Synthesis of Tris(trifluoromethyl)nickelates(II)—Coping with “The C2F5 Problem”
Abstract
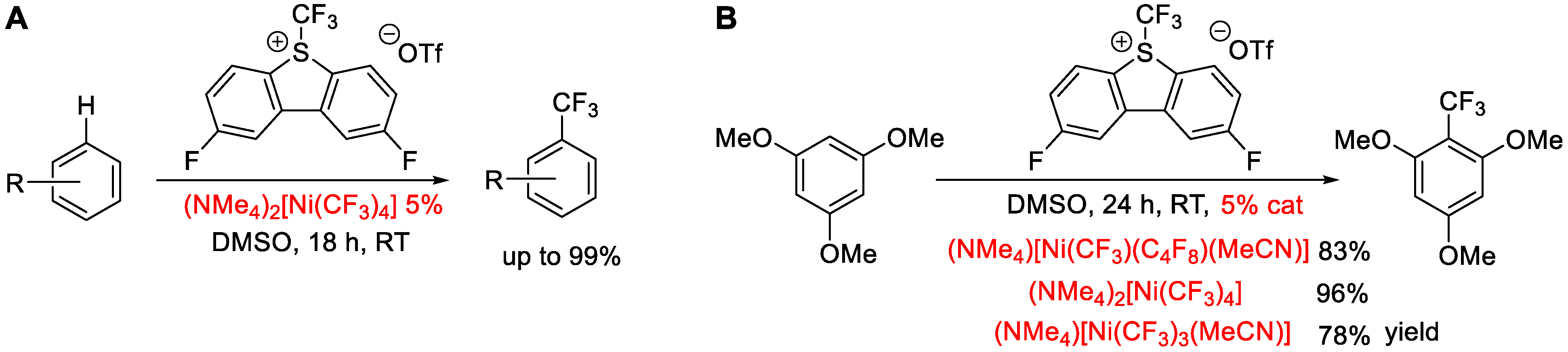
:1. Introduction
2. Results and Discussion
2.1. Synthesis
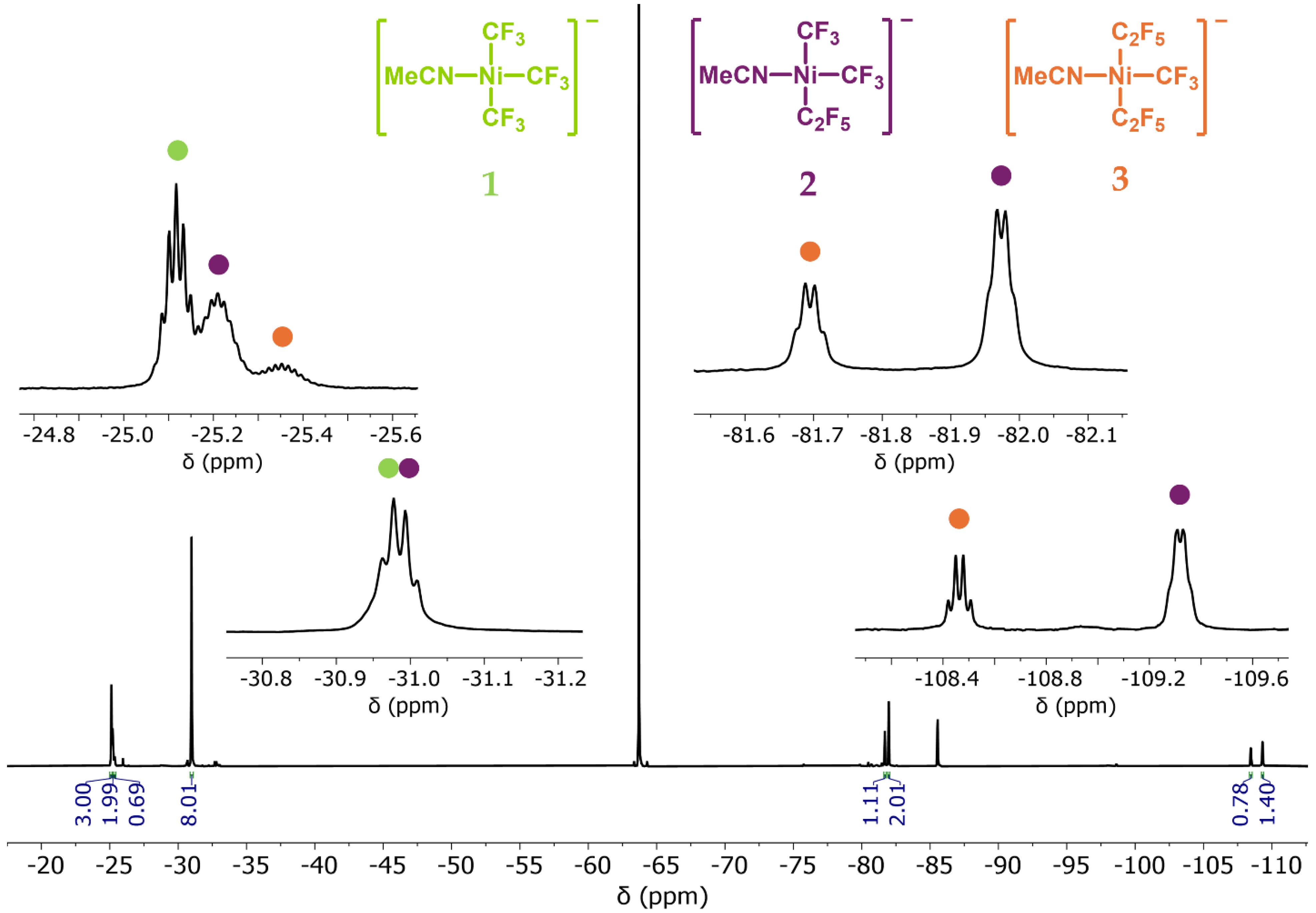
2.2. NMR Spectroscopy and Stereoselectivity
2.3. Single-Crystal X-ray Diffraction (sc-XRD)
2.4. Reaction with N-Heterocyclic Carbene (NHC) Ligands
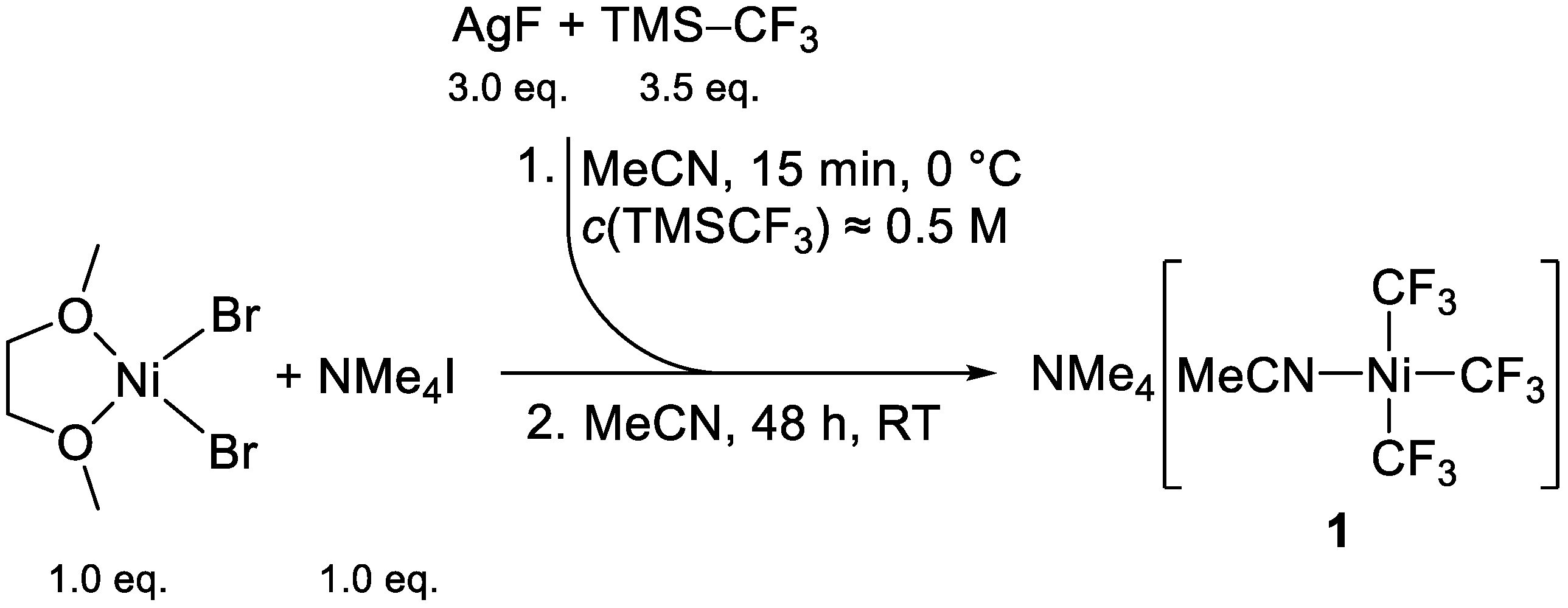
2.5. Mechanistic Considerations on the CF3 to C2F5 Conversion and Refined Reaction Procedure
3. Materials and Methods
3.1. NMR Spectroscopy
3.2. Single-Crystal X-ray Diffraction
3.3. General Synthesis Conditions
3.4. Materials
3.5. Synthetic Procedures
3.5.1. Preparation of (NMe4)[Ni(CF3)3(MeCN)] (1, Initial Procedure)
3.5.2. Preparation of (NMe4)[Ni(CF3)3(MeCN)] (1, Refined Procedure)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Purushotam, B.A.; Banerjee, D. Recent advances on non-precious metal-catalysed fluorination, difluoromethylation, trifluoromethylation, and perfluoroalkylation of N-heteroarenes. Org. Biomol. Chem. 2023, 21, 9298–9315. [Google Scholar] [CrossRef] [PubMed]
- Yerien, D.E.; Barata-Vallejo, S.; Postigo, A. Radical Perfluoroalkylation of Aliphatic Substrates. ACS Catal. 2023, 13, 7756–7794. [Google Scholar] [CrossRef]
- Baguia, H.; Evano, G. Direct Perfluoroalkylation of C–H Bonds in (Hetero)arenes. Chem.-Eur. J. 2022, 28, e202200975. [Google Scholar] [CrossRef] [PubMed]
- Deolka, S.; Govindarajan, R.; Vasylevskyi, S.; Roy, M.C.; Khusnutdinova, J.R.; Khaskin, E. Ligand-free nickel catalyzed perfluoroalkylation of arenes and heteroarenes. Chem. Sci. 2022, 13, 12971–12979. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Pazos, D.J.; Lasso, J.D.; Li, C.-J. Modern methods for the synthesis of perfluoroalkylated aromatics. Org. Biomol. Chem. 2021, 19, 7116–7128. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.P.; Philip, R.M.; Anilkumar, G. Nickel-catalysed fluoromethylation reactions. Catal. Sci. Technol. 2021, 11, 6317–6329. [Google Scholar] [CrossRef]
