Limited Domain SnSb@N-PC Composite Material as a High-Performance Anode for Sodium Ion Batteries
Abstract
1. Introduction
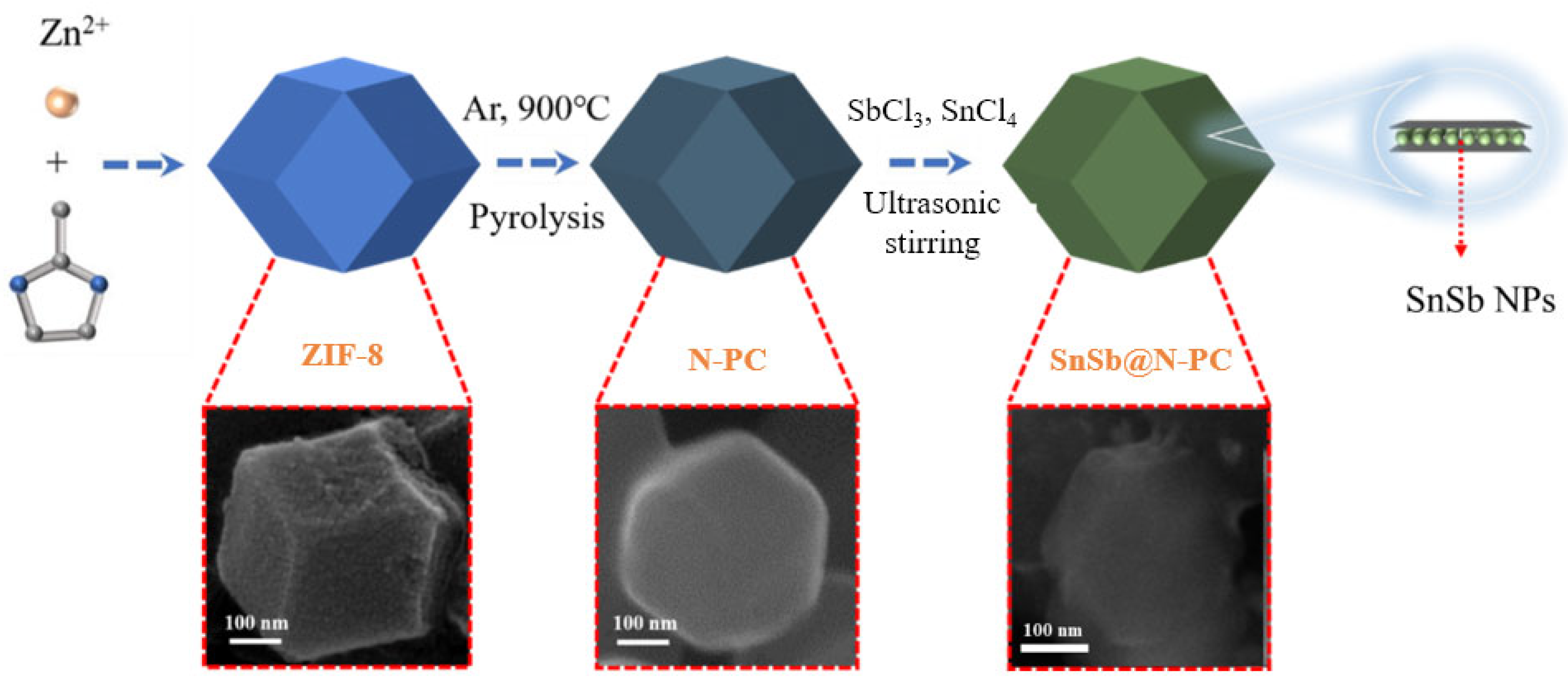
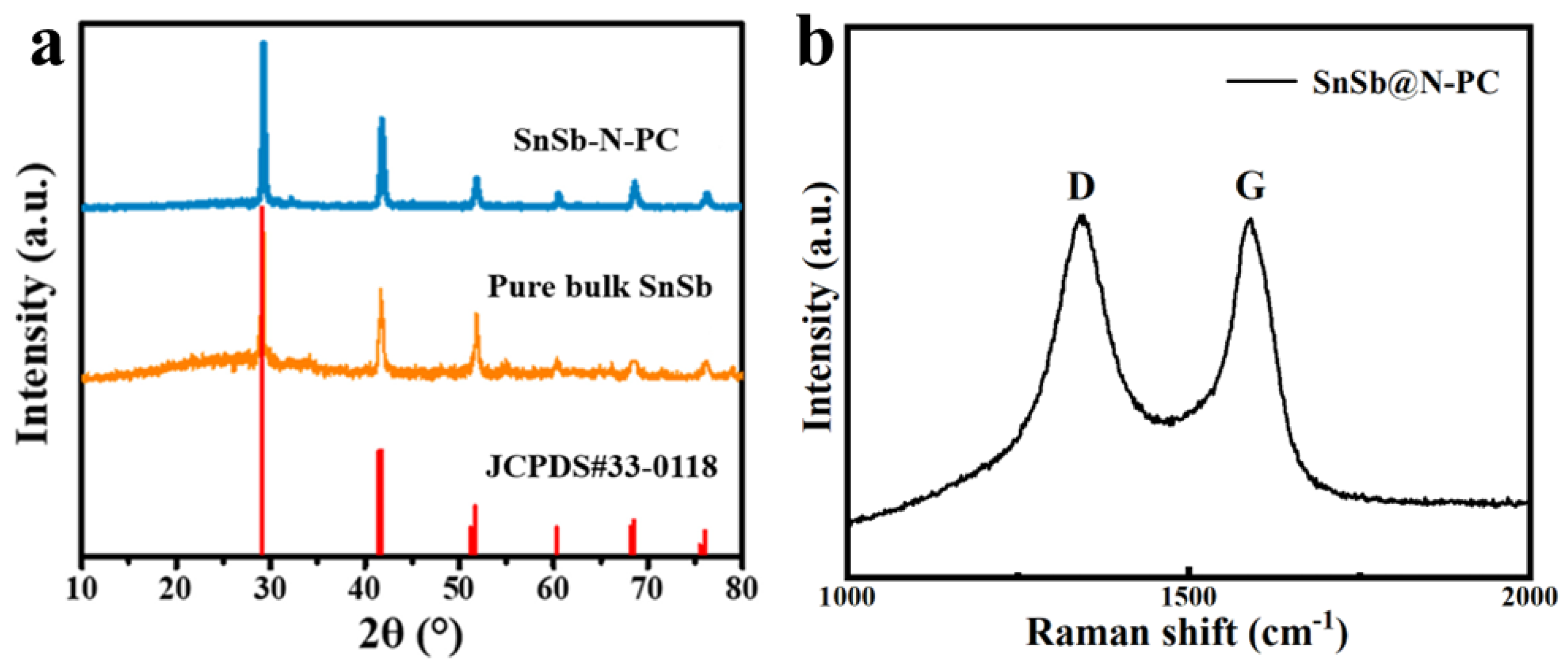
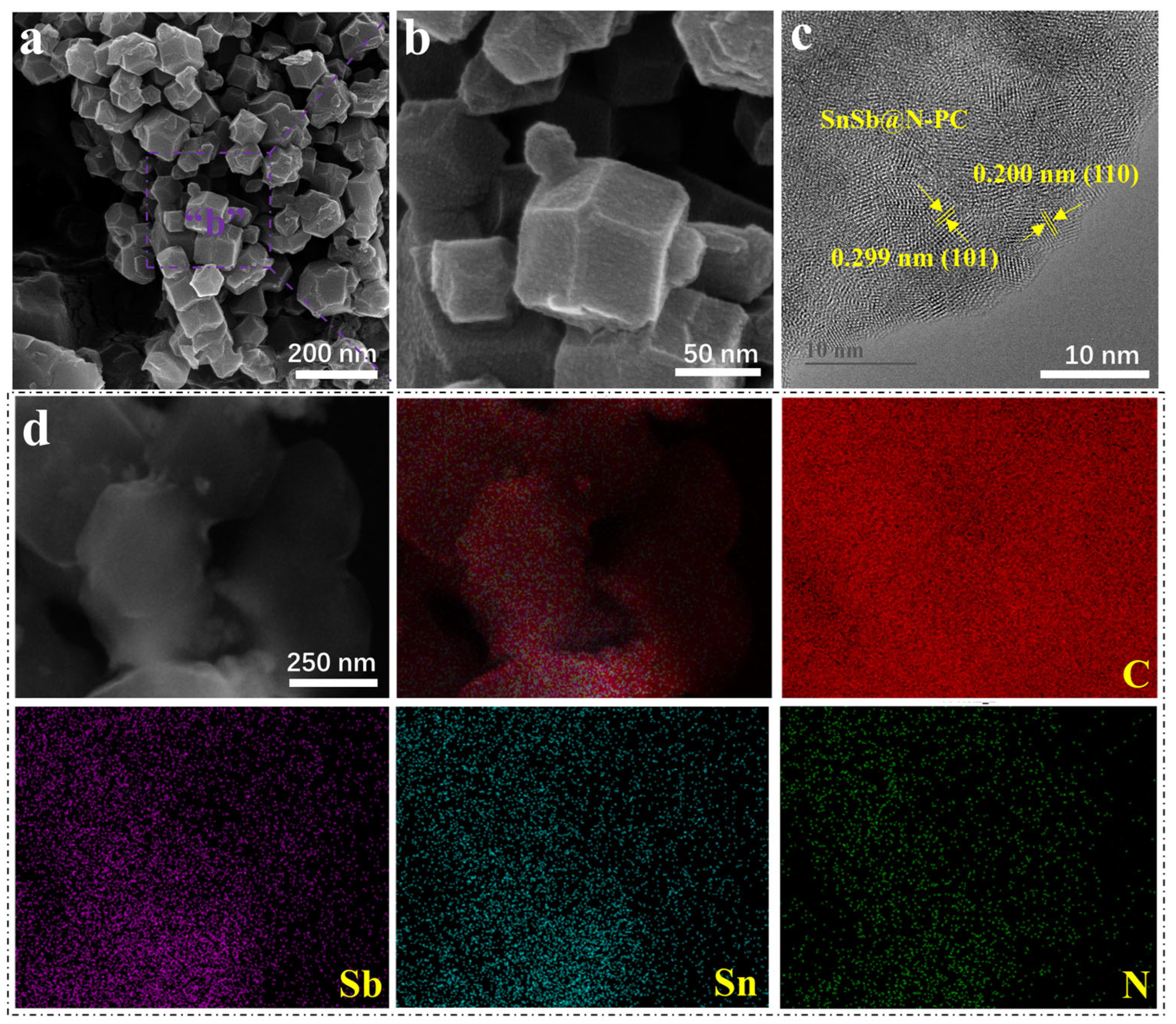
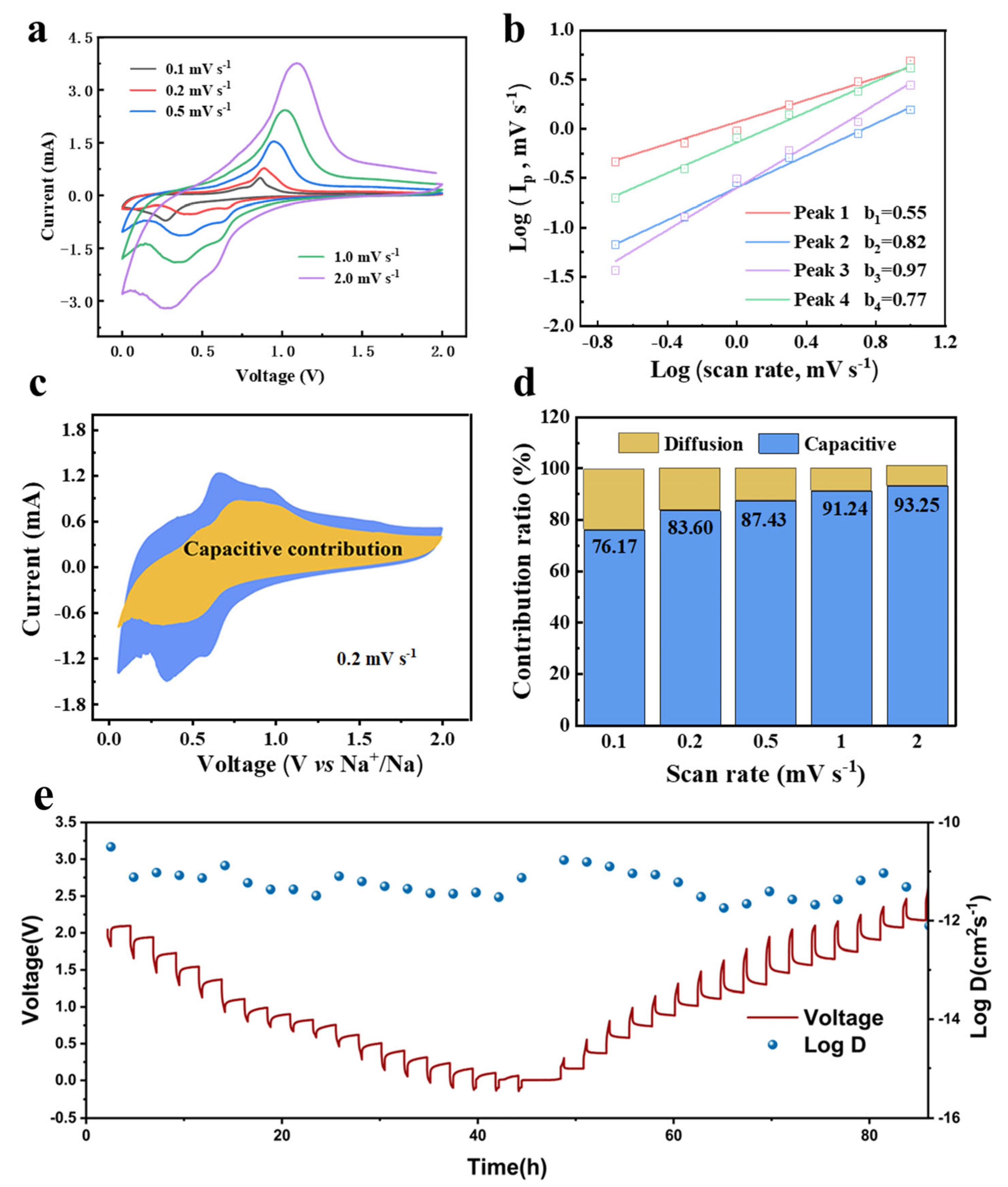
2. Results
3. Discussion
4. Materials and Methods
4.1. Preparation of SnSb@N-PC
4.2. Characterization
4.3. Electrochemical Measurements
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ge, J.; Fan, L.; Wang, J.; Zhang, Q.; Liu, Z.; Zhang, E.; Liu, Q.; Yu, X.; Lu, B. MoSe2/N-Doped Carbon as Anodes for Potassium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1801477. [Google Scholar] [CrossRef]
- Mu, J.; Zhao, Z.; Gao, X.W.; Liu, Z.M.; Luo, W.B.; Sun, Z.; Gu, Q.F.; Li, F. Bimetallic PdFe3 Nano-Alloy with Tunable Electron Configuration for Boosting Electrochemical Nitrogen Fixation. Adv. Energy Mater. 2023, 14, 2303558. [Google Scholar] [CrossRef]
- Zhao, L.K.; Gao, X.W.; Mu, J.; Luo, W.B.; Liu, Z.; Sun, Z.; Gu, Q.F.; Li, F. Durable Integrated K-Metal Anode with Enhanced Mass Transport through Potassiphilic Porous Interconnected Mediator. Adv. Funct. Mater. 2023, 33, 2304292. [Google Scholar] [CrossRef]
- Yu, W.; Ge, J.; Hu, Y.; Shen, D.; Luo, W.; Chen, S.; Wu, L.; Liu, Z.; Zhou, J.; Yang, H.; et al. Hybrid High-Performance Aqueous Batteries with Potassium-Based Cathode||Zinc Metal Anode. Sci. China Mater. 2022, 66, 923–931. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Ding, H.; Chen, S.; Yu, X.; Lu, B. Carbon Nanoscrolls for Aluminum Battery. ACS Nano 2018, 12, 8456–8466. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Jia, X.; Li, W.; Zhang, Q.; Fan, L.; Ding, H.; Yang, H.; Yu, X.; Li, X.; et al. Graphene Armored with a Crystal Carbon Shell for Ultrahigh-Performance Potassium Ion Batteries and Aluminum Batteries. ACS Nano 2019, 13, 10631–10642. [Google Scholar] [CrossRef]
- Wang, J.; Fan, L.; Liu, Z.; Chen, S.; Zhang, Q.; Wang, L.; Yang, H.; Yu, X.; Lu, B. In Situ Alloying Strategy for Exceptional Potassium Ion Batteries. ACS Nano 2019, 13, 3703–3713. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, T.; Ali, M.; Meng, Y.; Jin, Y.; Cui, Y.; Chen, W. Rechargeable Batteries for Grid Scale Energy Storage. Chem. Rev. 2022, 122, 16610–16751. [Google Scholar] [CrossRef]
