Research Progress of TiO2 Modification and Photodegradation of Organic Pollutants
Abstract
1. Introduction
2. Mechanism and Kinetics of Photodegradation of Organic Pollutants by TiO2 Photocatalyst
2.1. Mechanism of Photodegradation of Organic Pollutants by TiO2 Photocatalyst
2.2. Kinetics of Photodegradation of Organic Pollutants by TiO2 Photocatalyst
- Solution pH value has an important effect on photocatalytic degradation. The photocatalytic activity of TiO2 may vary under acidic and alkaline conditions.
- The amount of catalyst dosing will also affect the degradation rate. Less than or more than the optimal dosage will lead to a decrease in the degradation rate.
- Factors such as organic pollutant’s initial concentration and light intensity also affect the kinetic process of photocatalytic degradation.
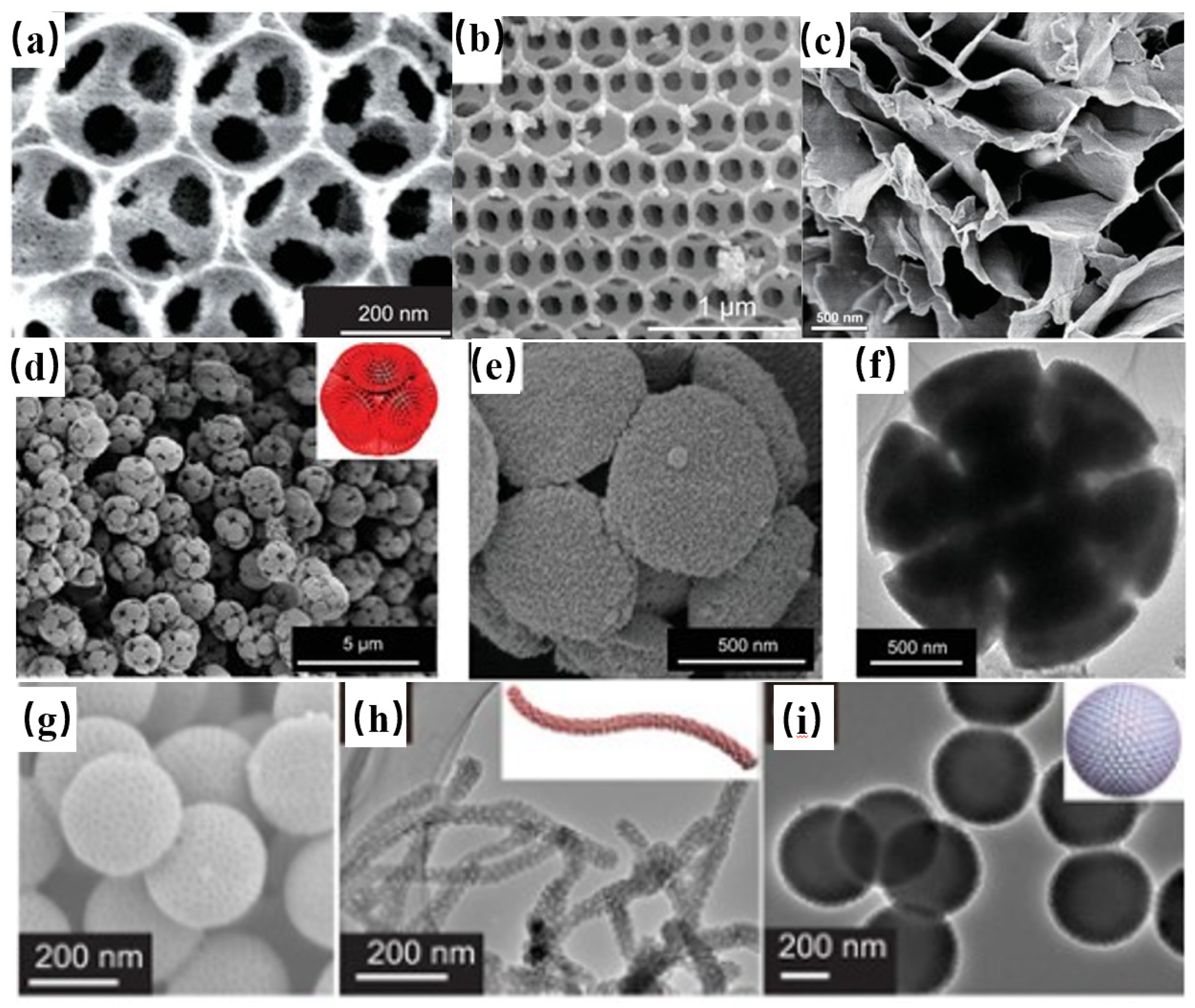
3. Synthesis Method of TiO2 Photocatalyst
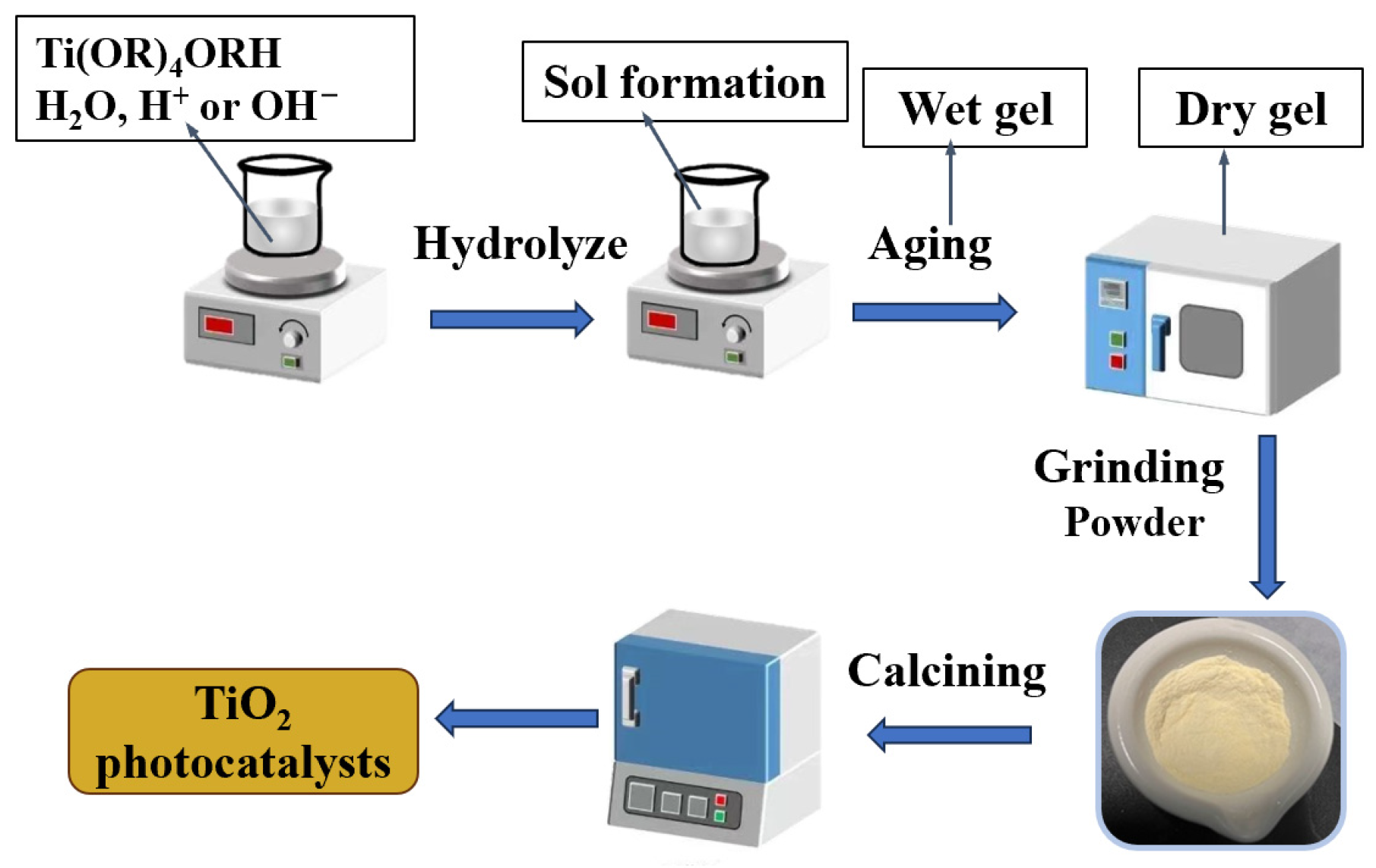
3.1. Sol-Gel Method
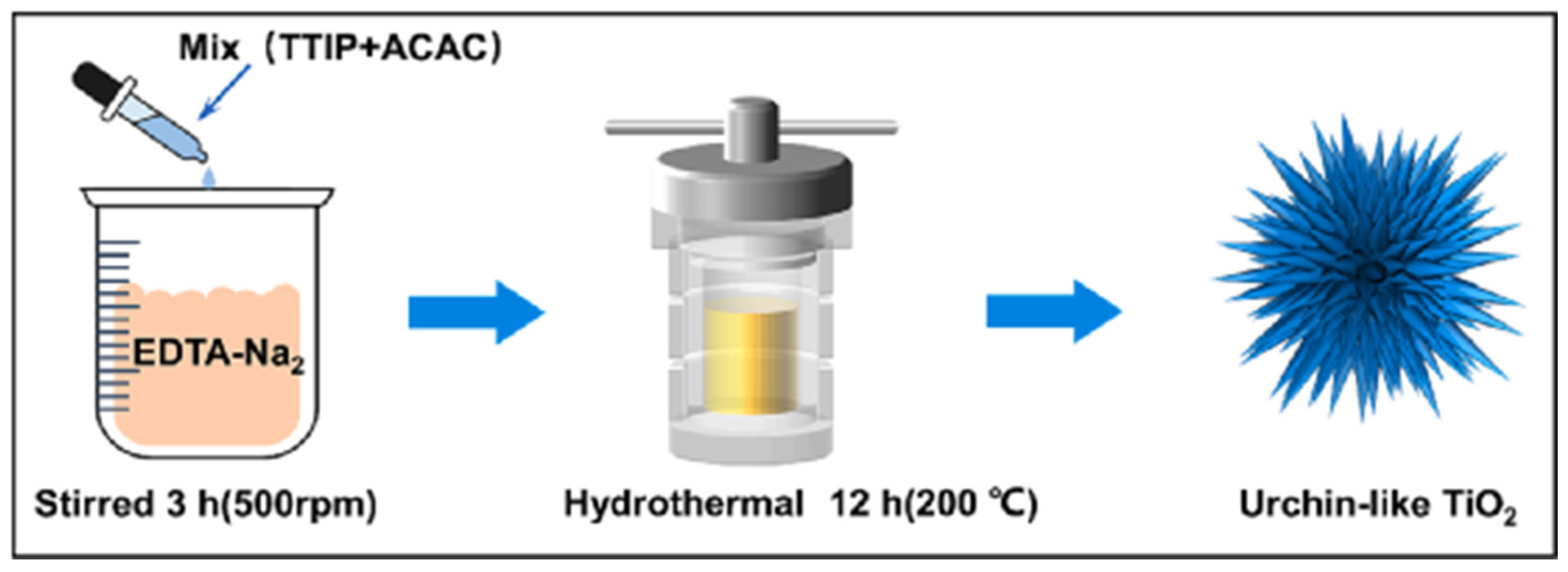
3.2. Hydrothermal Synthesis