- Kananovich, D.G. Copper-Catalyzed Trifluoromethylation Reactions. In Copper Catalysis in Organic Synthesis, 1st ed.; Anilkumar, G., Saranya, S., Eds.; Wiley-VCH: Weinheim, Germany, 2020; Chapter 17; pp. 367–393. [Google Scholar]
- Meucci, E.A.; Nguyen, S.N.; Camasso, N.M.; Chong, E.; Ariafard, A.; Canty, A.J.; Sanford, M.S. Nickel(IV)-Catalyzed C–H Trifluoromethylation of (Hetero)arenes. J. Am. Chem. Soc. 2019, 141, 12872–12879. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Vicic, D.A. Transition-Metal-Catalyzed Difluoromethylation, Difluoromethylenation, and Polydifluoromethylenation Reactions. In Organometallic Fluorine Chemistry; Series: Topics in Organometallic Chemistry; Braun, T., Hughes, R.P., Eds.; Springer: Cham, Switzerland, 2015; Volume 52, pp. 113–141. [Google Scholar] [CrossRef]
- Wang, H.; Vicic, D.A. Organometallic Aspects of Fluoroalkylation Reactions with Copper and Nickel. Synlett 2013, 24, 1887–1898. [Google Scholar] [CrossRef]
- Deolka, S.; Govindarajan, R.; Khaskin, E.; Vasylevskyi, S.; Bahri, J.; Fayzullin, R.R.; Roy, M.C.; Khusnutdinova, J.R. Oxygen transfer reactivity mediated by nickel perfluoroalkyl complexes using molecular oxygen as a terminal oxidant. Chem. Sci. 2023, 14, 7026–7035. [Google Scholar] [CrossRef]
- DiMucci, I.M.; Titus, C.J.; Nordlund, D.; Bour, J.R.; Chong, E.; Grigas, D.P.; Hu, C.-H.; Kosobokov, M.D.; Martin, C.D.; Mirica, L.M.; et al. Scrutinizing formally NiIV centers through the lenses of core spectroscopy, molecular orbital theory, and valence bond theory. Chem. Sci. 2023, 14, 6915–6929. [Google Scholar] [CrossRef]
