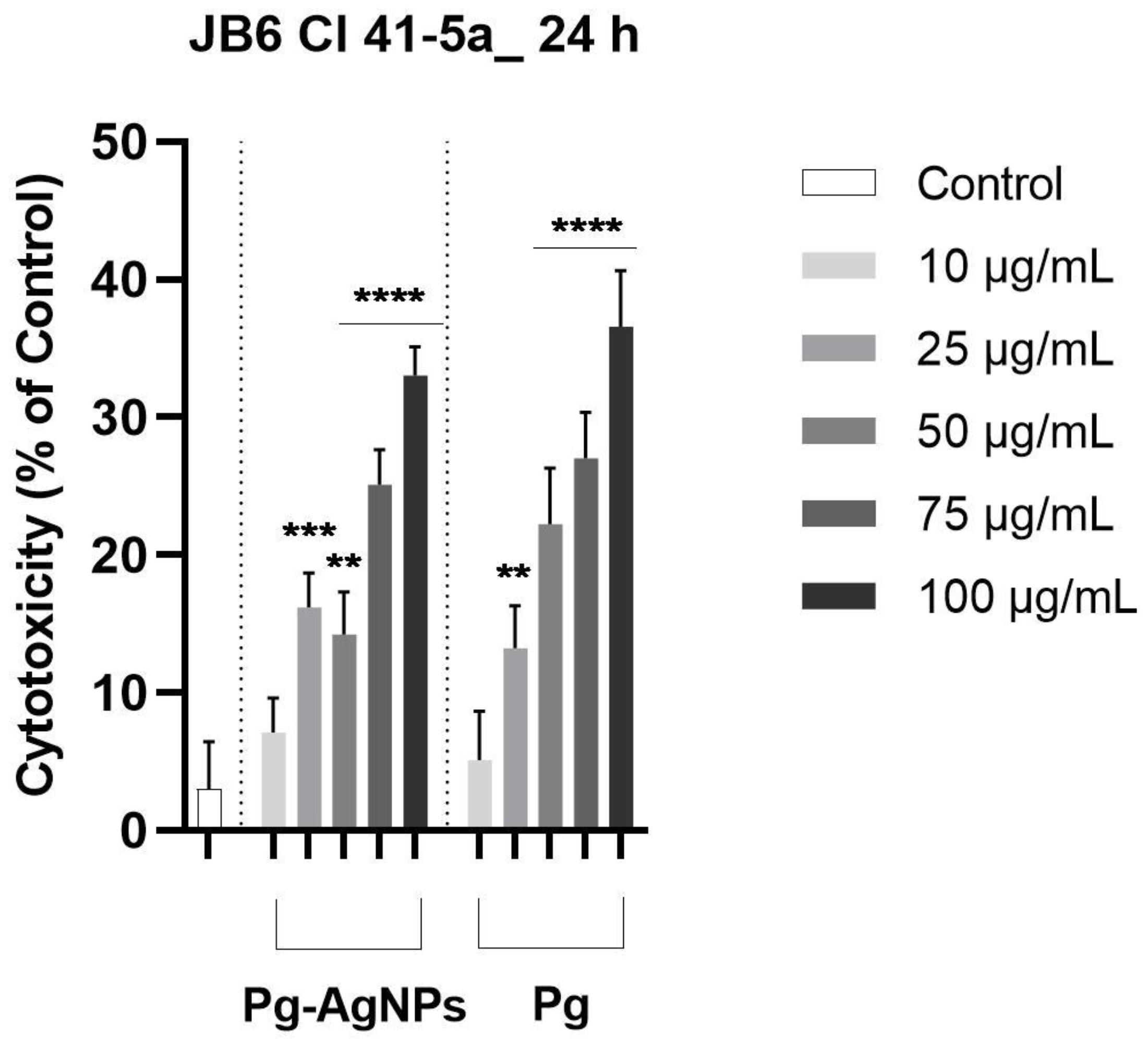
2.2.4. Cytotoxicity Assay
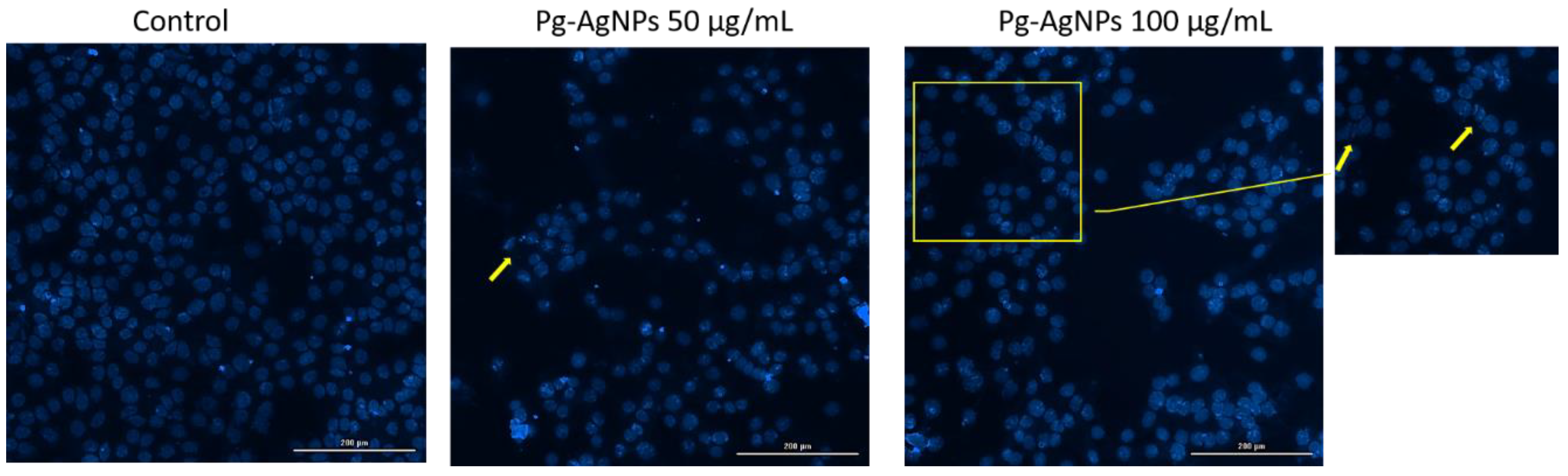
2.2.5. Nuclear Morphology Evaluation
2.3. In Ovo Toxicological Screening of Green Synthesized Pg-AgNPs
3. Discussion
4. Materials and Methods
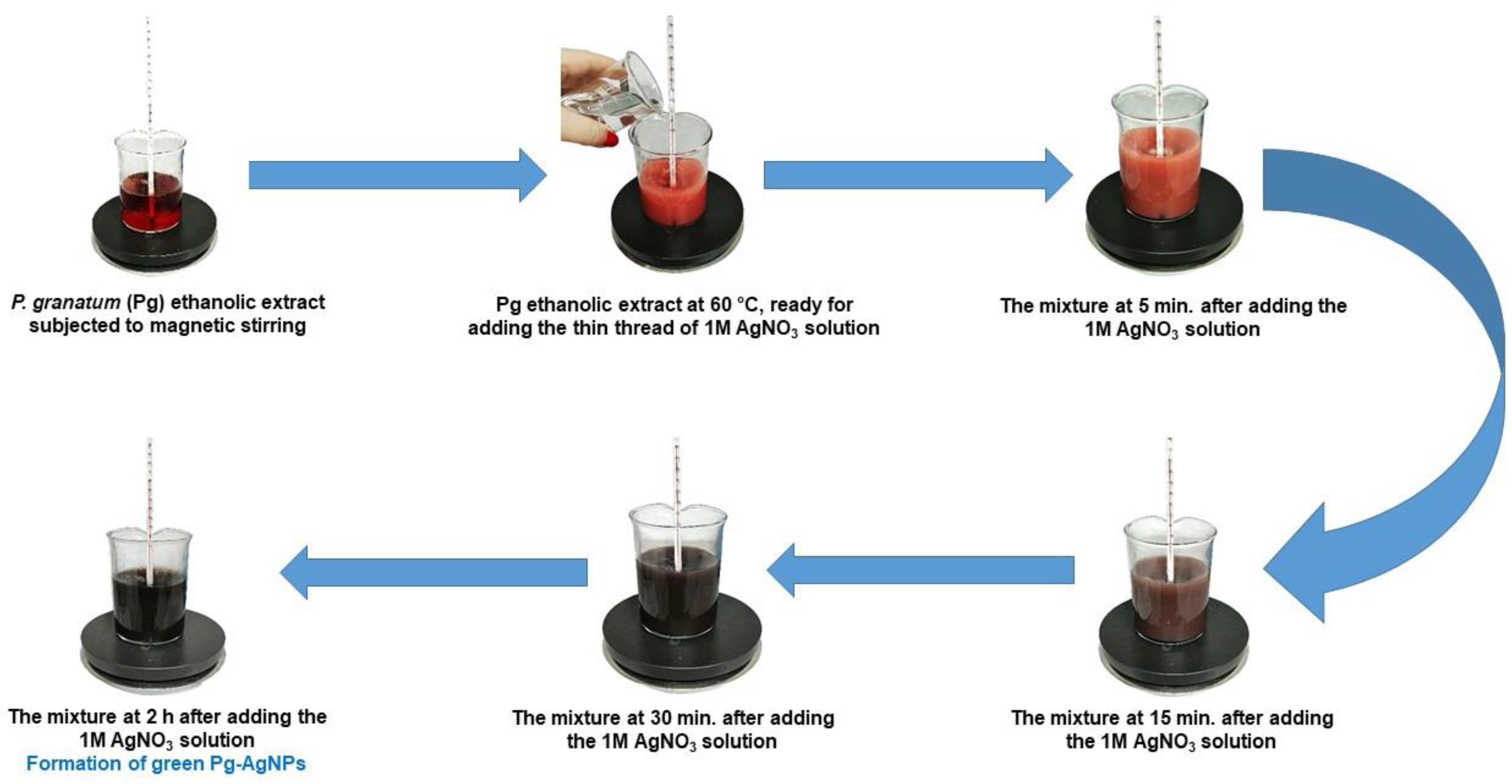
4.1. Biosynthesis of the Pg-AgNPs
4.2. Physicochemical Characterization of Green Synthesized Pg-AgNPs
4.3. In Vitro Toxicological Screening of Green Synthesized Pg-AgNPs
4.3.1. Specific Reagents and Equipment
4.3.2. Antimicrobial Activity
4.3.3. Cell Culture
4.3.4. Treatment Protocol
4.3.5. Cell Viability Test
4.3.6. Cellular Morphology Analysis
4.3.7. Evaluation of Cell Confluence and Cell Number
4.3.8. Assessment of Lactate Dehydrogenase (LDH) Leakage
4.3.9. Hoechst Nuclear Staining
4.4. In Ovo Toxicological Screening of Green Synthesized Pg-AgNPs
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Das, P.; Ghosh, S.; Nayak, B. Phyto-fabricated Nanoparticles and Their Anti-biofilm Activity: Progress and Current Status. Front. Nanotechnol. 2021, 3, 739286. [Google Scholar] [CrossRef]
- Yuan, L.; Ozcan, S. Green nanomaterials: On track for a sustainable future. Nano Today 2015, 10, 417–420. [Google Scholar]
- Ullah, A.; Lim, S.I. Plant extract-based synthesis of metallic nanomaterials, their applications, and safety concerns. Biotechnol. Bioeng. 2022, 119, 2273–2304. [Google Scholar] [CrossRef]
- Kumar, S.; Lather, V.; Pandita, D. Green synthesis of therapeutic nanoparticles: An expanding horizon. Nanomedicine 2015, 10, 2451–2471. [Google Scholar] [CrossRef]
- Hamrayev, H.; Shameli, K.; Korpayev, S. Green synthesis of zinc oxide nanoparticles and its biomedical applications: A review. J. Res. Nanosci. Nanotechnol. 2021, 1, 62–74. [Google Scholar] [CrossRef]
- Park, S.Y.; Lu, G.; Kim, B.; Song, W.C.; Park, G.; Choi, Y.W. A Comparative Study on Physicochemical, Photocatalytic, and Biological Properties of Silver Nanoparticles Formed Using Extracts of Different Parts of Cudrania tricuspidata. Nanomaterials 2020, 10, 1350. [Google Scholar] [CrossRef] [PubMed]
- Ranjani, S.; Shariq Ahmed, M.; Ruckmani, K.; Hemalatha, S. Green Nanocolloids Control Multi-Drug Resistant Pathogenic Bacteria. J. Clust. Sci. 2020, 31, 861–866. [Google Scholar] [CrossRef]
- Karimi, S.; Mahdavi Shahri, M. Medical and cytotoxicity effects of green synthesized silver nanoparticles using Achillea millefolium extract on MOLT-4 lymphoblastic leukemia cell line. J. Med. Virol. 2021, 93, 3899–3906. [Google Scholar] [CrossRef]
- Sani, S.; Nair, S. Studies on in vitro evaluation of antidiabetic potentials of watermelon and pomegranate peels. Bayero J. Pure Appl. Sci. 2017, 10, 32–35. [Google Scholar] [CrossRef]
- Jain, V.; Viswanatha, G.L.; Manohar, D.; Shivaprasad, H.N. Isolation of Antidiabetic Principle from Fruit Rinds of Punica granatum. Evid. Based Complement. Alternat. Med. 2012, 2012, 147202. [Google Scholar] [CrossRef]
- Vini, R.; Sreeja, S. Punica granatum and its therapeutic implications on breast carcinogenesis: A review. Biofactors 2015, 41, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Muharrami, L.; Munawaroh, F.; Ersam, T.; Santoso, M. Phytochemical screening of ethanolic extract: A preliminary test on five medicinal plants on bangkalan. J. Pena Sains 2020, 7, 96–102. [Google Scholar] [CrossRef]
- Dioguardi, M.; Ballini, A.; Sovereto, D.; Spirito, F.; Cazzolla, A.P.; Aiuto, R.; Crincoli, V.; Caloro, G.A.; Lo Muzio, L. Application of the Extracts of Punica granatum in Oral Cancer: Scoping Review. Dent. J. 2022, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.; Juyal, D.; Singh, M.; Sharma, S. In vivo and in vitro study to evaluate the anti-osteoporotic activity of Punica granatum seed, Bambusa arundinaceae leaves, and trichosanthes diocio fruit ethanolic extract. Indian J. Anim. Res. 2024, 58, 107–114. [Google Scholar] [CrossRef]
- Ajaikumar, K.B.; Asheef, M.; Babu, B.H.; Padikkala, J. The inhibition of gastric mucosal injury by Punica granatum L. (pomegranate) methanolic extract. J. Ethnopharmacol. 2005, 96, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Willian, N. Silver Nanoparticles (AgNPs) as Effective Disinfectants with Natural Source: A New Inspiration. IOP Conf. Ser. Earth Environ. Sci. 2023, 1148, 012002. [Google Scholar] [CrossRef]
- Celebioglu, H.U.; Imamoglu, R.; Tas, R. Biogenic synthesis of silver nanoparticles using trachystemon orientalis l. and their antibacterial activities. In Proceedings of the 13th International Conference on Nanomaterials—Research & Application, Brno, Czech Republic, 20–22 October 2021; pp. 329–336. [Google Scholar]
- Annu Ahmed, S.; Kaur, G.; Sharma, P.; Singh, S.; Ikram, S. Evaluation of the antioxidant, antibacterial and anticancer (lung cancer cell line A549) activity of Punica granatum mediated silver nanoparticles. Toxicol. Res. 2018, 7, 923–930. [Google Scholar] [CrossRef]