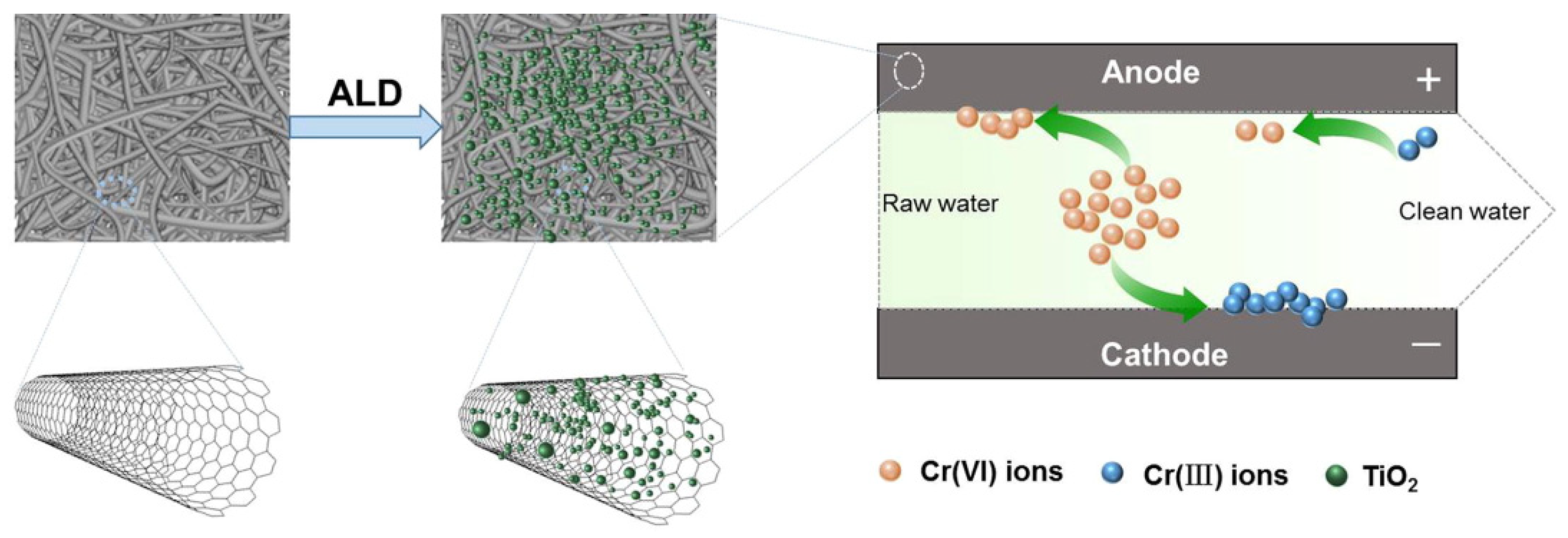
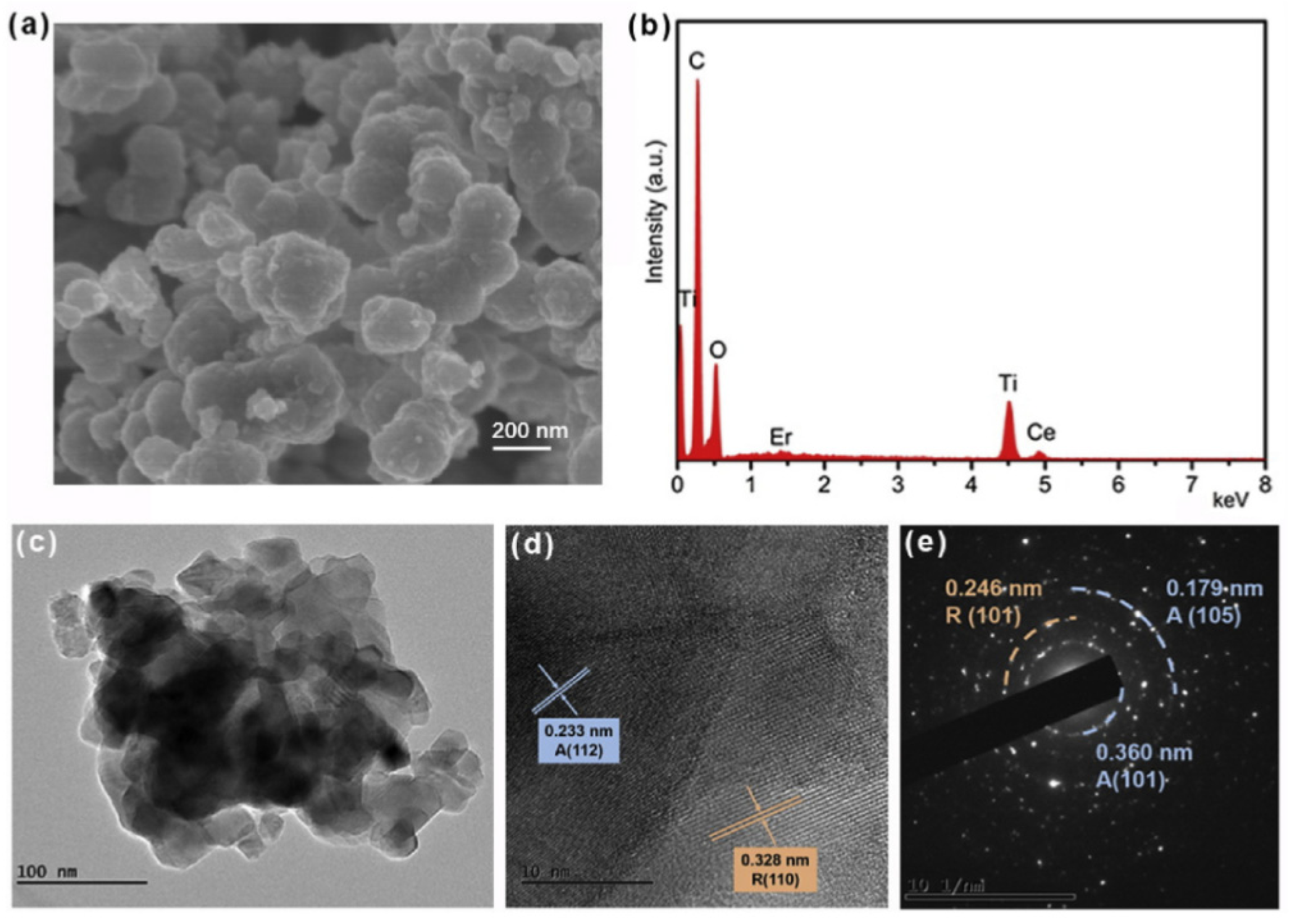
3.3. Atomic Layer Deposition Method
3.4. Microemulsion Method
3.5. Other Synthesis Methods
4. Modification Method of TiO2 Photocatalyst
4.1. Precious Metal-Doped Titanium Dioxide
4.2. Transition Metal-Doped Titanium Dioxide
4.2.1. Doping with a Single Transition Metal Elements
4.2.2. Co-Doping of Transition Metal Elements
4.3. Rare Earth Metal-Doped Titanium Dioxide
4.3.1. Doping with a Single Rare Earth Metal Element
4.3.2. Co-Doping with Rare Earth Metal Elements
4.3.3. Co-Doping of Rare Earth Elements with Other Elements
4.4. Compound Semiconductors Based on Titanium Dioxide
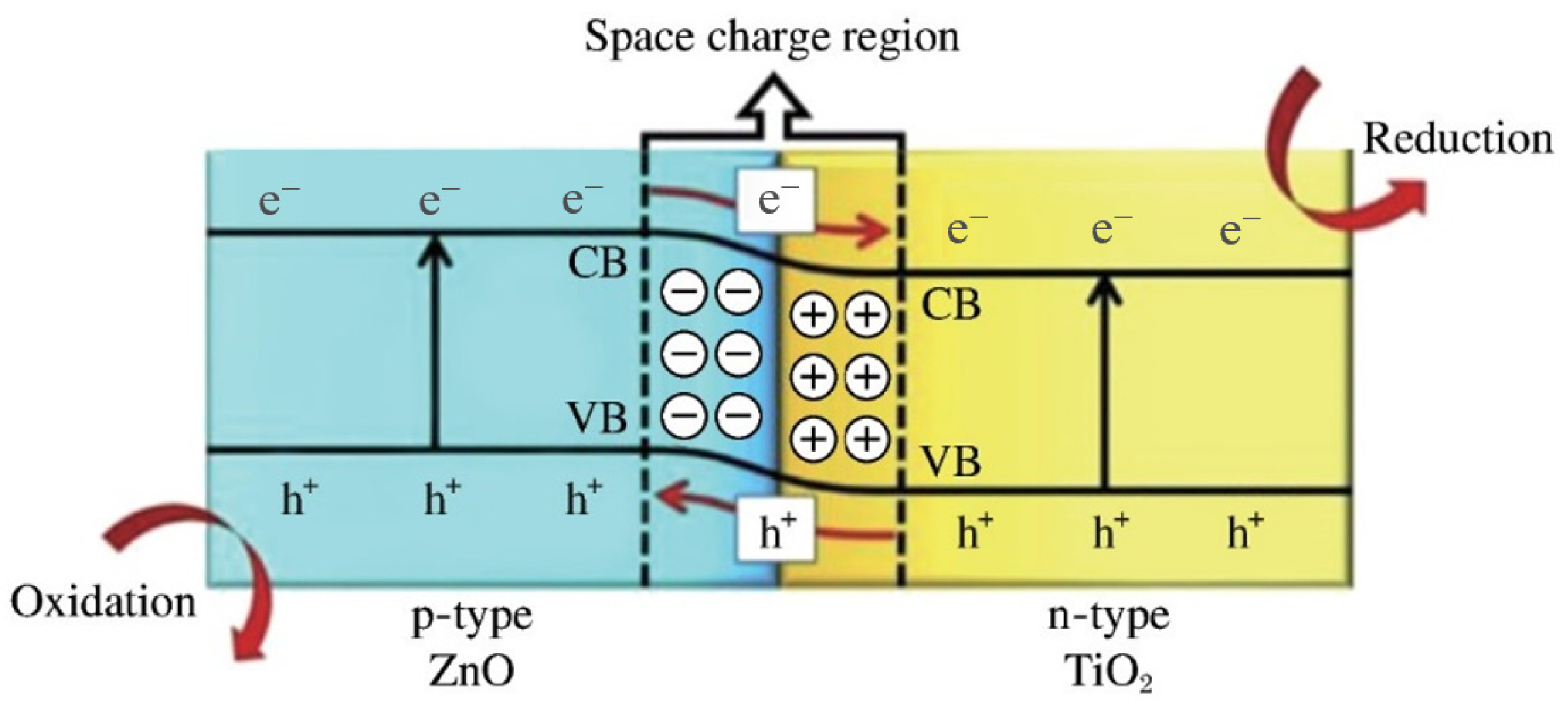
4.4.1. Titanium Dioxide Composites with Common Semiconductors
4.4.2. Titanium Dioxide Hybrid Materials with Graphene
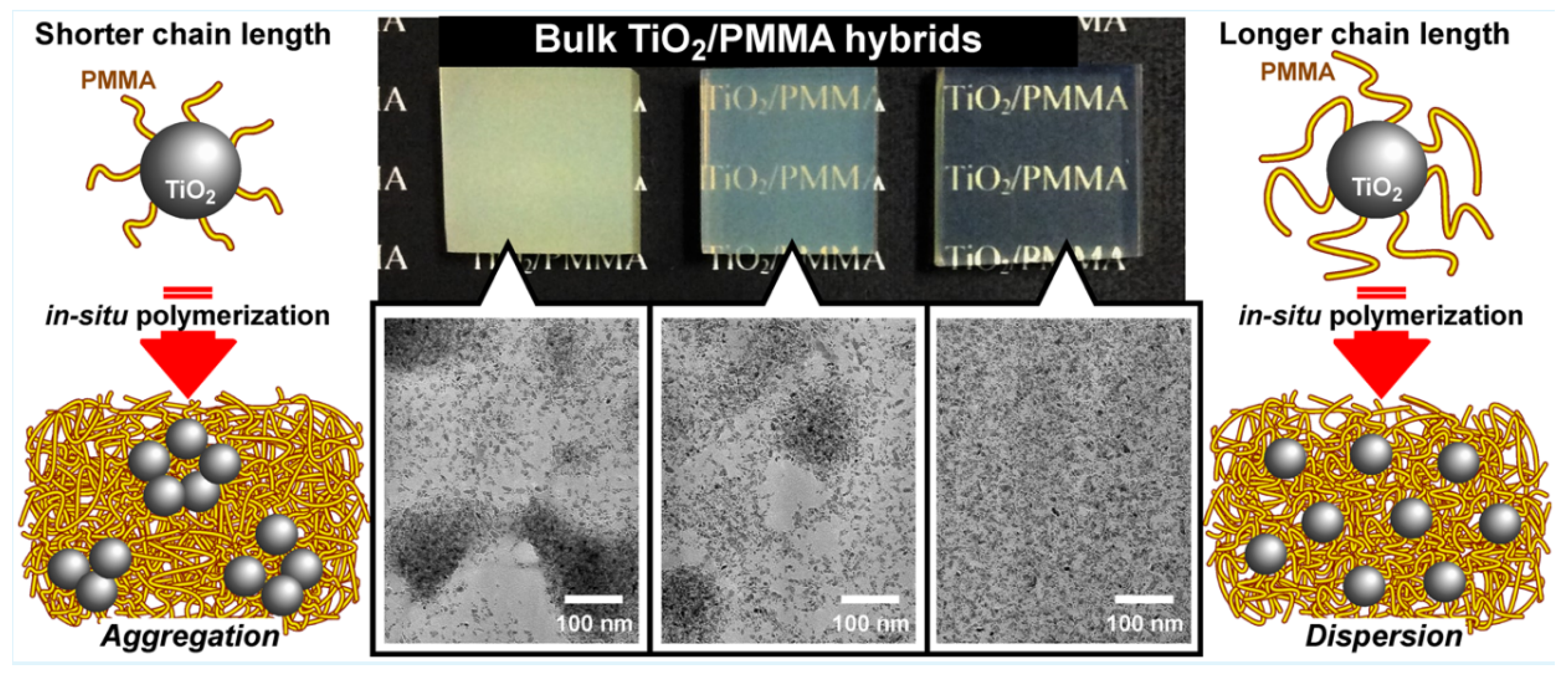
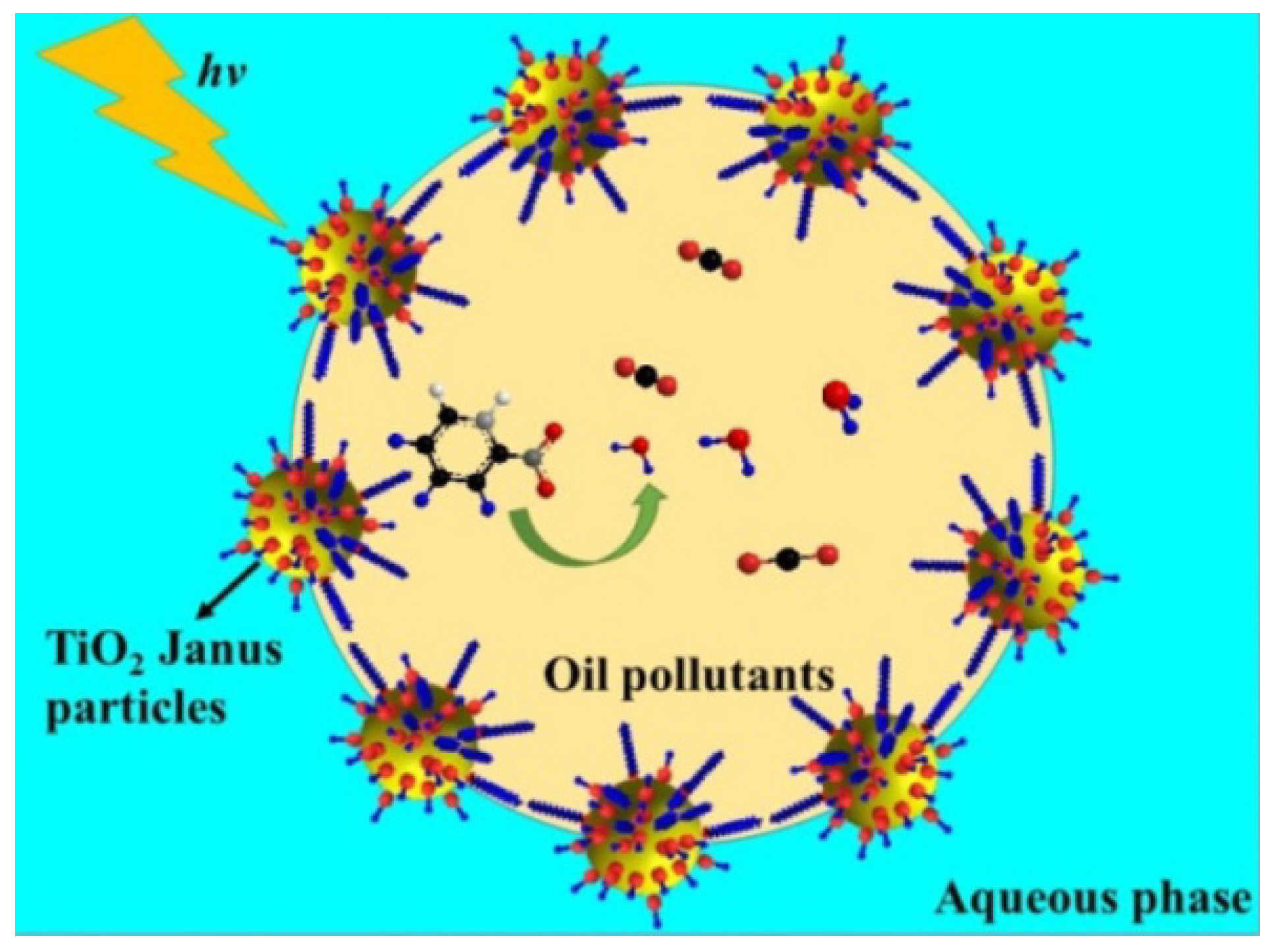
4.5. TiO2 Composite Polymers
5. Summary and Outlook
5.1. Summary of Research Progress
- By doping precious metals, transition metals, rare earth metals, or non-metallic elements, TiO2 can change the band structure, broaden its light absorption range, and improve the separation efficiency of photoelectron-hole pairs, which can significantly improve the photocatalytic activity of TiO2.
- TiO2 was mixed with other semiconductor materials to form a composite photocatalytic material with a heterogeneous structure. This modification method can make use of the synergistic effect between different materials to improve the utilization efficiency of photogenerated electron-hole pairs and enhance photocatalytic performance. For example, the composite of TiO2 with SiO2, ZnO, and other materials can form heterogeneous structures and improve photocatalytic efficiency.
- By introducing functional material polymer on the surface of TiO2, the surface properties of TiO2 are improved, and the photocatalytic performance is improved. Surface modification can increase the active sites on the surface of TiO2 and promote the separation and migration of photogenerated electrons and holes.