- Shreiber, S.T.; Puchall, G.I.; Vicic, D.A. Transformation of brucine into trifluoromethyl neobrucine using the homoleptic nickel catalyst [Ni(CF3)4]2−. Tetrahedron Lett. 2022, 97, 153795. [Google Scholar] [CrossRef]
- Shreiber, S.T.; Amin, F.; Schäfer, S.A.; Cramer, R.E.; Klein, A.; Vicic, D.A. Synthesis, structure, and electrochemical properties of [LNi(Rf)(C4F8)]− and [LNi(Rf)3]− complexes. Dalton Trans. 2022, 51, 5515–5523. [Google Scholar] [CrossRef] [PubMed]
- Shreiber, S.T.; Vicic, D.A. Solvated Nickel Complexes as Stoichiometric and Catalytic Perfluoroalkylation Agents. Angew. Chem. Int. Ed. 2021, 60, 18162–18167. [Google Scholar] [CrossRef] [PubMed]
- Shreiber, S.T.; DiMucci, I.M.; Khrizanforov, M.N.; Titus, C.J.; Nordlund, D.; Dudkina, Y.; Cramer, R.E.; Budnikova, Y.; Lancaster, K.M.; Vicic, D.A. [(MeCN)Ni(CF3)3]− and [Ni(CF3)4]2−: Foundations toward the Development of Trifluoromethylations at Unsupported Nickel. Inorg. Chem. 2020, 59, 9143–9151. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, A.C. Recent Development of Trifluoromethyl Reagents: A Review. J. Sci. Res. 2021, 13, 317–333. [Google Scholar] [CrossRef]
- Ravidas, C.M.T.; Shreiber, S.T.; Kletsch, L.; Klein, A.; Vicic, D.A. Nickel Perfluoroalkyl Complexes Supported by Simple Acetate Coligands. Organometallics 2024, 43, 706–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Synthesis and Application of Well-Defined Fluoroalkyl Zinc Complexes. Eur. J. Org. Chem. 2024, 2024, e202400307. [Google Scholar] [CrossRef]
- Tölke, K.; Porath, S.; Neumann, B.; Stammler, H.-G.; Hoge, B. Water-Stable Lewis Superacids as Precursors for (Pseudo)halogenogallate and -indate Salts. Eur. J. Inorg. Chem. 2024, 2024, e202400191. [Google Scholar] [CrossRef]
- Deolka, S.; Govindarajan, R.; Gridneva, T.; Roy, M.C.; Vasylevskyi, S.; Vardhanapu, P.K.; Khusnutdinova, J.R.; Khaskin, E. General High-Valent Nickel Metallocycle Catalyst for the Perfluoroalkylation of Heteroarenes and Peptides. ACS Catal. 2023, 13, 13127–13139. [Google Scholar] [CrossRef]