- Periakaruppan, R.; Manju, P.; Priya, C.; Ranjitha, P.; Gokul Raj, S.; Danaraj, J. Biosynthesis of Silica Nanoparticles Using the Leaf Extract of Punica granatum and Assessment of Its Antibacterial Activities Against Human Pathogens. Appl. Biochem. Biotechnol. 2022, 194, 5594–5605. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Bulut, O.; Some, S.; Mandal, A.K.; Yilmaz, M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019, 9, 2673–2702. [Google Scholar] [CrossRef]
- Singhal, M.; Chatterjee, S.; Kumar, A.; Syed, A.; Bahkali, A.H.; Gupta, N.; Nimesh, S. Exploring the Antibacterial and Antibiofilm Efficacy of Silver Nanoparticles Biosynthesized Using Punica granatum Leaves. Molecules 2021, 26, 5762. [Google Scholar] [CrossRef]
- Franzolin, M.R.; Courrol, D.D.S.; Silva, F.R.O.; Courrol, L.C. Antimicrobial Activity of Silver and Gold Nanoparticles Prepared by Photoreduction Process with Leaves and Fruit Extracts of Plinia cauliflora and Punica granatum. Molecules 2022, 27, 6860. [Google Scholar] [CrossRef] [PubMed]
- Saratale, G.D.; Saratale, R.G.; Kim, D.S.; Kim, D.Y.; Shin, H.S. Exploiting Fruit Waste Grape Pomace for Silver Nanoparticles Synthesis, Assessing Their Antioxidant, Antidiabetic Potential and Antibacterial Activity Against Human Pathogens: A Novel Approach. Nanomaterials 2020, 10, 1457. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Shin, H.S.; Kumar, G.; Benelli, G.; Kim, D.S.; Saratale, G.D. Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif. Cells Nanomed. Biotechnol. 2018, 46, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Emima Jeronsia, J.; Ragu, R.; Jerline Mary, A.; Jerome Das, S. Elucidating the structural, anticancer, and antibacterial traits of Punica granatum peel extracts-mediated Ag and Ag/GO nanocomposites. Microsc. Res. Tech. 2022, 85, 44–55. [Google Scholar]
- Saeidnia, S.; Manayi, A.; Abdollahi, M. From in vitro experiments to in vivo and clinical studies; Pros and cons. Curr. Drug Discov. Technol. 2015, 12, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Ruedinger, F.; Lavrentieva, A.; Blume, C.; Pepelanova, I.; Scheper, T. Hydrogels for 3D mammalian cell culture: A starting guide for laboratory practice. Appl. Microbiol. Biotechnol. 2015, 99, 623–636. [Google Scholar] [CrossRef] [PubMed]
- Moacă, E.A.; Watz, C.; Faur, A.C.; Lazăr, D.; Socoliuc, V.; Păcurariu, C.; Ianoș, R.; Rus, C.I.; Minda, D.; Barbu-Tudoran, L.; et al. Biologic Impact of Green Synthetized Magnetic Iron Oxide Nanoparticles on Two Different Lung Tumorigenic Monolayers and a 3D Normal Bronchial Model—EpiAirwayTM Microtissue. Pharmaceutics 2023, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Prodan-Bărbulescu, C.; Watz, C.G.; Moacă, E.A.; Faur, A.C.; Dehelean, C.A.; Faur, F.I.; Grigoriţă, L.O.; Maghiari, A.L.; Tuţac, P.; Duţă, C.; et al. A Preliminary Report Regarding the Morphological Changes of Nano-Enabled Pharmaceutical Formulation on Human Lung Carcinoma Monolayer and 3D Bronchial Microtissue. Medicina 2024, 60, 208. [Google Scholar] [CrossRef] [PubMed]
- Leighton, J.; Nassauer, J.; Tchao, R. The chick embryo in toxicology: An alternative to the rabbit eye. Food Chem. Toxicol. 1985, 23, 293–298. [Google Scholar] [CrossRef]
- Buhr, C.; Wiesmann, N.; Tanner, R.; Brieger, J.; Eckrich, J. The chorioallantoic membrane assay in nanotoxicological research—An alternative for in vivo experimentation. Nanomaterials 2020, 10, 2328. [Google Scholar] [CrossRef]
- Mangır, N.; Dikici, S.; Claeyssens, F.; MacNeil, S. Using ex ovo chick chorioallantoic membrane (cam) assay to evaluate the biocompatibility and angiogenic response to biomaterials. ACS Biomater. Sci. Eng. 2019, 5, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: A green expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Raveendran, P.; Fu, J.; Wallen, S.L. Completely “green” synthesis and stabilization of metal nanoparticles. J. Am. Chem. Soc. 2003, 125, 13940–13941. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Hashemi, S.A.; Ghasemi, Y.; Atapour, A.; Amani, A.M.; Dashtaki, A.S.; Babapoor, A.; Arjmand, O. Green synthesis of silver nanoparticles toward bio and medical applications: Review study. Artif. Cells Nanomed. Biotechnol. 2018, 46, S855–S872. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Sedaghat, S.; Moradi, O.; Salmanabadi, S.A. Review on green nano-biosynthesis of silver nanoparticles and their biological activities: With an emphasis on medicinal plants. Inorg. Nano Met. Chem. 2020, 51, 133–142. [Google Scholar] [CrossRef]