- Wang, F.; Wu, X.; Li, C.; Zhu, Y.; Fu, L.; Wu, Y.; Liu, X. Nanostructured positive electrode materials for post-lithium ion batteries. Energy Environ. Sci. 2016, 9, 3570–3611. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, B.; Sun, Q.; Wennersten, R. Application of energy storage in integrated energy systems—A solution to fluctuation and uncertainty of renewable energy. J. Energy Storage 2022, 52, 104812. [Google Scholar] [CrossRef]
- Li, J.; Fleetwood, J.; Hawley, W.B.; Kays, W. From Materials to Cell: State-of-the-Art and Prospective Technologies for Lithium-Ion Battery Electrode Processing. Chem. Rev. 2022, 122, 903–956. [Google Scholar] [CrossRef]
- Yuan, S.; Lai, Q.; Duan, X.; Wang, Q. Carbon-based materials as anode materials for lithium-ion batteries and lithium-ion capacitors: A review. J. Energy Storage 2023, 61, 106716. [Google Scholar] [CrossRef]
- Xiang, J.; Wei, Y.; Zhong, Y.; Yang, Y.; Cheng, H.; Yuan, L.; Xu, H.; Huang, Y. Building Practical High-Voltage Cathode Materials for Lithium-Ion Batteries. Adv. Mater. 2022, 34, 2200912. [Google Scholar] [CrossRef]
- Mohr, S.H.; Mudd, G.M.; Giurco, D. Lithium Resources and Production: Critical Assessment and Global Projections. Minerals 2012, 2, 65–84. [Google Scholar] [CrossRef]
- Grosjean, C.; Miranda, P.H.; Perrin, M.; Poggi, P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sustain. Energy Rev. 2012, 16, 1735–1744. [Google Scholar] [CrossRef]
- Wanger, T.C. The Lithium future—Resources, recycling, and the environment. Conserv. Lett. 2011, 4, 202–206. [Google Scholar] [CrossRef]
- Pavlovskii, A.A.; Pushnitsa, K.; Kosenko, A.; Novikov, P.; Popovich, A.A. A Minireview on the Regeneration of NCM Cathode Material Directly from Spent Lithium-Ion Batteries with Different Cathode Chemistries. Inorganics 2022, 10, 141. [Google Scholar] [CrossRef]
- Nayak, P.K.; Yang, L.; Brehm, W.; Adelhelm, P. From Lithium-Ion to Sodium-Ion Batteries: Advantages, Challenges, and Surprises. Angew. Chem. Int. Ed. 2018, 57, 102–120. [Google Scholar] [CrossRef]
- Gu, X.; Gao, X.-W.; Yang, D.; Gu, Q.; Song, Y.; Chen, H.; Ren, T.; Luo, W.-B. Two positive effects with one arrow: Modulating crystal and interfacial decoration towards high-potential cathode material. J. Energy Chem. 2024, 92, 216–223. [Google Scholar] [CrossRef]
- Mu, J.-J.; Liu, Z.-M.; Lai, Q.-S.; Wang, D.; Gao, X.-W.; Yang, D.-R.; Chen, H.; Luo, W.-B. An industrial pathway to emerging presodiation strategies for increasing the reversible ions in sodium-ion batteries and capacitors. Energy Mater. 2022, 2, 200043. [Google Scholar] [CrossRef]