- Briand, M.; Anselmi, E.; Dagousset, G.; Magnier, E. The Revival of Enantioselective Perfluoroalkylation—Update of New Synthetic Approaches from 2015–2022. Chem. Rec. 2023, 23, e202300114. [Google Scholar] [CrossRef]
- Keßler, M.; Kapiza, M.; Stammler, H.-G.; Neumann, B.; Röschenthaler, G.-V.; Hoge, B. Triorganylphosphoranides: Realization of an Unusual Structural Motif Utilizing Electron Withdrawing Pentafluoroethyl Groups. ChemPlusChem 2023, 88, e202200436. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Xie, Q.; Wang, X.; Wang, Q.; Ni, C.; Hu, J. Copper-mediated pentafluoroethylation of organoboronates and terminal alkynes with TMSCF3. Chem. Commun. 2022, 58, 5156–5159. [Google Scholar] [CrossRef] [PubMed]
- Rachor, S.G.; Müller, R.; Wittwer, P.; Kaupp, M.; Braun, T. Synthesis, Reactivity, and Bonding of Gold(I) Fluorido−Phosphine Complexes. Inorg. Chem. 2022, 61, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Tiessen, N.; Keßler, M.; Neumann, B.; Stammler, H.-G.; Hoge, B. Evidence of the Lewis-Amphoteric Character of Tris(pentafluoroethyl)silanide, [Si(C2F5)3]−. Angew. Chem. Int. Ed. 2021, 60, 12124–12131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; MacMillan, D.W.C. Metallaphotoredox Perfluoroalkylation of Organobromides. J. Am. Chem. Soc. 2020, 142, 19480–19486. [Google Scholar] [CrossRef] [PubMed]
- Cobos, C.J.; Hintzer, K.; Sölter, L.; Tellbach, E.; Thaler, A.; Troe, J. Shock wave and modelling study of the dissociation pathways of (C2F5)3N. Phys. Chem. Chem. Phys. 2019, 21, 9785–9792. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Li, L.; Zhu, Z.; Zhang, R.; Ni, C.; Hu, J. From C1 to C2: TMSCF3 as a Precursor for Pentafluoroethylation. Angew. Chem. Int. Ed. 2018, 57, 13211–13215. [Google Scholar] [CrossRef] [PubMed]