5.2. Future Research Direction and Development Trend
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, Y.; Wang, L.; Qiang, X.; Gu, W.; Ma, Z.; Wang, G. An Overview of Biological Mechanisms and Strategies for Treating Wastewater from Printing and Dyeing Processes. J. Water Process Eng. 2023, 55, 104242. [Google Scholar] [CrossRef]
- Ewuzie, U.; Saliu, O.D.; Dulta, K.; Ogunniyi, S.; Bajeh, A.O.; Iwuozor, K.O.; Ighalo, J.O. A Review on Treatment Technologies for Printing and Dyeing Wastewater (PDW). J. Water Process Eng. 2022, 50, 103273. [Google Scholar] [CrossRef]
- Dong, H.; Guo, T.; Zhang, W.; Ying, H.; Wang, P.; Wang, Y.; Chen, Y. Biochemical Characterization of A Novel Azoreductase from Streptomyces sp.: Application in Eco-Friendly Decolorization of Azo Dye Wastewater. Int. J. Biol. Macromol. 2019, 140, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Guan, D.; Sun, Z.; Zhang, Z.; Shan, Y.; Wu, Y.; Gong, C.; Ren, X. Osmotic Cleaning of Typical Inorganic and Organic Foulants on Reverse Osmosis Membrane for Textile Printing and Dyeing Wastewater Treatment. Chemosphere 2023, 336, 139162. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Yuan, J.; Cai, D.; Li, B.; Hu, D.; Li, J. Concentrating And Recycling of High-Concentration Printing and Dyeing Wastewater by A Disc Tube Reverse Osmosis-Fenton Oxidation/Low-Temperature Crystallization Process. Sep. Purif. Technol. 2021, 266, 118583. [Google Scholar] [CrossRef]
- Deng, D.; Lamssali, M.; Aryal, N.; Ofori-Boadu, A.; Jha, M.K.; Samuel, R.E. Textiles Wastewater Treatment Technology: A Review. Water Environ. Res. Res. Publ. Water Environ. Fed. 2020, 92, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of Various Recent Wastewater Dye Removal Methods: A Review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Li, W.; Mu, B.; Yang, Y. Feasibility Of Industrial-Scale Treatment of Dye Wastewater Via Bio-Adsorption Technology. Bioresour. Technol. 2019, 277, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Huang, L.; Zheng, Z.; Wei, J.; Qiu, Z.; Zeng, D. Enhanced Degradation Performance of Fe(75)B(12.5)Si(12.5) Amorphous Alloys On Azo Dye. Environ. Sci. Pollut. Res. Int. 2023, 30, 34428–34439. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, S.; Piña-Mondragón, S.; González-Barceló, Ó. Treatment of The Azo Dye Direct Blue 2 in A Biological Aerated Filter under Anaerobic/Aerobic Conditions. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2010, 61, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.; Damianovic, M.; Foresti, E.; Florencio, L.; Kato, M.T.; Gavazza, S. Biological Treatment of Real Textile Wastewater Containing Sulphate, Salinity, and Surfactant Through An Anaerobic-Aerobic System. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2022, 85, 2882–2898. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Z.; Du, W.; Liu, P.; Zhang, L.; Shi, F. Treatment of Wastewater Containing Methyl Orange Dye By Fluidized Three Dimensional Electrochemical Oxidation Process Integrated with Chemical Oxidation And Adsorption. J. Environ. Manag. 2022, 311, 114775. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, G.; Perumal, N.; Priya, A.K.; Rajendran, S. A Review of Graphene-Based Semiconductors for Photocatalytic Degradation of Pollutants in Wastewater. Chemosphere 2022, 300, 134391. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.E.M.; Moreira, A.J.; Nobrega, E.T.D.; Alencar, R.G.; Rabello, P.T.; Blaskievicz, S.F.; Marques, G.N.; Mascaro, L.H.; Paris, E.C.; Lemos, S.G.; et al. Hierarchical Structure of 3D Zno Experimentally Designed to Achieve High Performance in The Sertraline Photocatalysis in Natural Waters. Chem. Eng. J. 2023, 475, 146235. [Google Scholar] [CrossRef]
- Syahidatul Insyirah Mohd Foad, N.; Abu Bakar, F. Synthesis of TiO2 Photocatalyst with Tunable Optical Properties and Exposed Facet for Textile Wastewater Treatment. Results Opt. 2023, 13, 100545. [Google Scholar]
- Thuan, D.V.; Chu, T.T.H.; Thanh, H.D.T.; Le, M.V.; Ngo, H.L.; Le, C.L.; Thi, H.P. Adsorption and Photodegradation of Micropollutant in Wastewater by Photocatalyst TiO2/Rice Husk Biochar. Environ. Res. 2023, 236, 116789. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, A.M.; Yogendra, K.; Madhusudhana, N.; Mahadevan, K.M.; Veena, S.R. Efficient Photodegradation of Victoria Blue B and Acridine Orange Dyes by Nickel Oxide Nanoparticles. Mater. Today Proc. 2023, 92, 1616–1622. [Google Scholar] [CrossRef]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic Degradation of Organic Pollutants Using TiO2-Based Photocatalysts. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq, A.U.; Akram, N. Photocatalysis: An Effective Tool for Photodegradation of Dyes—A Review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Ul Haq, I.; Ahmad, W.; Ahmad, I.; Yaseen, M. Photocatalytic Oxidative Degradation of Hydrocarbon Pollutants In Refinery Wastewater Using TiO2 As Catalyst. Water Environ. Res. Res. Publ. Water Environ. Fed. 2020, 92, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Armaković, S.J.; Savanović, M.M.; Šiljegović, M.V.; Kisić, M.; Šćepanović, M.; Grujić-Brojčin, M.; Simić, N.; Gavanski, L.; Armaković, S. Self-Cleaning and Charge Transport Properties of Foils Coated with Acrylic Paint Containing TiO2 Nanoparticles. Inorganics 2024, 12, 35. [Google Scholar] [CrossRef]
- Ravelli, D.; Dondi, D.; Fagnoni, M.; Albini, A. Titanium Dioxide Photocatalysis: An Assessment of The Environmental Compatibility for The Case of The Functionalization of Heterocyclics. Appl. Catal. B Environ. 2010, 99, 442–447. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Song, Y.; Li, Z. TiO2 Homojunction with Au Nanoparticles Decorating as An Efficient and Stable Electrocatalyst for Hydrogen Evolution Reaction. Mater. Charact. 2019, 151, 286–291. [Google Scholar] [CrossRef]
- El Mragui, A.; Zegaoui, O.; Daou, I. Synthesis, Characterization and Photocatalytic Properties under Visible Light of Doped and Co-Doped TiO2-Based Nanoparticles. Mater. Today Proc. 2019, 13, 857–865. [Google Scholar] [CrossRef]
- El-Kholy, R.A.; Isawi, H.; Zaghlool, E.; Soliman, E.A.; Khalil, M.M.H.; Said, M.M.; El-Aassar, A.M. Preparation and Characterization of Rare Earth Element Nanoparticles for Enhanced Photocatalytic Degradation. Environ. Sci. Pollut. Res. Int. 2023, 30, 69514–69532. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.G.; Rao, K.S.R.K. Comparison Of Modification Strategies towards Enhanced Charge Carrier Separation and Photocatalytic Degradation Activity of Metal Oxide Semiconductors (TiO2, WO3 And Zno). Appl. Surf. Sci. 2017, 391, 124–148. [Google Scholar] [CrossRef]
- Srinithi, S.; Balakumar, V.; Chen, T.-W.; Chen, S.-M.; Akilarasan, M.; Lou, B.-S.; Yu, J. In-Situ Fabrication of TiO2-MWCNT Composite for An Efficient Electron Transfer Photocatalytic Rhodamine B Dye Degradation under UV–Visible Light. Diam. Relat. Mater. 2023, 138, 110245. [Google Scholar] [CrossRef]
- Makota, O.; Dutková, E.; Briančin, J.; Bednarcik, J.; Lisnichuk, M.; Yevchuk, I.; Melnyk, I. Advanced Photodegradation of Azo Dye Methyl Orange Using H2O2-Activated Fe3O4@SiO2 @ZnO Composite under UV Treatment. Molecules 2024, 29, 1190. [Google Scholar] [CrossRef] [PubMed]
- Rubesh Ashok Kumar, S.; Vasvini Mary, D.; Suganya Josephine, G.A. Design of solar-light-driven agglomerated cluster-like transition/rare-earth metal oxide-supported carbon-based nanomaterial for the degradation of azo dye. Chem. Phys. Impact 2024, 8, 100563. [Google Scholar]
- Kumar, S.R.A.; Mary, D.V.; Josephine, G.A.S.; Sivasamy, A. Hydrothermally synthesized WO3:CeO2 supported gC3N4 nanolayers for rapid photocatalytic degradation of azo dye under natural sunlight. Inorg. Chem. Commun. 2024, 164, 112366. [Google Scholar] [CrossRef]
- Luo, L.; Shen, J.; Jin, B. Construction of Zn0.5Cd0.5S/Bi4O5Br2 Heterojunction for Enhanced Photocatalytic Degradation of Tetracycline Hydrochloride. Inorganics 2024, 12, 127. [Google Scholar] [CrossRef]
- Nawaz, R.; Hanafiah, M.M.; Ali, M.; Anjum, M.; Baki, Z.A.; Mekkey, S.D.; Ullah, S.; Khurshid, S.; Ullah, H.; Arshad, U. Review of the performance and energy requirements of metals modified TiO2 materials based photocatalysis for phenolic compounds degradation: A case of agro-industrial effluent. J. Environ. Chem. Eng. 2024, 12, 112766. [Google Scholar] [CrossRef]