- Farouk, S.M.; Abu-Hussien, S.H.; Abd-Elhalim, B.T.; Mohamed, R.M.; Arabe, N.M.; Hussain, A.A.T.; Mostafa, M.E.; Hemdan, B.; El-Sayed, S.M.; Bakry, A.; et al. Biosynthesis and characterization of silver nanoparticles from Punica granatum (pomegranate) peel waste and its application to inhibit foodborne pathogens. Sci. Rep. 2023, 13, 19469. [Google Scholar] [CrossRef] [PubMed]
- Devanesan, S.; AlSalhi, M.S.; Balaji, R.V.; Ranjitsingh, A.J.A.; Ahamed, A.; Alfuraydi, A.A.; AlQahtani, F.Y.; Aleanizy, F.S.; Othman, A.H. Antimicrobial and Cytotoxicity Effects of Synthesized Silver Nanoparticles from Punica granatum Peel Extract. Nanoscale Res. Lett. 2018, 13, 315. [Google Scholar] [CrossRef] [PubMed]
- Rouhollah, H.; Marzieh, R. Green synthesis of silver nanoparticles using extract of oak fruit hull (Jaft): Synthesis and in vitro cytotoxic effect on MCF-7 cells. Int. J. Breast Cancer 2015, 2015, 846743. [Google Scholar]
- Arya, G.; Kumari, R.M.; Sharma, N.; Gupta, N.; Kumar, A.; Chatterjee, S.; Nimesh, S. Catalytic, antibacterial and antibiofilm efficacy of biosynthesised silver nanoparticles using Prosopis juliflora leaf extract along with their wound healing potential. J. Photochem. Photobiol. B Biol. 2019, 190, 50–58. [Google Scholar] [CrossRef]
- Khorrami, S.; Kamali, F.; Zarrabi, A. Bacteriostatic activity of aquatic extract of black peel pomegranate and silver nanoparticles biosynthesized by using the extract. Biocatal. Agric. Biotechnol. 2020, 25, 101620. [Google Scholar] [CrossRef]
- Khorrami, S.; Abdollahi, Z.; Eshaghi, G.; Khosravi, A.; Bidram, E.; Zarrabi, A. An improved method for fabrication of Ag-GO nanocomposite with controlled anticancer and anti-bacterial behavior; A comparative study. Sci. Rep. 2019, 9, 9167. [Google Scholar] [CrossRef]
- Allafchian, A.R.; Mirahmadi-Zare, S.Z.; Jalali, S.A.H.; Hashemi, S.S.; Vahabi, M.R. Green synthesis of silver nanoparticles using phlomis leaf extract and investigation of their antibacterial activity. J. Nanostructure Chem. 2016, 6, 129–135. [Google Scholar] [CrossRef]
- Khaleghi, M.; Khorrami, S.; Ravan, H. Identification of Bacillus thuringiensis bacterial strain isolated from the mine soil as a robust agent in the biosynthesis of silver nanoparticles with strong antibacterial and anti-biofilm activities. Biocatal. Agric. Biotechnol. 2019, 18, 101047. [Google Scholar] [CrossRef]
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef]
- Raja, S.; Ramesh, V.; Thivaharan, V. Green biosynthesis of silver nanoparticles using Calliandra haematocephala leaf extract, their antibacterial activity and hydrogen peroxide sensing capability. Arab. J. Chem. 2017, 10, 253–261. [Google Scholar] [CrossRef]
- Ritu-Verma, K.K.; Das, A.; Chandra, P. Phytochemical-based synthesis of silver nanoparticle: Mechanism and potential applications. BioNanoScience 2023, 13, 1359–1380. [Google Scholar] [CrossRef]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 298–309. [Google Scholar] [CrossRef]
- Das, B.; De, A.; Das, M.; Das, S.; Samanta, A. A new exploration of Dregea volubilis flowers: Focusing on antioxidant and antidiabetic properties. S. Afr. J. Bot. 2017, 109, 16–24. [Google Scholar] [CrossRef]
- Zia, M.; Gul, S.; Akhtar, J.; Haq, I.U.; Abbasi, B.H.; Hussain, A.; Naz, S.; Chaudhary, M.F. Green synthesis of silver nanoparticles from grape and tomato juices and evaluation of biological activities. IET Nanobiotechnol. 2017, 11, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Giri, N.; Natarajan, R.; Gunasekaran, S.; Shreemathi, S. 13C NMR and FTIR spectroscopic study of blend behavior of PVP and nano silver particles. Arch. Appl. Sci. Res. 2011, 3, 624–630. [Google Scholar]
- Yong, N.L.; Ahmad, A.; Mohammad, A.W. Synthesis and characterization of silver oxide nanoparticles by a novel method. Int. J. Sci. Eng. Res. 2013, 4, 155–158. [Google Scholar]
- Perry, H.R.; Green, W.D.; Maloney, O.J. Perry’s Chemical Engineers’ Handbook, 7th ed.; McGraw Hill: New York, NY, USA, 1999. [Google Scholar]
- Fadel, Q.J.; Al-Mashhedy, L.A.M. Biosynthesis of silver nanoparticles using peel extract of Raphanus sativus L. Biotechnol. Ind. J. 2017, 13, 120. [Google Scholar]
- Mojgan, G.; Noshin, M.; Mehdi, M.K.; Samira, B.; Masoud, S.N. Biosynthesis and characterization of silver nanoparticles prepared from two novel natural precursors by facile thermal decomposition methods. Sci. Rep. 2016, 6, 32539. [Google Scholar]
- Chand, K.; Abro, M.I.; Aftab, U.; Shah, A.H.; Lakhan, M.N.; Cao, D.; Mehdi, G.; Mohamed, A.M.A. Green synthesis characterization and antimicrobial activity against: Staphylococcus aureus of silver nanoparticles using extracts of neem, onion and tomato. RSC Adv. 2019, 9, 17002–17015. [Google Scholar] [CrossRef]