- Yang, S.; Wang, B.; Lv, Q.; Zhang, N.; Zhang, Z.; Jing, Y.; Li, J.; Chen, R.; Wu, B.; Xu, P.; et al. Recent Advances in Cathodes for All-Solid-State Lithium-Sulfur Batteries. Chin. Chem. Lett. 2023, 34, 107783. [Google Scholar] [CrossRef]
- Ye, H.; Lei, D.; Shen, L.; Ni, B.; Li, B.; Kang, F.; He, Y.-B. In-Situ Polymerized Cross-Linked Binder for Cathode in Lithium-Sulfur Batteries. Chin. Chem. Lett. 2020, 31, 570–574. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, Z.; Huang, Z.; Guo, Y.; Zhang, S.; Zhao, Y.; Zhi, C. Recent Advances for Zn-Gas Batteries Beyond Zn-Air/Oxygen Battery. Chin. Chem. Lett. 2023, 34, 107600. [Google Scholar] [CrossRef]
- Wang, Q.; Li, J.; Jin, H.; Xin, S.; Gao, H. Prussian-Blue Materials: Revealing New Opportunities for Rechargeable Batteries. InfoMat 2022, 4, e12311. [Google Scholar] [CrossRef]
- Cao, L.; Gao, X.; Zhang, B.; Ou, X.; Zhang, J.; Luo, W.-B. Bimetallic Sulfide Sb2S3@FeS2 Hollow Nanorods as High-Performance Anode Materials for Sodium-Ion Batteries. ACS Nano 2020, 14, 3610–3620. [Google Scholar] [CrossRef]
- Bai, X.; Wu, N.; Yu, G.; Li, T. Recent Advances in Anode Materials for Sodium-Ion Batteries. Inorganics 2023, 11, 289. [Google Scholar] [CrossRef]
- Fedoseeva, Y.V.; Shlyakhova, E.V.; Stolyarova, S.G.; Vorfolomeeva, A.A.; Nishchakova, A.D.; Grebenkina, M.A.; Makarova, A.A.; Kovalenko, K.A.; Okotrub, A.V.; Bulusheva, L.G. Electrochemical Performance of Potassium Hydroxide and Ammonia Activated Porous Nitrogen-Doped Carbon in Sodium-Ion Batteries and Supercapacitors. Inorganics 2022, 10, 198. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, R.; Mu, D.; Tan, G.; Gao, H.; Li, N.; Chen, R.; Wu, F. Progress in Electrolyte and Interface of Hard Carbon and Graphite Anode for Sodium-Ion Battery. Carbon Energy 2022, 4, 458–479. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, X.; Li, J.; Jin, H.; Gao, H. Minimizing the Interfacial Resistance for a Solid-State Lithium Battery Running at Room Temperature. Chem. Eng. J. 2022, 488, 137740. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Jiang, Y.; Gui, Q.; Ba, D.; Li, Y.; Liu, J. Binder-Free Electrodes for Advanced Potassium-Ion Batteries: A Review. Chin. Chem. Lett. 2021, 32, 1299–1308. [Google Scholar] [CrossRef]
- Yang, M.; Sun, Z.; Nie, P.; Yu, H.; Zhao, C.; Yu, M.; Luo, Z.; Geng, H.; Wu, X. SbPS4: A Novel Anode for High-Performance Sodium-Ion Batteries. Chin. Chem. Lett. 2022, 33, 470–474. [Google Scholar] [CrossRef]