- Zhao, H.; Dong, J.; Xie, Y.; Meng, L.; Shen, S.; Chen, J.G.; Hu, D.; Yang, G. Construction of thin-shell TiO2 vesicles inspired by the shell-deposition of diatoms for chlorophyll-sensitized photocatalyst. Solid State Sci. 2024, 152, 107520. [Google Scholar] [CrossRef]
- Eddy, D.R.; Permana, M.D.; Sakti, L.K.; Sheha, G.A.N.; Solihudin; Hidayat, S.; Takei, T.; Kumada, N.; Rahayu, I. Heterophase Polymorph of TiO2 (Anatase, Rutile, Brookite, TiO2 (B)) for Efficient Photocatalyst: Fabrication and Activity. Nanomaterials 2023, 13, 704. [Google Scholar] [CrossRef] [PubMed]
- Razdan, N.K.; Bhan, A. Catalytic site ensembles: A context to reexamine the Langmuir-Hinshelwood kinetic description. J. Catal. 2021, 404, 726–744. [Google Scholar] [CrossRef]
- Arikal, D.; Kallingal, A. Photocatalytic degradation of azo and anthraquinone dye using TiO2/MgO nanocomposite immobilized chitosan hydrogels. Environ. Technol. 2021, 42, 2278–2291. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, Y.; He, H.; Xu, L.; Li, W.; Zhao, D. Recent advances in the synthesis of hierarchically mesoporous TiO2 materials for energy and environmental applications. Natl. Sci. Rev. 2020, 7, 1702–1725. [Google Scholar] [CrossRef] [PubMed]
- Naas, L.A.; Bouaouina, B.; Bensouici, F.; Mokeddem, K.; Abaidia, S.E. Effect of TiN thin films deposited by oblique angle sputter deposition on sol-gel coated TiO2 layers for photocatalytic applications. Thin Solid Film. 2024, 793, 140275. [Google Scholar] [CrossRef]
- Jamil, A.; Sawaira, T.; Ali, A. Ce-TiO2 nanoparticles with surface-confined Ce3+/Ce4+ redox pairs for rapid sunlight-driven elimination of organic contaminants from water. Environ. Nanotechnol. Monit. Manag. 2024, 21, 100946. [Google Scholar] [CrossRef]
- Huo, J.; Xiao, Y.; Yang, H. Ultrasmall TiO2/C nanoparticles with oxygen vacancy-enriched as an anode material for advanced Li-ion hybrid capacitors. J. Energy Storage 2024, 89, 111586. [Google Scholar] [CrossRef]
- Fermeli, P.N.; Lagopati, N.; Gatou, A.M. Biocompatible PANI-Encapsulated Chemically Modified Nano-TiO2 Particles for Visible-Light Photocatalytic Applications. Nanomaterials 2024, 14, 642. [Google Scholar] [CrossRef] [PubMed]
- Parashar, D.; Achari, G.; Kumar, M. Multi-antibiotics removal under UV-A light using sol-gel prepared TiO2: Central composite design, effect of persulfate addition and degradation pathway study. Chemosphere 2023, 341, 140025. [Google Scholar] [CrossRef] [PubMed]
- Gnanasekaran, L.; Priya, A.K.; Ghfar, A.A.; Sekar, K.; Santhamoorthy, M.; Arthi, M.; Soto-Moscoso, M. The influence of heterostructured TiO2/ZnO nanomaterials for the removal of azo dye pollutant. Chemosphere 2022, 308, 136161. [Google Scholar] [CrossRef] [PubMed]
- Ioannidou, T.; Anagnostopoulou, M.; Vasiliadou, I.A.; Marchal, C.; Alexandridou, E.O.; Keller, V.; Christoforidis, K.C. Mixed phase anatase nanosheets/brookite nanorods TiO2 photocatalysts for enhanced gas phase CO2 photoreduction and H2 production. J. Environ. Chem. Eng. 2024, 12, 111644. [Google Scholar] [CrossRef]
- Rawat, J.; Sharma, H.; Dwivedi, C. Microwave-assisted synthesis of carbon quantum dots and their integration with TiO2 nanotubes for enhanced photocatalytic degradation. Diam. Relat. Mater. 2024, 144, 111050. [Google Scholar] [CrossRef]
- Matakgane, M.; Mokoena, T.; Kroon, R. Robust upconversion luminescence of Ho3+/Yb3+ co-doped TiO2 nanophosphors manifested by crystallinity. J. Mol. Struct. 2024, 1305, 137747. [Google Scholar] [CrossRef]
- Yang, W.; Jia, N.; Xu, W.; Bu, Q. Fabrication of novel 3D urchin-like mixed-phase TiO2 for efficient degradation of trimethoprim. Mater. Lett. 2023, 351, 135001. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, X.; Zhang, L.; Zhu, J.; Li, S.; Hu, P.; Feng, J.; Yan, W. Insight into the effect of surface carboxyl and amino groups on the adsorption of titanium dioxide for acid red G. Front. Chem. Sci. Eng. 2021, 15, 1147–1157. [Google Scholar] [CrossRef]
- Jialin, L.; Zhonghao, W.; Furui, C. Self-assembly, structure and catalytic activity of Ni3 on TiO2: A triple-atom catalyst for hydrogen evolution. Appl. Surf. Sci. 2024, 643, 158719. [Google Scholar]
- Abidi, M.; Assadi, A.; Bouzaza, A. Photocatalytic indoor/outdoor air treatment and bacterial inactivation on CuO/TiO2 prepared by HiPIMS on polyester cloth under low intensity visible light. Appl. Catal. B Environ. 2019, 259, 118074. [Google Scholar] [CrossRef]
- Cao, Y.-Q.; Zi, T.-Q.; Zhao, X.-R.; Liu, C.; Ren, Q.; Fang, J.-B.; Li, W.-M.; Li, A.-D. Enhanced visible light photocatalytic activity of Fe2O3 modified TiO2 prepared by atomic layer deposition. Sci. Rep. 2020, 10, 13437. [Google Scholar] [CrossRef]
- Feng, J.; Xiong, S.; Ren, L.; Wang, Y. Atomic layer deposition of TiO2 on carbon-nanotubes membrane for capacitive deionization removal of chromium from water. Chin. J. Chem. Eng. 2022, 45, 15–21. [Google Scholar] [CrossRef]
- Ostyn, N.R.; Sree, S.P.; Li, J.; Feng, J.Y.; Roeffaers, M.B.; De Feyter, S.; Dendooven, J.; Detavernier, C.; Martens, J.A. Covalent graphite modification by low-temperature photocatalytic oxidation using a titanium dioxide thin film prepared by atomic layer deposition. Catal. Sci. Technol. 2021, 11, 6724–6731. [Google Scholar] [CrossRef]
- Ke, W.; Qin, X.; Vazquez, Y.; Lee, I.; Zaera, F. Direct characterization of interface sites in Au/TiO2 catalysts prepared using atomic layer deposition. Chem Catal. 2024, 4, 100977. [Google Scholar] [CrossRef]
- Lys, A.; Gnilitskyi, I.; Coy, E.; Jancelewicz, M.; Gogotsi, O.; Iatsunskyi, I. Highly regular laser-induced periodic silicon surface modified by MXene and ALD TiO2 for organic pollutants degradation. Appl. Surf. Sci. 2023, 640, 15833. [Google Scholar] [CrossRef]
- Domenico, R.; Francesca, D.A.; Irene, B.; Paola, B.M.; Luca, D.P. Easy way to produce iron-doped titania nanoparticles via the solid-state method and investigation their photocatalytic activity. J. Mater. Res. 2023, 38, 1282–1292. [Google Scholar]
- Sun, L.; Zhou, Q.; Mao, J.; Ouyang, X.; Yuan, Z.; Song, X.; Gong, W.; Mei, S.; Xu, W. Study on Photocatalytic Degradation of Acid Red 73 by Fe3O4@TiO2 Exposed (001) Facets. Appl. Sci. 2022, 12, 3574. [Google Scholar] [CrossRef]
- Yang, J.X.; Chang, W.; Huang, L.; Qin, J.; Li, Y.F. Preparation and properties of titanium dioxide nanofibers by microemulsion electrospinning. Chem. Ind. Eng. 2022, 39, 21–28. [Google Scholar]
- Sun, L.; Wang, G.; Bi, S.; Li, H.; Zhan, H.; Liu, W. Synergistic modulation of oxygen vacancies and heterojunction structure in Pd@CN@TiO2 promotes efficient photocatalytic Suzuki–Miyaura C–C coupling reactions. J. Appl. Organomet. Chem. 2024, 38, e7353. [Google Scholar] [CrossRef]
- Hongying, L.; Bicheng, Z.; Jian, S.; Haiming, G.; Jiaguo, Y.; Liuyang, Z. Photocatalytic hydrogen production from seawater by TiO2/RuO2 hybrid nanofiber with enhanced light absorption. J. Colloid Interface Sci. 2024, 654, 1010–1019. [Google Scholar]
- Andrea, B.S.; Roberto, F.; Armeli, I.M.T.; Javier, L.-T.F.; José, U.F.; Salvatore, S. H2 production through glycerol photoreforming using one-pot prepared TiO2-rGO-Au photocatalysts. Mol. Catal. 2023, 547, 113346. [Google Scholar]
- Djeda, R.; Mailhot, G.; Prevot, V. Porous Layered Double Hydroxide/TiO2 Photocatalysts for the Photocatalytic Degradation of Orange II. ChemEngineering 2020, 4, 39. [Google Scholar] [CrossRef]
- Zhao, Y.; Ge, H.; Kondo, Y.; Kuwahara, Y.; Mori, K.; Yamashita, H. Photosynthesis of hydrogen peroxide in a two-phase system by hydrophobic Au nanoparticle-deposited plasmonic TiO2 catalysts. Catal. Today 2024, 431, 114558. [Google Scholar] [CrossRef]
- Li, S.; Hu, M.; Chen, X.; Sui, S.; Jin, L.; Geng, Y.; Jiang, J.; Liu, A. The Performance And Functionalization of Modified Cementitious Materials Via Nano Titanium-Dioxide: A Review. Case Stud. Constr. Mater. 2023, 19, E02414. [Google Scholar] [CrossRef]
- Khitous, A.; Noel, L.; Molinaro, C.; Vidal, L.; Grée, S.; Soppera, O. Sol-Gel TiO2 Thin Film on Au Nanoparticles for Heterogeneous Plasmonic Photocatalysis. ACS Appl. Mater. Interfaces 2024, 16, 10856–10866. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qiu, F.; Xu, W.; Cao, S.; Zhu, H. Recent Progress In Enhancing Photocatalytic Efficiency of TiO2-Based Materials. Appl. Catal. A-Gen. 2015, 495, 131–140. [Google Scholar] [CrossRef]