- Mostafa, Y.S.; Alamri, S.A.; Alrumman, S.A.; Hashem, M.; Baka, Z.A. Green synthesis of silver nanoparticles using pomegranate and orange peel extracts and their antifungal activity against alternaria solani, the causal agent of early blight disease of tomato. Plants 2021, 10, 2363. [Google Scholar] [CrossRef]
- Mansoor, S.; Zahoor, I.; Baba, T.R.; Padder, S.A.; Bhat, Z.A.; Koul, A.M.; Jiang, L. Fabrication of silver nanoparticles against fungal pathogens. Front. Nanotechnol. 2021, 3, 859. [Google Scholar] [CrossRef]
- Javan Bakht Dalir, S.; Djahaniani, H.; Nabati, F.; Hekmati, M. Characterization and the evaluation of antimicrobial activities of silver nanoparticles biosynthesized from Carya illinoinensis leaf extract. Heliyon 2020, 6, e03624. [Google Scholar] [CrossRef]
- Raheem, H.Q.; Al-Thahab, A.; Abd, F.G. Antibacterial activity of silver nanoparticles extracted from Proteus mirabilis and healing the wound in rabbit. Biochem. Cell. Arch. 2018, 18, 97–104. [Google Scholar]
- Parvekar, P.; Palaskar, J.; Metgud, S.; Maria, R.; Dutta, S. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of silver nanoparticles against Staphylococcus aureus. Biomater. Investig. Dent. 2020, 7, 105–109. [Google Scholar] [CrossRef]
- Wypij, M.; Czarnecka, J.; Swiecimska, M.; Dahm, H.; Rai, M.; Golinska, P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J. Microbiol. Biotechnol. 2018, 34, 23. [Google Scholar] [CrossRef]
- Manjunatha, D.; Megha, G.T.; Nagaraju, S.; Akarsh, S.; Nandish, G.; Sowmya, H.V.; Thippeswamy, B. Eco-friendly synthesized silver nanoparticles from endophytic fungus Phyllosticta owaniana: KUMBMDBT-32 and evaluation of biomedical properties. Arch. Microbiol. 2023, 205, 217. [Google Scholar] [CrossRef]
- Erdoğan, Ö.; Paşa, S.; Çevik, Ö. Green synthesis and characterization of anticancer effected silver nanoparticles with silverberry (Elaeagnus angustifolia) fruit aqueous extract. Int. J. Pure Appl. Sci. 2021, 7, 391–400. [Google Scholar] [CrossRef]
- Ryman-Rasmussen, J.P.; Riviere, J.E.; Monteiro-Riviere, N.A. Variables influencing interactions of untargeted quantum dot nanoparticles with skin cells and identification of biochemical modulators. Nano Lett. 2007, 7, 1344–1348. [Google Scholar] [CrossRef]
- Bolzinger, M.; Briançon, S.; Chevalier, Y. Nanoparticles through the skin: Managing conflicting results of inorganic and organic particles in cosmetics and pharmaceutics. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2011, 3, 463–478. [Google Scholar] [CrossRef]
- Hamdy, M.; Elbehairi, S.; Shati, A.; Abd-Rabboh, H.; Alfaifi, M.; Fawy, K.; Ibrahium, H.A.; Alamri, S.; Awwad, N.S. Cytotoxic potential of bio-silica conjugate with different sizes of silver nanoparticles for cancer cell death. Materials 2022, 15, 4074. [Google Scholar] [CrossRef] [PubMed]
- Anish, S.; Mahesh Kumar, D. Evaluation of the In Vitro Cytotoxicity of Silver Nanoparticles on PBMC Cells Using MTT Assay. Ann. Int. Med. Dent. Res. 2022, 8, 185–191. [Google Scholar]
- Radko, L.; Stypuła-Trębas, S.; Posyniak, A.; Żyro, D.; Ochocki, J. Silver(I) Complexes of the Pharmaceutical Agents Metronidazole and 4-Hydroxymethylpyridine: Comparison of Cytotoxic Profile for Potential Clinical Application. Molecules 2019, 24, 1949. [Google Scholar] [CrossRef]
- Stryjska, K.; Radko, L.; Chęcińska, L.; Kusz, J.; Posyniak, A.; Ochocki, J. Synthesis, Spectroscopy, Light Stability, Single-Crystal Analysis, and In Vitro Cytotoxic Activity on HepG2 Liver Cancer of Two Novel Silver(I) Complexes of Miconazole. Int. J. Mol. Sci. 2020, 21, 3629. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.A.; Berretta, A.A.; Torres, E.C.; Buszinski, A.F.M.; Fernandes, G.L.; Mendes-Gouvêa, C.C.; De Souza-Neto, F.N.; Gorup, L.F.; De Camargo, E.R.; Barbosa, D.B. Antimicrobial Potential and Cytotoxicity of Silver Nanoparticles Phytosynthesized by Pomegranate Peel Extract. Antibiotics 2018, 7, 51. [Google Scholar] [CrossRef]
- Sarkar, S.; Kotteeswaran, V. Green synthesis of silver nanoparticles from aqueous leaf extract of Pomegranate (Punica granatum) and their anticancer activity on human cervical cancer cells. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025014. [Google Scholar] [CrossRef]
- Padmaa Paarakh, M.; Ani Jose, P. In vitro, anticancer activity of silver nanoparticle synthesized from Punica granatum dried peel against cancer cell lines. Indian J. Nat. Prod. Resour. 2020, 11, 224–238. [Google Scholar]
- Saad, P.G.; Castelino, R.D.; Ravi, V.; Al-Amri, I.S.; Khan, S.A. Green synthesis of silver nanoparticles using Omani pomegranate peel extract and two polyphenolic natural products: Characterization and comparison of their antioxidant, antibacterial, and cytotoxic activities. Beni-Suef Univ. J. Basic. Appl. Sci. 2021, 10, 29. [Google Scholar] [CrossRef]