- Mu, J.; Gao, X.-W.; Liu, Z.; Luo, W.-B.; Sun, Z.; Gu, Q.; Li, F. Boosting Nitrogen Electrocatalytic Fixation by Three-Dimensional TiO2-&N& Nanowire Arrays. J. Energy Chem. 2022, 75, 293–300. [Google Scholar]
- Wang, D.; Liu, Z.; Gao, X.-W.; Gu, Q.; Zhao, L.; Luo, W.-B. Massive Anionic Fluorine Substitution Two-Dimensional δ-MnO2 Nanosheets for High-Performance Aqueous Zinc-Ion Battery. J. Energy Storage 2023, 72, 108740. [Google Scholar] [CrossRef]
- Li, J.-G.; Mu, J.-J.; Liu, Z.-M.; Lai, Q.-S.; Zhao, L.-K.; Gao, X.-W.; Yang, D.-R.; Chen, H.; Luo, W.-B. Boosting Potassium-Based Dual Ion Battery With High Energy Density and Long Lifespan by Red Phosphorous. J. Power Sources 2023, 571, 233054. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, W.; Shih, K.; Wang, J.; Wang, Z.; Guo, H.; Yan, G.; Li, X.; Song, L. A MoS2 Coating Strategy to Improve the Comprehensive Electrochemical Performance of LiVPO4F. J. Power Sources 2016, 315, 294–301. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Yan, G.; Li, H.; Peng, W.; Li, X.; Song, L.; Shih, K. Improving the Electrochemical Performance of Lithium Vanadium Fluorophosphate Cathode Material: Focus on Interfacial Stability. J. Power Sources 2016, 329, 553–557. [Google Scholar] [CrossRef]
- Wang, S.-S.; Liu, Z.-M.; Gao, X.-W.; Wang, X.-C.; Chen, H.; Luo, W.-B. Layer-Structured Multitransition-Metal Oxide Cathode Materials for Potassium-Ion Batteries with Long Cycling Lifespan and Superior Rate Capability. ACS Appl. Mater. Interfaces 2023, 15, 57165–57173. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Zhou, J.; Han, K.; Lu, B. Insights into Metal/Metalloid-Based Alloying Anodes for Potassium Ion Batteries. ACS Mater. Lett. 2021, 3, 1572–1598. [Google Scholar] [CrossRef]
- Cheng, N.; Fan, L.; Liu, Z.; Chen, S.; Zhang, E.; Zhao, J.; Yang, H.; Yu, X.; Lu, B. Fluorine Atom-Inducing Graphene Oxide in situ Coating SnPO Composites as Anode for Sodium Ion Batteries. Mater. Today Energy 2019, 11, 174–181. [Google Scholar] [CrossRef]
- Zhang, W.; Mao, J.; Li, S.; Chen, Z.; Guo, Z. Phosphorus-Based Alloy Materials for Advanced Potassium-Ion Battery Anode. J. Am. Chem. Soc. 2017, 139, 3316–3319. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Luo, Y.; Qiu, W.; Liu, P.; Tong, Y. A review of carbon materials and their composites with alloy metals for sodium ion battery anodes. Carbon 2016, 98, 162–178. [Google Scholar] [CrossRef]
- Borah, R.; Hughson, F.R.; Johnston, J.; Nann, T. On battery materials and methods. Mater. Today Adv. 2020, 6, 100046. [Google Scholar] [CrossRef]