- Hou, W.; Hung, W.H.; Pavaskar, P.; Goeppert, A.; Aykol, M.; Cronin, S.B.J.A.C. Photocatalytic Conversion of CO2 to Hydrocarbon Fuels Via Plasmon-Enhanced Absorption and Metallic Interband Transitions. ACS Catal. 2011, 1, 929–936. [Google Scholar] [CrossRef]
- Bhatti, M.A.; Shah, A.A.; Almaani, K.F.; Tahira, A.; Chandio, A.D.; Willander, M.; Nur, O.; Mugheri, A.Q.; Bhatti, A.L.; Waryani, B.; et al. TiO2/ZnO Nanocomposite Material For Efficient Degradation Of Methylene Blue. J. Nanosci. Nanotechnol. 2021, 21, 2511–2519. [Google Scholar] [CrossRef] [PubMed]
- Chacon-Argaez, U.; Cedeño-Caero, L.; Cadena-Nava, R.D.; Ramirez-Acosta, K.; Moyado, S.F.; Sánchez-López, P.; Alonso Núñez, G. Photocatalytic Activity and Biocide Properties of Ag-TiO2 Composites on Cotton Fabrics. Materials 2023, 16, 4513. [Google Scholar] [CrossRef] [PubMed]
- Borrego PÉRez, J.A.; Morales, E.R.; Paraguay Delgado, F.; Meza Avendaño, C.A.; Alonso Guzman, E.M.; Mathews, N.R. Ag Nanoparticle Dispersed TiO2 Thin Films by Single Step Sol-Gel Process: Evaluation of The Physical Properties and Photocatalytic Degradation. Vacuum 2023, 215, 112276. [Google Scholar] [CrossRef]
- Majeed, A.; Ibrahim, A.H.; Al-Rawi, S.S.; Iqbal, M.A.; Kashif, M.; Yousif, M.; Abidin, Z.U.; Ali, S.; Arbaz, M.; Hussain, S.A. Green Organo-Photooxidative Method for the Degradation of Methylene Blue Dye. ACS Omega 2024, 9, 12069–12083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jin, X.; Chen, F.; Kuang, X.; Min, J.; Duan, H.; Li, J.; Chen, J. Oxygen Vacancy Induced Interaction between Pt and TiO2 to Improve The Oxygen Reduction Performance. J. Colloid Interface Sci. 2023, 650, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, A. Optical And Electrochemical Study On The Performance Of Au@ TiO2 Core-Shell Heterostructured Nanoparticles as Photocatalyst for Photodegradation of Methylene Blue under Solar-Light Irradiation. Int. J. Electrochem. Sci. 2022, 17, 220636. [Google Scholar] [CrossRef]
- Zuo, J.; Ma, X.; Tan, C.; Xia, Z.; Zhang, Y.; Yu, S.; Li, Y.; Li, Y.; Li, J. Preparation of Au-RGO/TiO2 Nanotubes and Study on The Photocatalytic Degradation of Ciprofloxacin. Anal. Methods Adv. Methods Appl. 2023, 15, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Martins, P.M.; Queirós, J.M.; Tavares, C.J.; Vilas-Vilela, J.L.; Lanceros-Méndez, S.; Reguera, J. Size Effect in Hybrid TiO2:Au Nanostars for Photocatalytic Water Remediation Applications. Int. J. Mol. Sci. 2022, 23, 13741. [Google Scholar] [CrossRef] [PubMed]
- Bhuskute, B.D.; Ali-Löytty, H.; Honkanen, M.; Salminen, T.; Valden, M. Influence of The Photodeposition Sequence on The Photocatalytic Activity of Plasmonic Ag-Au/TiO2 Nanocomposites. Nanoscale Adv. 2022, 4, 4335–4343. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Aguilar, C.A.; Palos-Barba, V.; Thangarasu, P.; Koodali, R.T. Visible Light Driven Photo-Degradation of Congo Red by TiO2-ZnO/Ag: DFT Approach on Synergetic Effect on Band Gap Energy. Chemosphere 2018, 213, 481–497. [Google Scholar] [CrossRef] [PubMed]
- Stojadinović, S.; Radić, N.; Vasilić, R.; Tadić, N.; Tsanev, A. Photocatalytic Degradation of Methyl Orange in The Presence of Transition Metals (Mn, Ni, Co) Modified TiO2 Coatings Formed by Plasma Electrolytic Oxidation. Solid State Sci. 2022, 129, 106896. [Google Scholar] [CrossRef]
- Belošević-Čavor, J.; Koteski, V.; Umićević, A.; Ivanovski, V. Effect of 5d Transition Metals Doping on The Photocatalytic Properties of Rutile TiO2. Comput. Mater. Sci. 2018, 151, 328–337. [Google Scholar] [CrossRef]
- Meshram, A.A.; Sontakke, S.M. Rapid Degradation of Metamitron And Highly Complex Mixture of Pollutants Using MIL-53(Al) Integrated Combustion Synthesized TiO2. Adv. Powder Technol. 2021, 32, 3125–3135. [Google Scholar] [CrossRef]
- Zhu, Y. Enhanced Photoelectrochemical Properties of Fe-TiO2 Nanotube Films: A Combined Experimental and Theoretical Study. Int. J. Electrochem. Sci. 2021, 16, 210662. [Google Scholar] [CrossRef]
- Mingmongkol, Y.; Trinh, D.T.T.; Phuinthiang, P.; Channei, D.; Ratananikom, K.; Nakaruk, A.; Khanitchaidecha, W. Enhanced Photocatalytic and Photokilling Activities of Cu-Doped TiO2 Nanoparticles. Nanomater 2022, 12, 1198. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.N.; Babji, P.; Parvatamma, B.; Naidu, T.M. Decontamination of Pesticide Residues in Water Samples Using Copper and Zinc Co-Doped Titania Nanocatalyst. Environ. Eng. Manag. J. 2020, 19, 721–731. [Google Scholar] [CrossRef]
- Sukhadeve, G.K.; Gedam, R.S. Visible Light Assisted Photocatalytic Degradation of Mixture of Reactive Ternary Dye Solution by Zn–Fe Co-Doped TiO2 Nanoparticles. Chemosphere 2023, 341, 139990. [Google Scholar] [CrossRef]
- Liu, X. Preparation of Rare Earth Doped Modified TiO2 Nanocrystalline Materials and Their Photocatalytic Properties. Master’s Thesis, Changji College, Xinjiang, China, 2022. [Google Scholar]
- Shi, Z.M.; Jin, L.N. Influence of La3+/Ce3+-Doping on Phase Transformation and Crystal Growth in TiO2-15wt% Zno Gels. J. Non-Cryst. Solids 2009, 355, 213–220. [Google Scholar] [CrossRef]
- Ricci, P.C.; Carbonaro, C.M.; Geddo Lehmann, A.; Congiu, F.; Puxeddu, B.; Cappelletti, G.; Spadavecchia, F. Structure and Photoluminescence of TiO2 Nanocrystals Doped and Co-Doped with N and Rare Earths (Y3+, Pr3+). J. Alloys Compd. 2013, 561, 109–113. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Yang, O.B.; Kim, H.-C.; Khil, M.-S. TiO2 Nanofibers Doped with Rare Earth Elements and Their Photocatalytic Activity. Ceram. Int. 2012, 38, 5925–5930. [Google Scholar] [CrossRef]
- Wen, M.; Yang, H.; Tong, L.; Yuan, L. Study on Degradation of Gold-Cyanide Wastewater by Rare-Earth La Doped TiO2 -MnO2 Composite Photocatalyst. Mol. Catal. 2023, 550, 113506. [Google Scholar] [CrossRef]
- ReszczyŃska, J.; Grzyb, T.; Sobczak, J.W.; Lisowski, W.; Gazda, M.; Ohtani, B.; Zaleska, A. Visible Light Activity of Rare Earth Metal Doped (Er3+, Yb3+ Or Er3+/Yb3+) Titania Photocatalysts. Appl. Catal. B Environ. 2015, 163, 40–49. [Google Scholar] [CrossRef]
- Tang, X.; Xue, Q.; Qi, X.; Cheng, C.; Yang, M.; Yang, T.; Chen, F.; Qiu, F.; Quan, X. DFT and Experimental Study on Visible-Light Driven Photocatalysis of Rare-Earth-Doped TiO2. Vacuum 2022, 200, 110972. [Google Scholar] [CrossRef]
- Zhou, F.; Yan, C.; Sun, Q.; Komarneni, S. TiO2/Sepiolite Nanocomposites Doped with Rare Earth Ions: Preparation, Characterization and Visible Light Photocatalytic Activity. Microporous Mesoporous Mater. 2019, 274, 25–32. [Google Scholar] [CrossRef]
- Kobwittaya, K.; Oishi, Y.; Torikai, T.; Yada, M.; Watari, T.; Luitel, H.N. Bright Red Upconversion Luminescence from Er3+ and Yb3+ Co-Doped Zno-TiO2 Composite Phosphor Powder. Ceram. Int. 2017, 43, 13505–13515. [Google Scholar] [CrossRef]
- Ćurković, L.; Briševac, D.; Ljubas, D.; Mandić, V.; Gabelica, I. Synthesis, Characterization, and Photocatalytic Properties of Sol-Gel Ce-TiO2 Films. Processes 2024, 12, 1144. [Google Scholar] [CrossRef]
- Benammar, I.; Salhi, R.; Deschanvres, J.-L.; Maalej, R. The Effect Of Rare Earth (Er, Yb) Element Doping On The Crystallization Of Y2Ti2O7 Pyrochlore Nanoparticles Developed by Hydrothermal-Assisted-Sol-Gel Method. J. Solid State Chem. 2023, 320, 123856. [Google Scholar] [CrossRef]
- Arévalo-Pérez, J.C.; Cruz-Romero, D.D.L.; Cordero-García, A.; Lobato-García, C.E.; Aguilar-Elguezabal, A.; Torres-Torres, J.G. Photodegradation of 17 A-Methyltestosterone Using TiO2-Gd3+ and TiO2-Sm3+ Photocatalysts and Simulated Solar Radiation As An Activation Source. Chemosphere 2020, 249, 126497. [Google Scholar] [CrossRef] [PubMed]
- Kuwik, M.; Kowalska, K.; Pisarska, J.; Kochanowicz, M.; Żmojda, J.; Dorosz, J.; Dorosz, D.; Pisarski, W.A. Influence of TiO2 Concentration on Near-Infrared Emission of Germanate Glasses Doped with Tm3+ and Tm3+/Ho3+ Ions. Ceram. Int. 2023, 49, 41090–41097. [Google Scholar] [CrossRef]
- Alzahrani, J.S.; Alrowaili, Z.A.; Eke, C.; Olarinoye, I.O.; Al-Buriahi, M.S. Characterization and Applications of Highly Optical Transparency Tellurite Glasses Doped with Er3+, Tm3+, And Nd3+. Optik 2023, 281, 170825. [Google Scholar] [CrossRef]