- Durgawale, P.P.; Datkhile, K.D. Study of in-vitro anti-cancer and anti-oxidative properties of methanolic extract of Punica granatum flowers. Der Pharm. Lett. 2016, 8, 197–201. [Google Scholar]
- Li, Y.; Yang, F.; Zheng, W.; Hu, M.; Wang, J.; Ma, S.; Deng, Y.; Luo, Y.; Ye, T.; Yin, W. Punica granatum (pomegranate) leaves extract induces apoptosis through mitochondrial intrinsic pathway and inhibits migration and invasion in non-small cell lung cancer in vitro. Biomed. Pharmacother. 2016, 80, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Shirode, A.B.; Kovvuru, P.; Chittur, S.V.; Henning, S.M.; Heber, D.; Reliene, R. Antipro-liferative effects of pomegranate extract in MCF7 breast cancer cells is associated with reduced DNA repair gene expression and induction of double strand breaks. Mol. Carcinog. 2014, 53, 458–470. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Sanchez, M.A.; Davalos, A.; Gonzalez-Sarrias, A.; Casas-Agustench, P.; Visioli, F.; Monedero-Saiz, T.; García-Talavera, N.V.; Gómez-Sánchez, M.B.; Sánchez-Álvarez, C.; García-Albert, A.M.; et al. MicroRNAs expression in normal and malignant colon tissues as biomarkers of colorectal cancer and in response to pomegranate extracts consumption: Critical issues to discern between modulatory effects and potential artifacts. Mol. Nutr. Food Res. 2015, 59, 1973–1986. [Google Scholar] [CrossRef]
- Zhou, B.; Yi, H.; Tan, J.; Wu, Y.; Liu, G.; Qiu, Z. Anti-proliferative effects of polyphenols from pomegranate rind (Punica granatum L.) on EJ bladder cancer cells via regulation of p53/miR-34a axis. Phytother. Res. 2015, 29, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Rettig, M.B.; Heber, D.; An, J.; Seeram, N.P.; Rao, J.Y.; Liu, H.; Klatte, T.; Belldegrun, A.; Moro, A.; Henning, S.M.; et al. Pomegranate extract inhibits androgen-independent prostate cancer growth through a nuclear factor-κB-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2662–2671. [Google Scholar] [CrossRef] [PubMed]
- Joseph, M.M.; Aravind, S.R.; Varghese, S.; Mini, S.; Sreelekha, T.T. Evaluation of antioxidant, antitumor and immunomodulatory properties of polysaccharide isolated from fruit rind of Punica granatum. Mol. Med. Rep. 2012, 5, 489–496. [Google Scholar]
- Veeragoni, D.; Deshpande, S.; Rachamalla, H.; Ande, A.; Puvvada, N.; Mutheneni, S. In vitro and in vivo anticancer and genotoxicity profiles of green synthesized and chemically synthesized silver nanoparticles. ACS Appl. Bio Mater. 2022, 5, 2324–2339. [Google Scholar] [CrossRef]
- Santhanam, N.; Arivazhagan, A.; Kennedy, K.; Swaminathan, S. Silver nanoparticles synthesized from aloe barbadensis leaf extract induces g0/g1 cell cycle arrest in thp-1 acute monocytic leukemia cells. Asian J. Pharm. Pharmacol. 2019, 5, 701–713. [Google Scholar] [CrossRef]
- Mohamad Sukri, S.N.A.; Shameli, K.; Teow, S.Y.; Chew, J.; Ooi, L.T.; Lee-Kiun Soon, M.; Ismail, N.A.; Moeini, H. Enhanced antibacterial and anticancer activities of plant extract mediated green synthesized zinc oxide-silver nanoparticles. Front. Microbiol. 2023, 14, 1194292. [Google Scholar] [CrossRef] [PubMed]
- Ramya, E.; Narayana Rao, D. Cytotoxicity Studies of Fruit-Extracted Metal Nanostructures. In Cytotoxicity—Understanding Cellular Damage and Response; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Baharara, J.; Namvar, F.; Ramezani, T.; Hosseini, N.; Mohamad, R. Green synthesis of silver nanoparticles using achillea biebersteinii flower extract and its anti-angiogenic properties in the rat aortic ring model. Molecules 2014, 19, 4624–4634. [Google Scholar] [CrossRef] [PubMed]
- Kuri, P.; Pion, E.; Mahl, L.; Kainz, P.; Schwarz, S.; Brochhausen, C.; Aung, T.; Haerteis, S. Deep learning-based image analysis for the quantification of tumor-induced angiogenesis in the 3d in vivo tumor model—Establishment and addition to laser speckle contrast imaging (lsci). Cells 2022, 11, 2321. [Google Scholar] [CrossRef] [PubMed]
- Decker, S.; Arango-Ospina, M.; Rehder, F.; Moghaddam, A.; Simon, R.; Merle, C.; Renkawitz, T.; Boccaccini, A.R.; Westhauser, F. In vitro and in ovo impact of the ionic dissolution products of boron-doped bioactive silicate glasses on cell viability, osteogenesis, and angiogenesis. Sci. Rep. 2022, 12, 8510. [Google Scholar] [CrossRef] [PubMed]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine, and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Baltazar, A.J.; Reyes-López, S.Y.; Larrañaga, D.; Estévez, M.; Pérez, R. Green synthesis of silver nanoparticles using a Melissa officinalis leaf extract with antibacterial properties. Results Phys. 2017, 7, 2639–2643. [Google Scholar] [CrossRef]
- Characteristic of IR Absorption Frequencies of Organic Functional Groups. Available online: http://www2.ups.edu/faculty/hanson/Spectroscopy/IR/IRfrequencies.html (accessed on 11 April 2024).