- Palomares, V.; Casas-Cabanas, M.; Castillo-Martínez, E.; Han, M.H.; Rojo, T. Update on Na-based battery materials. A growing research path. Energy Environ. Sci 2013, 6, 2312–2337. [Google Scholar] [CrossRef]
- Wang, L.; Tian, H.; Yao, X.; Cai, Y.; Gao, Z.; Su, Z. Research Progress and Modification Measures of Anode and Cathode Materials for Sodium-Ion Batteries. ChemElectroChem 2024, 11, e202300414. [Google Scholar] [CrossRef]
- Shi, H.; Gao, X.-W.; Wang, X.; Chen, H.; Han, W.; Gu, Q.; Liu, Z.; Luo, W.-B. Surface Residual Alkali Reverse Utilization: Stabilizing the Lay-Structured Oxide Cathode for High Stability Potassium Ion Batteries. Chem. Eng. J. 2024, 484, 149574. [Google Scholar] [CrossRef]
- Xie, H.; Tan, X.; Luber, E.J.; Olsen, B.C.; Kalisvaart, W.P.; Jungjohann, K.L.; Mitlin, D.; Buriak, J.M. β-SnSb for Sodium Ion Battery Anodes: Phase Transformations Responsible for Enhanced Cycling Stability Revealed by In Situ TEM. ACS Energy Lett. 2018, 3, 1670–1676. [Google Scholar] [CrossRef]
- Zhang, D.M.; Jia, J.H.; Yang, C.C.; Jiang, Q. Fe7Se8 Nanoparticles Anchored on N-doped Carbon Nanofibers as High-Rate Anode for Sodium-Ion Batteries. Energy Storage Mater. 2020, 24, 439–449. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, Z.; Wang, C.C.; Yang, C.C.; Jiang, Q. MOF-Derived Fe7S8 Nanoparticles/N-Doped Carbon Nanofibers as an Ultra-Stable Anode for Sodium-Ion Batteries. Small 2021, 17, 2102349. [Google Scholar] [CrossRef]
- Choi, J.-H.; Ha, C.-W.; Choi, H.-Y.; Lee, S.-M. Carbon Embedded SnSb Composite Tailored by Carbothermal Reduction Process as High Performance Anode for Sodium-Ion Batteries. J. Ind. Eng. Chem. 2018, 60, 451–457. [Google Scholar] [CrossRef]
- Choi, J.-H.; Ha, C.-W.; Choi, H.-Y.; Seong, J.-W.; Park, C.-M.; Lee, S.-M. Porous Carbon-Free SnSb Anodes for High-Performance Na-Ion Batteries. J. Power Sources 2018, 386, 34–39. [Google Scholar] [CrossRef]
- He, M.; Walter, M.; Kravchyk, K.V.; Erni, R.; Widmer, R.; Kovalenko, M.V. Monodisperse SnSb Nanocrystals for Li-ion and Na-ion Battery Anodes: Synergy and Dissonance Between Sn and Sb. Nanoscale 2015, 7, 455–459. [Google Scholar] [CrossRef]
- Jia, H.; Dirican, M.; Chen, C.; Zhu, J.; Zhu, P.; Yan, C.; Li, Y.; Dong, X.; Guo, J.; Zhang, X. Reduced Graphene Oxide-Incorporated SnSb@CNF Composites as Anodes for High-Performance Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 9696–9703. [Google Scholar] [CrossRef]