- Pascariu, P.; Cojocaru, C.; Homocianu, M.; Samoila, P. Tuning of Sm3+ and Er3+-doped TiO2 nanofibers for enhancement of the photocatalytic performance: Optimization of the photodegradation conditions. J. Environ. Manag. 2022, 316, 115317. [Google Scholar] [CrossRef] [PubMed]
- Guetni, I.; Belaiche, M.; Ferdi, C.A.; Oulhakem, O.; Alaoui, K.B.; Zaoui, F.; Bahije, L. Novel modified nanophotocatalysts of TiO2 nanoparticles and TiO2/Alginate beads with lanthanides [La, Sm, Y] to degrade the Azo dye Orange G under UV-VIS radiation. Mater. Sci. Semicond. Process. 2024, 174, 108193. [Google Scholar] [CrossRef]
- Ren, Y.; Han, Y.; Li, Z.; Liu, X.; Zhu, S.; Liang, Y.; Yeung, K.W.K.; Wu, S. Ce and Er Co-doped TiO2 for rapid bacteria- killing using visible light. Bioact. Mater. 2020, 5, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Seshaiah, K.V.; Dileep, R.K.; Easwaramoorthi, R.; Ganapathy, V.; Kumar, R.S.S. Deciphering the role of (Er3+/Nd3+) co-doping effect on TiO2 as an improved electron transport layer in perovskite solar cells. Sol. Energy 2023, 262, 111801. [Google Scholar]
- Guo, Y.T.; Wang, H.Q. Research on Rare Earth Lanthanum-Doped Nanotitanium Dioxide Composite Fresh Packaging Films. Mater. Guide 2018, 32, 4357–4362. [Google Scholar]
- Li, J.; Zhang, X.; Tan, Z.; Wei, X.; Zhou, X. Effect of Lanthanum and Graphene Oxide Doping on The Photocatalytic Performance of TiO2. Chem. New Mater. 2023, 51, 159–163+168. [Google Scholar]
- Alanazi, H.E.; Emran, K.M. Nd-Gd–Platinum Doped TiO2 Nanotube Arrays Catalyst for Water Splitting in Alkaline Medium. Int. J. Electrochem. Sci. 2023, 18, 100112. [Google Scholar] [CrossRef]
- Mei, Q.F.; Zhang, F.; Wang, N.; Lu, W.; Su, X.; Wang, W.; Wu, R. Titanium Dioxide-Based Z-Type Heterojunction Photocatalysts. J. Inorg. Chem. 2019, 35, 1321–1339. [Google Scholar]
- Qi, K.; Liu, N.; Cui, N.; Song, J.; Ma, Y.; Chen, Q. Design of Modified Titanium Dioxide Photocatalyst. J. Shenyang Norm. Univ. (Nat. Sci. Ed.) 2021, 39, 103–108. [Google Scholar]
- Hou, J.; Wang, Y.; Zhou, J.; Lu, Y.; Liu, Y.; Lv, X. Photocatalytic Degradation Of Methylene Blue Using A ZnO/TiO2 Heterojunction Nanomesh Electrode. Surf. Interfaces 2021, 22, 100889. [Google Scholar] [CrossRef]
- Wang, D. Preparation and Photocatalytic Properties of TiO2/ZnO Micro- and Nanomaterials and Core-Shell Structures. Ph.D. Thesis, University of Chinese Academy of Sciences (Changchun Institute of Optical Precision Machinery and Physics, Chinese Academy of Sciences), Beijing, China, 2018. [Google Scholar]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced Photocatalytic Performance of Direct Z-Scheme G-C3N4-TiO2 Photocatalysts for The Decomposition of Formaldehyde in Air. Phys. Chem. Chem. Phys. PCCP 2013, 15, 16883–16890. [Google Scholar] [CrossRef] [PubMed]
- Wongburapachart, C.; Pornaroontham, P.; Kim, K.; Rangsunvigit, P. Photocatalytic Degradation of Acid Orange 7 by NiO-TiO2/TiO2 Bilayer Film Photo-Chargeable Catalysts. Coatings 2023, 13, 141. [Google Scholar] [CrossRef]
- Bai, N.; Liu, X.; Li, Z.; Ke, X.; Zhang, K.; Wu, Q. High-efficiency TiO2/ZnO nanocomposites photocatalysts by sol–gel and hydrothermal methods. J. Sol-Gel Sci. Technol. 2021, 99, 92–100. [Google Scholar] [CrossRef]
- Wittawat, R.; Rittipun, R.; Jarasfah, M.; Nattaporn, B. Synthesis of ZnO/TiO2 Spherical Particles for Blue Light Screening by Ultrasonic Spray Pyrolysis. Mater. Today Commun. 2020, 24, 101126. [Google Scholar]
- Wang, S.; Li, X.; Li, D. Microwave Assisted Synthesis Of ZnO-TiO2 and Its Visible Light Catalytic Denitrification Activity. J. Fuel Chem. Technol. 2023, 51, 589–598. [Google Scholar] [CrossRef]
- Ali, M.M.; Haque, M.J.; Kabir, M.H.; Kaiyum, M.A.; Rahman, M.S. Nano Synthesis Of ZnO–TiO2 Composites by Sol-Gel Method and Evaluation of Their Antibacterial, Optical and Photocatalytic Activities. Results Mater. 2021, 11, 100199. [Google Scholar] [CrossRef]
- Abumousa, R.A.; Bououdina, M.; Ben Aissa, M.A.; Khezami, L.; Modwi, A. Efficient photocatalytic degradation of Congo red and other dyes by ternary TiO2/Y2O3@g-C3N4 nanohybrid. J. Mater. Sci. Mater. Electron. 2024, 35, 486. [Google Scholar] [CrossRef]
- Heltina, D.; Imamatul Mastura, D.; Amri, A.; Peratenta Sembiring, M. Komalasari, Comparison of Synthesis Methods on TiO2-Graphene Composites for Photodegradation of Compound Waste. Mater. Today Proc. 2023, 87, 293–298. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wu, J. Preparation of TiO2/Graphene Oxide and Their Photocatalytic Properties at Room Temperature. J. Fuel Chem. Technol. 2022, 50, 1307–1316. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Kowalczyk, Ł.; Zgrzebnicki, M.; Tryba, B.; Przepiórski, J.; Morawski, A.W. Methylene Blue Decomposition on TiO2/Reduced Graphene Oxide Hybrid Photocatalysts Obtained by A Two-Step Hydrothermal and Calcination Synthesis. Catal. Today 2020, 357, 630–637. [Google Scholar] [CrossRef]
- Zhou, M.; Jiang, Y.; Xie, Y.; Xie, D.L.; Mei, Y. Research progress on the preparation and modification of titanium dioxide nanoparticles and their application in polymer matrix composites. J. Compos. Mater. 2022, 39, 2089–2105. [Google Scholar]
- Maeda, S.; Fujita, M.; Idota, N.; Matsukawa, K.; Sugahara, Y. Preparation of Transparent Bulk TiO2/Pmma Hybrids with Improved Refractive Indices Via An In Situ Polymerization Process Using TiO2 Nanoparticles Bearing Pmma Chains Grown by Surface-Initiated Atom Transfer Radical Polymerization. ACS Appl. Mater. Interfaces 2016, 8, 34762–34769. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Q.; Liu, Y. Preparation of amphiphilic TiO2 Janus particles with highly enhanced photocatalytic activity. Chin. J. Catal. 2019, 40, 786–794. [Google Scholar] [CrossRef]
- Nair, V.R.; Shetty Kodialbail, V. Floating bed reactor for visible light induced photocatalytic degradation of Acid Yellow 17 using polyaniline-TiO2 nanocomposites immobilized on polystyrene cubes. Environ. Sci. Pollut. Res. 2020, 27, 14441–14453. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.; Chen, X.; Tsai, W. Poly(Methyl Methacrylate)/Titanium Dioxide (Pmma/TiO2) Nanocomposite with Shark-Skin Structure for Preventing Biofilm Formation. Mater. Lett. 2021, 285, 129098. [Google Scholar] [CrossRef]
- Neves, J.C.; Mohallem, N.D.; Viana, M. Polydimethylsiloxanes-Modified TiO2 Coatings: The Role of Structural, Morphological and Optical Characteristics in A Self-Cleaning Surface. Ceram. Int. 2020, 46 Pt B, 11606–11616. [Google Scholar] [CrossRef]
- Abdul, M.; Shahid, R.M.; Ghulam, M.M.; Tehreem, A.; Imran, S.; Maryum, R.; Aqsa, N. Fabrication of Ag–TiO2 nanocomposite employing dielectric barrier discharge plasma for photodegradation of methylene blue. Phys. B Condens. Matter 2023, 665, 414995. [Google Scholar]
- Dong, S.; Tebbutt, G.T.; Millar, R.; Grobert, N.; Maciejewska, B.M. Hierarchical porosity design enables highly recyclable and efficient Au/TiO2 composite fibers for photodegradation of organic pollutants. Mater. Des. 2023, 234, 112318. [Google Scholar] [CrossRef]
- Reguero-Márquez, G.A.; Lunagómez-Rocha, M.A.; Cervantes-Uribe, A.; Del Angel, G.; Rangel, I.; Torres-Torres, J.G.; González, F.; Godavarthi, S.; Arevalo-Perez, J.C.; de Los Monteros, A.E.; et al. Photodegradation of 2,4-D (dichlorophenoxyacetic acid) with Rh/TiO2; comparative study with other noble metals (Ru, Pt, and Au). RSC Adv. 2022, 12, 25711–25721. [Google Scholar] [CrossRef] [PubMed]
- Solmaz, F.; Aziz, H.; Rafael, L. Preparation of TiO2/Fe-MOF n–n heterojunction photocatalysts for visible-light degradation of tetracycline hydrochloride. Chemosphere 2023, 336, 139101. [Google Scholar]
- Muhammad, I.; Rab, N.; Akbar, K.J.; Habib, U.; Tahir, H.; Stanislaw, L.; Saifur, R.; Jerzy, J.; Alsaiar, M.A.; Asif, K.M.K.; et al. Synthesis and Characterization of Manganese-Modified Black TiO2 Nanoparticles and Their Performance Evaluation for the Photodegradation of Phenolic Compounds from Wastewater. Materials 2021, 14, 7422. [Google Scholar] [CrossRef]