- Semenescu, A.D.; Moacă, E.A.; Iftode, A.; Dehelean, C.A.; Tchiakpe-Antal, D.S.; Vlase, L.; Vlase, A.M.; Muntean, D.; Chioibaş, R. Phytochemical and Nutraceutical Screening of Ethanol and Ethyl Acetate Phases of Romanian Galium verum Herba (Rubiaceae). Molecules 2023, 28, 7804. [Google Scholar] [CrossRef] [PubMed]
- Arendrup, M.C.; Cuenca-Estrella, M.; Lass-Flörl, C.; Hope, W. EUCAST technical note on the EUCAST definitive document EDef 7.2: Method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for yeasts EDef 7.2 (EUCAST-AFST). Clin. Microbiol. Infect. 2012, 18, E246–E247. [Google Scholar] [CrossRef]
- M07Ed11; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018.
- Luepke, N.P. Hen’s Egg Chorioallantoic Membrane Test for Irritation Potential. Food Chem. Toxicol. 1985, 23, 287–291. [Google Scholar] [CrossRef]
- Batista-Duharte, A.; Murillo, G.J.; Betancourt, J.E.; Oliver, P.; Damiana, T. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
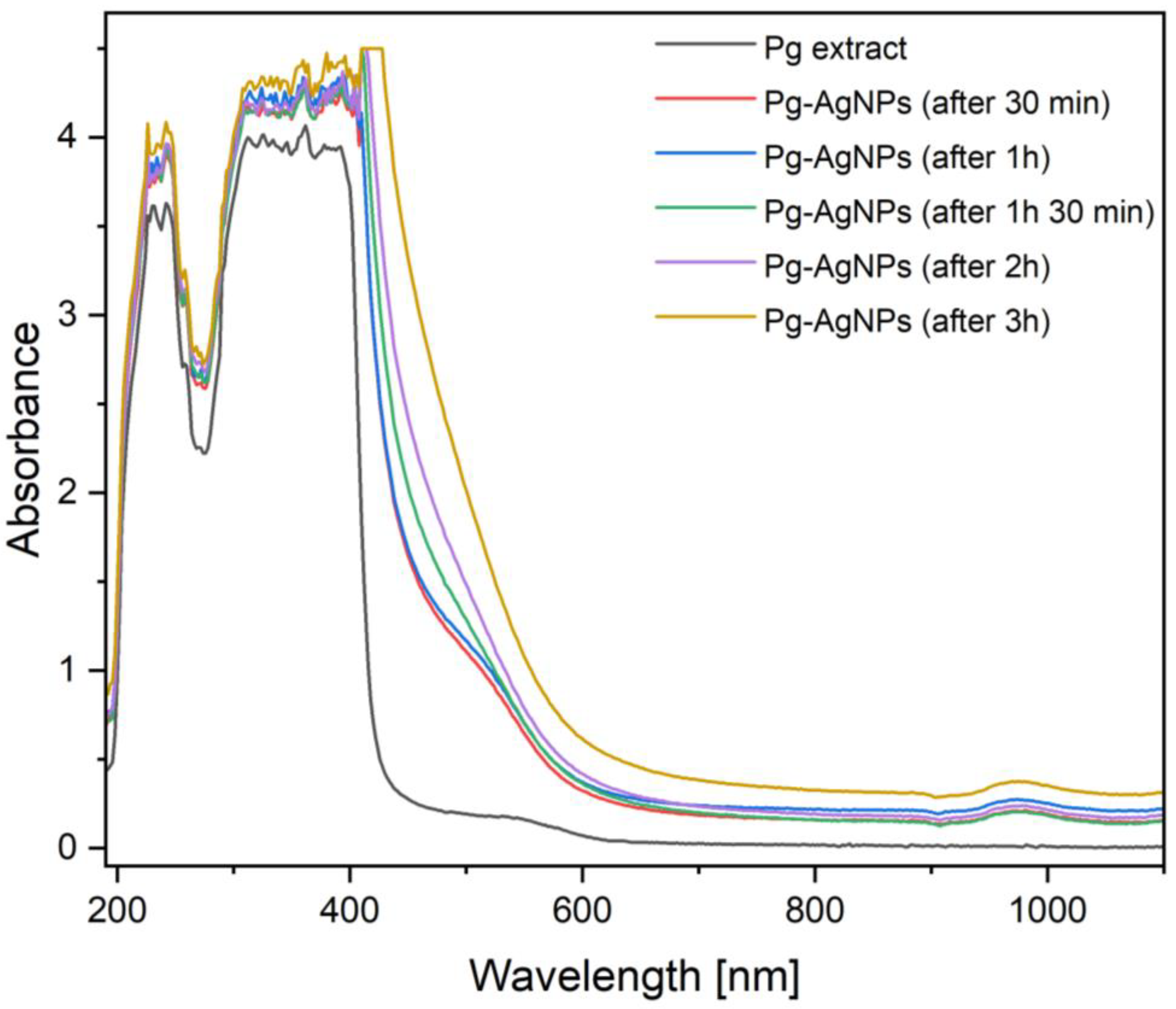


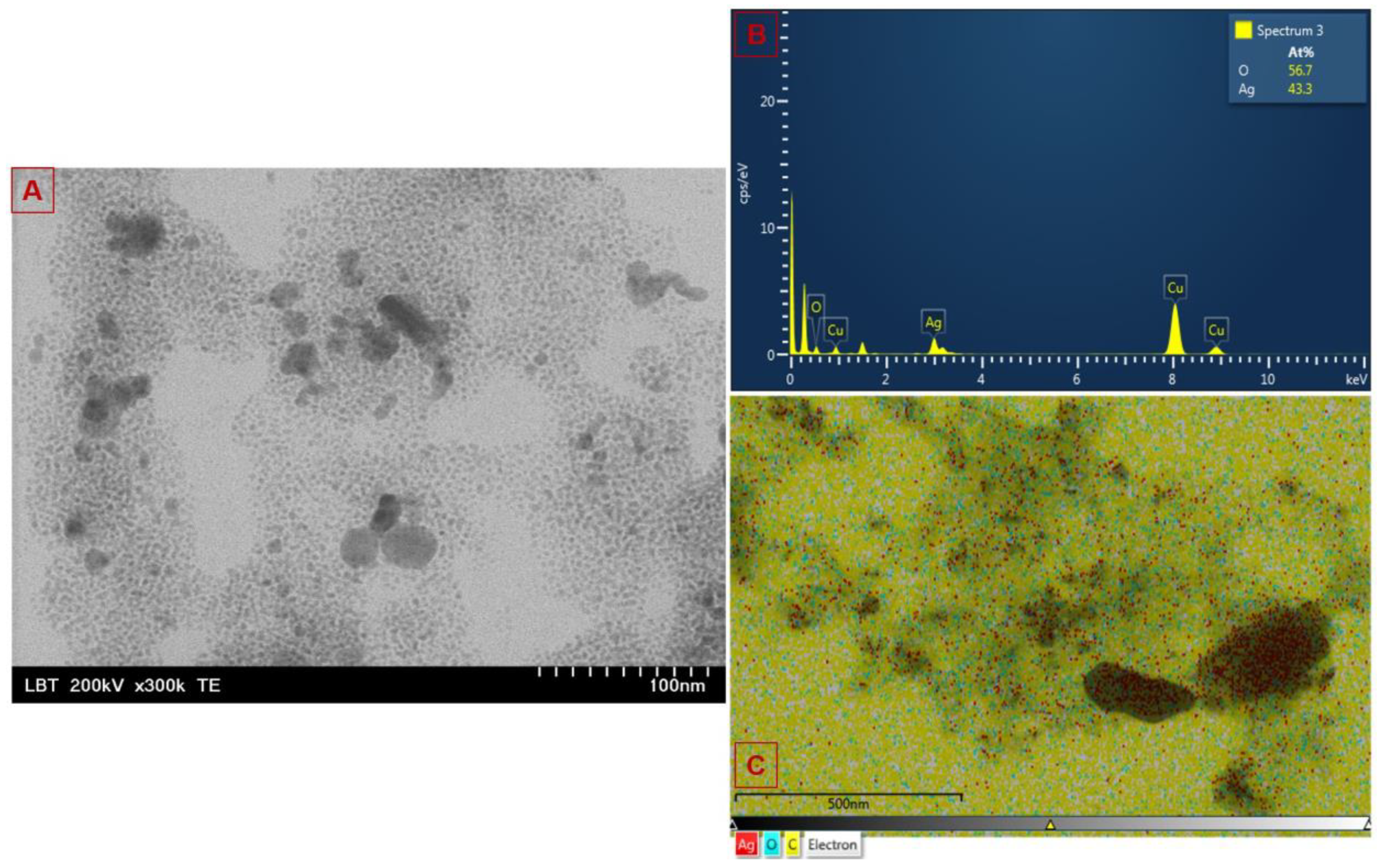
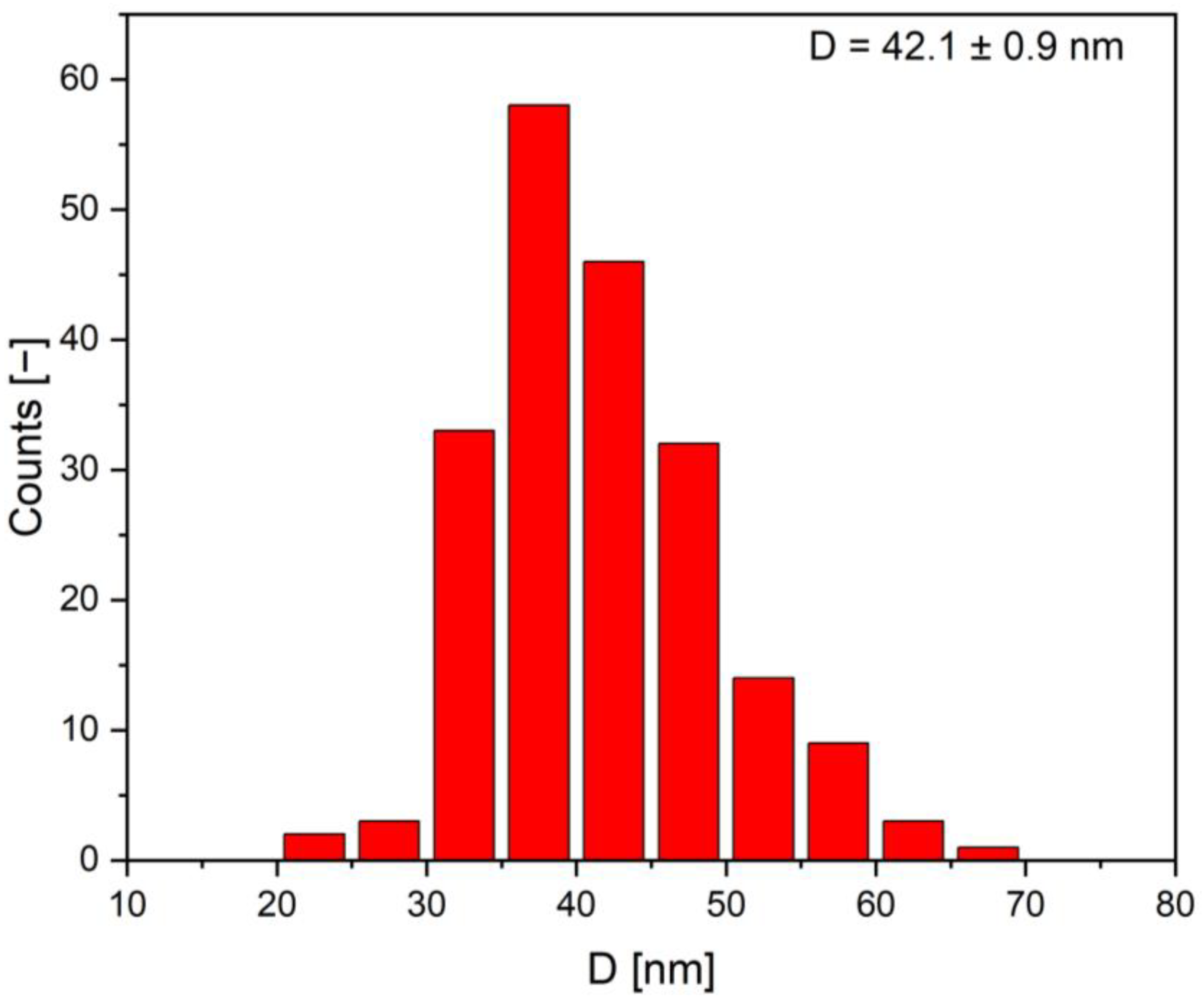
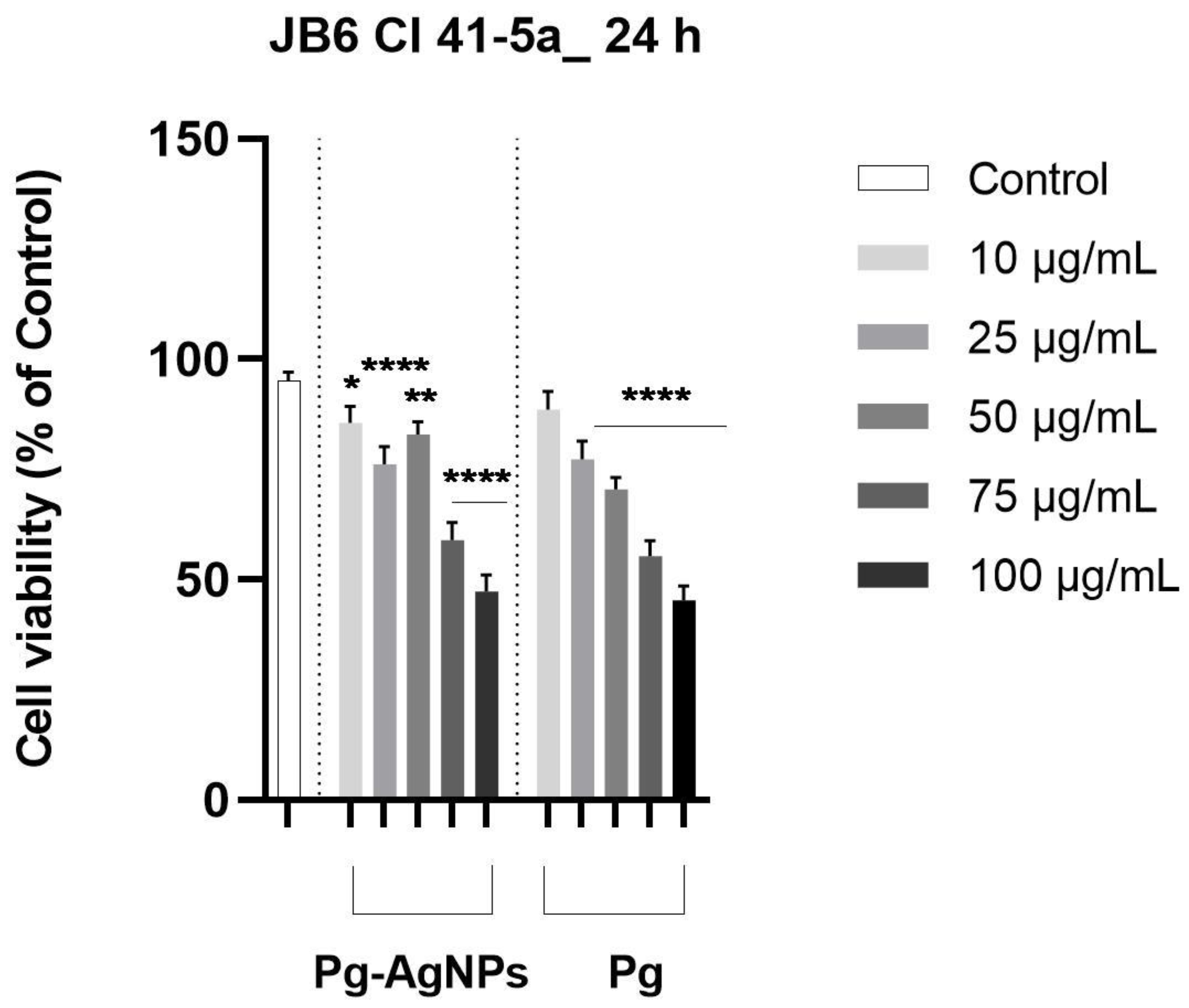

| Sample (Band Color in the Spectrum) | Absorbance | Wavelength (nm) | Time Interval |
|---|---|---|---|
| Pg peel ethanolic extract | 4.067 | 362 | before adding 1 M AgNO3 solution |
| Pg-AgNPs (red band) | 4.284 | 362 | after 30 min from adding 1 M AgNO3 solution |
| Pg-AgNPs (blue band) | 4.343 | 396 | after 1 h from adding 1 M AgNO3 solution |
| Pg-AgNPs (green band) | 4.500 | 410 | after 1 h 30 min from adding 1 M AgNO3 solution |
| Pg-AgNPs (violet band) | 4.500 | 410 | after 2 h from adding 1 M AgNO3 solution |
| Pg-AgNPs (ocher band) | 4.500 | 410 | after 3 h from adding 1 M AgNO3 solution |
| Absorption Peak/cm Pg-AgNPs/Pg Extract | Frequency Range/cm | Functional Group | Compound Class | Observation |
|---|---|---|---|---|
| 3402.43/3377.36 | 3550–3200 | O-H stretch | alcohol | intermolecular bonded band appearance—strong, broad |
| 3400–3300 | N-H stretching | aliphatic primary amine | band appearance—medium | |
| 2924.09/2937.59 | 3300–2500 | O-H stretch | carboxylic acids | usually centered on 3000/cm |
| 3200–2700 | O-H stretch | alcohols | intramolecular bonded | |