- Cheng, D.; Wei, A.; Ye, L.; Xu, G.; Tan, L.; Lu, B.; Chen, Y. Interfacial Bonding of SnSb Alloys with Graphene toward Ultrafast and Cycle-Stable Na-Ion Battery Anodes. ACS Sustain. Chem. Eng. 2022, 10, 12177–12187. [Google Scholar] [CrossRef]
- Ji, L.; Gu, M.; Shao, Y.; Li, X.; Engelhard, M.H.; Arey, B.W.; Wang, W.; Nie, Z.; Xiao, J.; Wang, C.; et al. Controlling SEI Formation on SnSb-Porous Carbon Nanofi bers for Improved Na Ion Storage. Adv. Mater. 2014, 26, 2901–2908. [Google Scholar] [CrossRef]
- Yue, L.; Jayapal, M.; Cheng, X.; Zhang, T.; Chen, J.; Ma, X.; Dai, X.; Lu, H.; Guan, R.; Zhang, W. Highly Dispersed Ultra-Small Nano Sn-SnSb Nanoparticles Anchored on Ndoped Graphene Sheets as High Performance Anode for Sodium Ion Batteries. Appl. Surf. Sci. 2020, 512, 145686. [Google Scholar] [CrossRef]
- Li, C.; Pei, Y.R.; Zhao, M.; Yang, C.C.; Jiang, Q. Sodium Storage Performance of Ultrasmall SnSb Nanoparticles. Chem. Eng. J. 2021, 40, 129617. [Google Scholar] [CrossRef]
- Darwiche, A.; Sougrati, M.T.; Fraisse, B.; Stievano, L.; Monconduit, L. Facile Synthesis and Long Cycle Life of SnSb as Negative Electrode Material for Na-Ion Batteries. Electrochem. Commun. 2013, 32, 18–21. [Google Scholar] [CrossRef]
- Lu, Y.C.; Dimov, N.; Okada, S.; Bui, T.H. SnSb Alloy Blended with Hard Carbon as Anode for Na-Ion Batteries. Energies 2018, 11, 1614. [Google Scholar] [CrossRef]
- Bian, Y.H.; Gao, X.W.; Zhao, L.K.; Liu, Z.; Gu, Q.; Luo, W.B. Enhanced Polysulfides Adsorption and Conversion for High Coulombic Efficiency Sodium-Ion Batteries. Batter. Supercaps 2023, 6, e202300227. [Google Scholar] [CrossRef]
- Lai, Q.S.; Mu, J.J.; Liu, Z.M.; Zhao, L.K.; Gao, X.W.; Yang, D.R.; Chen, H.; Luo, W.B. Tunnel-Type Na2Ti6O13@Carbon Nanowires as Anode Materials for Low-Temperature Sodium-Ion Batteries. Batter. Supercaps 2023, 6, e202200549. [Google Scholar] [CrossRef]
- Yang, D.; Gao, X.W.; Gao, G.; Lai, Q.; Ren, T.; Gu, Q.; Liu, Z.; Luo, W.B. Local Electronic Structure Constructing of Layer-Structured Oxide Cathode Material for High-Voltage Sodium-Ion Batteries. Carbon Energy 2024, e574. [Google Scholar] [CrossRef]
- Zhao, L.-K.; Gao, X.-W.; Gu, Q.; Ge, X.; Ding, Z.; Liu, Z.; Luo, W.-B. Realizing a Dendrite-Free Metallic-Potassium Anode using Reactive Prewetting Chemistry. eScience 2023, 4, 100201. [Google Scholar] [CrossRef]