- Eleni, E.; Zoi, C.; Athanasios, T.; Kyriakos, B.; Pavlina, T.; Daniel, S.; Anastasia, K.; Lambropoulou, D.A. Photocatalytic degradation of a mixture of eight antibiotics using Cu-modified TiO2 photocatalysts: Kinetics, mineralization, antimicrobial activity elimination and disinfection. J. Environ. Chem. Eng. 2021, 9, 105295. [Google Scholar]
- Cho, H.; Joo, H.; Kim, H.; Kim, J.-E.; Kang, K.-S.; Yoon, J. Improved photoelectrochemical properties of TiO2 nanotubes doped with Er and effects on hydrogen production from water splitting. Chemosphere 2021, 267, 129289. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, Y.N.; Magdalane, C.M.; Hepsibha, S.; Ramalingam, G.; Kumar, B.A.; Kumar, L.B.; Sangaraju, S. Europium decorated hierarchical TiO2 heterojunction nanostructure with enhanced UV light photocatalytic activity for degradation of toxic industrial effluent. Inorg. Chem. Commun. 2023, 157, 111339. [Google Scholar] [CrossRef]
- Hu, L.; Xing, M.; He, X.; Yang, K.; Zhu, J.; Wang, J.; He, J.; Shi, J. Photocatalytic degradation of tetracycline hydrochloride by ZnO/TiO2 composite photocatalyst. J. Mater. Sci. Mater. Electron. 2023, 34, 2273. [Google Scholar] [CrossRef]
- Huang, F.; Hao, H.; Sheng, W.; Lang, X. Dye-TiO2/SiO2 assembly photocatalysis for blue light-initiated selective aerobic oxidation of organic sulfides. Chem. Eng. J. 2021, 423, 129419. [Google Scholar] [CrossRef]
- Yang, J.; Huang, Q.; Sun, Y.; An, G.; Li, X.; Mao, J.; Wei, C.; Yang, B.; Li, D.; Tao, T. Photocatalytic oxidation of formaldehyde under visible light using BiVO4-TiO2 synthesized via ultrasonic blending. Environ. Sci. Pollut. Res. Int. 2024, 31, 30085–30098. [Google Scholar] [CrossRef] [PubMed]
- Paula, L.F.; Hofer, M.; Lacerda, V.P.B. Unraveling the photocatalytic properties of TiO2/WO3 mixed oxides. Photochem. Photobiol. Sci. 2019, 18, 2469–2483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, C.; Shi, X.; Feng, Q.; Shen, T.; Wang, S. Modulation of the Structure of the Conjugated Polymer TMP and the Effect of Its Structure on the Catalytic Performance of TMP–TiO2 under Visible Light: Catalyst Preparation, Performance and Mechanism. Materials 2023, 16, 1563. [Google Scholar] [CrossRef] [PubMed]
- Abu-Melha, S. Distinguishable photocatalytic activity of nano polyaniline with quantum dots metal oxide as photocatalysts for photodegradation of Dianix blue dye and different industrial pollutants. Polyhedron 2024, 252, 116781. [Google Scholar] [CrossRef]
- Meng, A.; Cheng, B.; Tan, H.; Fan, J.; Su, C.; Yu, J. TiO2/polydopamine S-scheme heterojunction photocatalyst with enhanced CO2-reduction selectivity. Appl. Catal. B Environ. 2021, 289, 120039. [Google Scholar] [CrossRef]



| Preparation Methods | Reaction Principle | Advantages | Disadvantages |
|---|---|---|---|
| sol-gel method | Inorganic salts and alcohol salts are hydrolyzed in distilled water, polymerized into a gel after hydrolysis, dried in a vacuum, and cured by high-temperature calcination. | Good dispersion; easy to control the reaction; simple process; low cost and cost-effective. | Long preparation time; many operating steps. |
| hydrothermal synthesis | In a closed system, using water as a solvent, the mixture reacts under certain temperature conditions. | Mild reaction conditions; high purity, good dispersion, crystalline form, controllable shape; environmentally friendly. | High equipment requirements; technically difficult and costly. |
| atomic layer deposition | A chemical vapor phase thin film deposition technique in which a substance is deposited on the surface of a substrate layer by layer in a single-atom-film format. | High accuracy; high atom utilization. | Expensive equipment; cumbersome process; difficult to promote industrialization. |
| microemulsion method | Mutually incompatible liquids form microreactors in the presence of surfactants for the preparation of nanomaterials. | Good dispersion of prepared samples; mild conditions; improved precursor reaction rate. | Poor purity; precursors may not be soluble; complex preparation. |
| Modification Methods of TiO2 | Doping Agents | The Surface Area (Before~After/m2 g−1) | Bandgap Energy (Before~After/eV) | Catalytic Effect | Reference |
|---|---|---|---|---|---|
| Precious Metal-Doped TiO2 | Ag | —— | 3.15~2.31 | The degradation rate of methylene blue under visible light was 93%. | [126] |
| Au | 17.8~28.7 | 3.15~2.9 | Methyl orange is completely dissolved within 90 min. | [127] | |
| Pt | 42~68 | 3.24~2.92 | The degradation rate of Dichlo-Rophenoxyacid (2,4-D) was 99%. | [128] | |
| Transition Metal-Doped TiO2 | Fe | —— | 3.22~3.20 | The removal rate of pollutants reached 97% within 240 min. | [129] |
| Mn | 50~93.35 | 3.20~2.21 | The degradation rate of pollutants increased from 48.17% to 60.12%. | [130] | |
| Cu | 43~46 | 3.08~2.78 | The reduction rate of organic carbon within 6 h is 75%. | [131] | |
| Rare Earth Metal-Doped TiO2 | La | —— | 3.16~3.12 | The degradation rate of p-azo dye orange-yellow G was 96.49% in 105 min under UV-VIS spectral radiation. | [100] |
| Er | —— | 3.15~2.69 | The degradation rate of methylene blue was 80% under visible light. | [132] | |
| Eu | —— | 3.43~3.40 | The degradation rate of Congo red reached 97%. | [133] | |
| TiO2 Composites with Common Semiconductors | ZnO | 50.05~107.98 | 3.26~2.76 | Under sunlight irradiation, when pH is 5.8, the degradation efficiency of the dye is the highest, which is 92%. | [134] |
| SiO2 | 217~256 | 3.22~3.22 | At 300 W Xenon lamp irradiation for 60 min, the degradation efficiency of TC is 96%. | [135] | |
| BiVO4 | 60.6~95.3 | 3.2~3.03 | The degradation rate of formaldehyde reached 97.1%. | [136] | |
| WO3 | 95~117 | 3.0~2.6 | Under no light conditions, the degradation rate of pollutants reached 22%. | [137] | |
| TiO2 composite polymers | Triformyl chlorine-melamine polymer (TMP) | 13~17 | 3.78~2.82 | It can degrade 96.1% RhB. | [138] |
| Polyaniline titanium Dioxide quantum Dots (PAN-TiQD) | —— | 2.95~2.82 | The degradation rate of Dianix blue dye reached 91%. | [139] | |
| Polydopamine (PDA) | —— | 3.22~3.15 | The photocatalytic CO2 reduction yield of CH4 by the composite was up to 1.50 μmol/g·h, which was 5 times that of pure TiO2. | [140] |
| Modification Methods | Advantages | Disadvantages |
|---|---|---|
| TiO2-doped noble metals | High specific surface area; good surface activity; good stability; characteristics of multiphase catalysis. | Inefficient use of visible light; Expensive precious metals. |
| TiO2-doped transition metal | The presence of polyvalent transition metals promotes chemical reactions, modulates the electronic structure of TiO2 to improve its photocatalytic properties, and extends the light absorption range. | Prone to focusing; not environmentally friendly. |
| TiO2-doped rare earth metals | Good stability; high catalytic activity; expanding the range of TiO2 light absorption and promoting photocatalytic reactions. | Complicated operating procedures; not easy to recycle. |
| TiO2 compound semiconductors | Formation of heterojunctions to expand the light absorption range of the material and improve photocatalytic efficiency. Reduction in electron-hole complex reaction. | The complexity of design and preparation. |
| TiO2 composite polymers | More environmentally friendly; Mechanical properties will be improved; Can be repeated many times. | The dispersion is not good; May degrade the polymer matrix. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, T.; Zha, J.; Hu, Y.; Chen, Q.; Zhang, J.; Luo, X. Research Progress of TiO2 Modification and Photodegradation of Organic Pollutants. Inorganics 2024, 12, 178. https://doi.org/10.3390/inorganics12070178
Mao T, Zha J, Hu Y, Chen Q, Zhang J, Luo X. Research Progress of TiO2 Modification and Photodegradation of Organic Pollutants. Inorganics. 2024; 12(7):178. https://doi.org/10.3390/inorganics12070178
Chicago/Turabian StyleMao, Tan, Junyan Zha, Ying Hu, Qian Chen, Jiaming Zhang, and Xueke Luo. 2024. "Research Progress of TiO2 Modification and Photodegradation of Organic Pollutants" Inorganics 12, no. 7: 178. https://doi.org/10.3390/inorganics12070178
APA StyleMao, T., Zha, J., Hu, Y., Chen, Q., Zhang, J., & Luo, X. (2024). Research Progress of TiO2 Modification and Photodegradation of Organic Pollutants. Inorganics, 12(7), 178. https://doi.org/10.3390/inorganics12070178