| 3000–2800 | N-H stretch | amine salt | band appearance—strong, broad | |
| 3000–2840 | C-H stretch | alkane | band appearance—medium | |
| 1716.65/1730.15 | 2000–1650 | C-H bending | aromatic compounds | band appearance—weak |
| 1740–1720 | C=O stretch | aldehyde | band appearance—strong | |
| 1730–1715 | C=O stretch | α, β—unsaturated ester | or formats | |
| 1725–1705 | C=O stretch | aliphatic ketone | or cyclohexanone, cyclopentenone | |
| 1720–1706 | C=O stretch | carboxylic acid | dimer | |
| 1600.92/1620.21 | 1650–1600 | C=C stretch | conjugated alkene | band appearance—medium |
| 1650–1580 | N-H bending | amine | band appearance—medium | |
| 1650–1566 | C=C stretch | cyclic alkene | band appearance—medium | |
| -/1444.68 | 1450 | C-H bending | methyl group | band appearance—medium |
| 1400–1600 | C=C stretch | aromatic compounds | band appearance—medium weak, multiple bands | |
| 1384.89/- | 1345–1385 | N-O stretch | nitro compounds | band appearance—strong, two bands |
| 1390–1380 | C-H bending | aldehyde | band appearance—medium | |
| 1385–1380 | C-H bending | alkane | gem dimethyl | |
| 1420–1330 | O-H bending | alcohol | band appearance—medium | |
| 1400–1000 | C-F stretch | fluoro compound | band appearance—strong | |
| 1390–1310 | O-H bending | phenol | band appearance—medium | |
| -/1230.58 | 1250–1020 | C-N stretch | amine | band appearance—medium |
| 1275–1200 | C-O stretch | alkyl aryl ether | band appearance—strong | |
| 1400–1000 | C-F stretch | fluoro compound | band appearance—strong | |
| 1068.56/- | 1250–1020 | C-N stretch | amine | band appearance—medium |
| 1085–1050 | C-O stretch | primary alcohol | band appearance—strong | |
| 1035.77/1045.42 | 1250–1020 | C-N stretch | amine | band appearance—medium |
| 1050–1040 | CO-O-CO stretch | anhydride | band appearance—strong, broad | |
| -/877.61 | 880 ± 20 | C-H bending | 1,2,4-trisubstituted 1,3-disubstituted | band appearance—strong |
| 831.32/817.82 | 850–550 | C-Cl stretch | halo compound | band appearance—strong |
| 840–790 | C=C bending | alkene | trisubstituted | |
| 810 ± 20 | C-H bending | 1,4-disubstituted 1,2,3,4-tetrasubstituted | band appearance—strong | |
| 773.46/779.24 | 850–550 | C-Cl stretch | halo compound | band appearance—strong |