- Zhao, L.-K.; Gao, X.-W.; Ren, T.-Z.; Wang, D.; Wang, D.-W.; Liu, Z.-M.; Chen, H.; Luo, W.-B. Regulating Ion Transport Behaviors toward Dendrite-Free Potassium Metal Batteries: Recent Advances and Perspectives. Rare Met. 2024, 43, 1435–1460. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, J.; Lu, B. Plum Pudding Model Inspired KVPO4F@3DC as High-Voltage and Hyperstable Cathode for Potassium Ion Batteries. Sci. Bull. 2020, 65, 1242–1251. [Google Scholar] [CrossRef]
- Wang, J.; Wang, B.; Liu, Z.; Fan, L.; Zhang, Q.; Ding, H.; Wang, L.; Yang, H.; Yu, X.; Lu, B. Nature of Bimetallic Oxide Sb2MoO6/rGO Anode for High-Performance Potassium-Ion Batteries. Adv. Sci. 2019, 6, 1900904. [Google Scholar] [CrossRef]
- Cheng, N.; Zhao, J.; Fan, L.; Liu, Z.; Chen, S.; Ding, H.; Yu, X.; Liu, Z.; Lu, B. Sb-MOFs Derived Sb Nanoparticles@Porous Carbon for High Performance Potassium-Ion Batteries Anode. Chem. Commun. 2019, 55, 12511–12514. [Google Scholar] [CrossRef]
- Ding, H.; Wang, J.; Fan, L.; Liu, Z.; Jia, X.; Yu, X.; Lu, B. Sn-Sb compounds with novel structure for stable potassium storage. Chem. Eng. J. 2020, 395, 125147. [Google Scholar] [CrossRef]
- Jia, M.; Jin, Y.; Zhao, C.; Zhao, P.; Jia, M. High Electrochemical Sodium Storage Performance of ZnSe/CoSe@N-Doped Porous Carbon Synthesized by the in-Situselenization of ZIF-8/67 Polyhedron. Appl. Surf. Sci. 2020, 518, 146259. [Google Scholar] [CrossRef]
- Chu, R.; Song, H.; Ullah, Z.; Guan, Z.; Zhang, Y.; Zhao, L.; Chen, M.; Li, W.; Li, Q.; Liu, L. ZIF-8 Derived Nitrogen-Doped Carbon Composites Boost the Rate Performance of Organic Cathodes for Sodium Ion Batteries. Electrochim. Acta 2020, 362, 137115. [Google Scholar] [CrossRef]
- He, Y.; Wang, L.; Dong, C.; Li, C.; Ding, X.; Qian, Y.; Xu, L. In-Situ Rooting ZnSe/N-Doped Hollow Carbon Architectures as High-Rate and Long-Life Anode Materials for Half/Full Sodium-Ion and Potassium-Ion Batteries. Energy Storage Mater. 2019, 23, 35–45. [Google Scholar] [CrossRef]
- Alam, M.W.; BaQais, A.; Nahvi, I.; Yasin, A.; Shajahan, S. Hydrothermally Synthesized Fluorine Added O3-NaFe1-xMgxO2 Cathodes for Sodium Ion Batteries. Inorganics 2023, 11, 37. [Google Scholar] [CrossRef]
- Fei, H.; Wu, P.; He, L.; Li, H. Facile Synthesis of Hollow V2O5 Microspheres for Lithium-Ion Batteries with Improved Performance. Inorganics 2024, 12, 37. [Google Scholar] [CrossRef]
- Li, T.; Yu, G.; Song, M.; Zhang, Q.; Li, Y.; Bai, X. Facile Synthesis of Nb-Doped CoTiO3 Hexagonal Microprisms as Promising Anode Materials for Lithium-Ion Batteries. Inorganics 2023, 11, 10. [Google Scholar] [CrossRef]