| 755 ± 20 | C-H bending | 1,2-disubstituted monosubstituted | band appearance—strong | |
| 524.64/- | 690–515 | C-Br stretch | halo compound | band appearance—strong |
| 600–500 | C-I stretch | halo compound | band appearance—strong |
| Pg-AgNPs Concentration (μg/mL) | S. aureus (+) ATCC 25923 | E. coli (−) ATCC 25922 | P. aeruginosa (−) ATCC 27853 | |||
|---|---|---|---|---|---|---|
| MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | MIC (μg/mL) | MBC (μg/mL) | |
| 25 | 284 | 562 | 62 | NA | 55 | NA |
| 50 | 193 | 329 | 50 | NA | 47 | NA |
| 75 | 146 | 256 | 34 | NA | 31 | NA |
| 100 | 97 | 164 | 18 | 10 | 15 | 10 |
| Sample/Concentration | Hemorrhage (tH) (s) | Lysis (tL) (s) | Coagulation (tC) (s) | IS |
|---|---|---|---|---|
| H2Od | 300 | 300 | 300 | 0.07 |
| SDS 1% | 20 | 38 | 45 | 18.5 |
| Pg-AgNPs, 10 μg/mL | 44 | 140 | 300 | 8.07 |
| Pg-AgNPs, 50 μg/mL | 300 | 300 | 240 | 1.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarău, O.S.; Moacă, E.-A.; Semenescu, A.-D.; Dumitru, R.; Jijie, A.-R.; Poenaru, M.; Dehelean, C.-A.; Chevereşan, A. Physicochemical and Toxicological Screening of Silver Nanoparticle Biosynthesis from Punica granatum Peel Extract. Inorganics 2024, 12, 160. https://doi.org/10.3390/inorganics12060160
Sarău OS, Moacă E-A, Semenescu A-D, Dumitru R, Jijie A-R, Poenaru M, Dehelean C-A, Chevereşan A. Physicochemical and Toxicological Screening of Silver Nanoparticle Biosynthesis from Punica granatum Peel Extract. Inorganics. 2024; 12(6):160. https://doi.org/10.3390/inorganics12060160
Chicago/Turabian StyleSarău, Oana Silvana, Elena-Alina Moacă, Alexandra-Denisa Semenescu, Raluca Dumitru, Alex-Robert Jijie, Marioara Poenaru, Cristina-Adriana Dehelean, and Adelina Chevereşan. 2024. "Physicochemical and Toxicological Screening of Silver Nanoparticle Biosynthesis from Punica granatum Peel Extract" Inorganics 12, no. 6: 160. https://doi.org/10.3390/inorganics12060160
APA StyleSarău, O. S., Moacă, E.-A., Semenescu, A.-D., Dumitru, R., Jijie, A.-R., Poenaru, M., Dehelean, C.-A., & Chevereşan, A. (2024). Physicochemical and Toxicological Screening of Silver Nanoparticle Biosynthesis from Punica granatum Peel Extract. Inorganics, 12(6), 160. https://doi.org/10.3390/inorganics12060160