- Venezia, E.; Salimi, P.; Liang, S.; Fugattini, S.; Carbone, L.; Zaccaria, R.P. Comparative Study of Lithium Halide-Based Electrolytes for Application in Lithium-Sulfur Batteries. Inorganics 2023, 11, 86. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Ali, Z.; Li, N.; Liu, Q.; Fan, W.; Ma, J.; Sun, R. Oxygen-doped Carbon Nitride Nanocages with Efficient Photon-to-Electron Conversion for Selective Oxidation of Xylose/Xylan to Yield Xylonic Acid. Pap. Biomater. 2023, 8, 53–65. [Google Scholar] [CrossRef]
- Ou, X.; Xiong, X.; Zheng, F.; Yang, C.; Lin, Z.; Hu, R.; Jin, C.; Chen, Y.; Liu, M. In Situ X-ray diffraction characterization of NbS2 nanosheets as the anode material for sodium ion batteries. J. Power Sources 2016, 325, 410–416. [Google Scholar] [CrossRef]
- Ni, Q.; Jiang, H.; Sandstrom, S.; Bai, Y.; Ren, H.; Wu, X.; Guo, Q.; Yu, D.; Wu, C.; Ji, X. A Na3V2(PO4)2O1.6F1.4 Cathode of Zn-Ion Battery Enabled by a Water-in-Bisalt Electrolyte. Adv. Funct. Mater. 2020, 30, 2003511. [Google Scholar] [CrossRef]
- Li, W.; Hu, S.; Luo, X.; Li, Z.; Sun, X.; Li, M.; Liu, F.; Yu, Y. Confined Amorphous Red Phosphorus in MOF-Derived N-Doped Microporous Carbon as a Superior Anode for Sodium-Ion Battery. Adv. Mater. 2017, 29, 1605820. [Google Scholar] [CrossRef]
- Zhou, L.-F.; Gao, X.-W.; Du, T.; Gong, H.; Liu, L.-Y.; Luo, W.-B. Two-dimensional NbSSe as anode material for low-temperature sodium-ion batteries. Chem. Eng. J. 2022, 435, 134838. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Ren, H.; Fu, S.; Yang, W.; Li, Y.; Jiao, Y.; Zhang, B. Limited Domain SnSb@N-PC Composite Material as a High-Performance Anode for Sodium Ion Batteries. Inorganics 2024, 12, 162. https://doi.org/10.3390/inorganics12060162
Liu Z, Ren H, Fu S, Yang W, Li Y, Jiao Y, Zhang B. Limited Domain SnSb@N-PC Composite Material as a High-Performance Anode for Sodium Ion Batteries. Inorganics. 2024; 12(6):162. https://doi.org/10.3390/inorganics12060162
Chicago/Turabian StyleLiu, Zhaomeng, Hailong Ren, Shizheng Fu, Wentao Yang, Yihua Li, Yang Jiao, and Botao Zhang. 2024. "Limited Domain SnSb@N-PC Composite Material as a High-Performance Anode for Sodium Ion Batteries" Inorganics 12, no. 6: 162. https://doi.org/10.3390/inorganics12060162
APA StyleLiu, Z., Ren, H., Fu, S., Yang, W., Li, Y., Jiao, Y., & Zhang, B. (2024). Limited Domain SnSb@N-PC Composite Material as a High-Performance Anode for Sodium Ion Batteries. Inorganics, 12(6), 162. https://doi.org/10.3390/inorganics12060162