- Mestre, J.; Castillón, S.; Boutureira, O. “Ligandless” Pentafluoroethylation of Unactivated (Hetero)aryl and Alkenyl Halides Enabled by the Controlled Self-Condensation of TMSCF3-Derived CuCF3. J. Org. Chem. 2019, 84, 15087–15097. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.; Li, L.; Ni, C.; Hu, J. Pentafluoroethylation of Arenediazonium Tetrafluoroborates Using On-Site Generated Tetrafluoroethylene. Chin. J. Chem. 2019, 37, 1131–1136. [Google Scholar] [CrossRef]
- Li, L.; Ni, C.; Xie, Q.; Hu, M.; Wang, F.; Hu, J. TMSCF3 as a Convenient Source of CF2=CF2 for Pentafluoroethylation, (Aryloxy)tetrafluoroethylation, and Tetrafluoroethylation. Angew. Chem. Int. Ed. 2017, 56, 9971–9975. [Google Scholar] [CrossRef]
- Sladojevich, F.; McNeill, E.; Börgel, J.; Zheng, S.-L.; Ritter, T. Condensed-Phase, Halogen-Bonded CF3I and C2F5I Adducts for Perfluoroalkylation Reactions. Angew. Chem. Int. Ed. 2015, 54, 3712–3716. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.; Young, R.J. Future challenges and opportunities with fluorine in drugs? Med. Chem. Res. 2023, 32, 1231–1234. [Google Scholar] [CrossRef]
- O’Hagan, D. Polar Organofluorine Substituents: Multivicinal Fluorines on Alkyl Chains and Alicyclic Rings. Chem.-Eur. J. 2020, 26, 7981–7997. [Google Scholar] [CrossRef]
- Fujiwara, T.; O’Hagan, D. Successful fluorine-containing herbicide agrochemicals. J. Fluor. Chem. 2014, 167, 16–29. [Google Scholar] [CrossRef]
- Prchalová, E.; Štěpánek, O.; Smrček, S.; Kotora, M. Medicinal Applications of Perfluoroalkylated Chain-Containing Compounds. Future Med. Chem. 2014, 6, 1201–1229. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.R. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 2007, 955–964. [Google Scholar] [CrossRef]
- Zhang, C.-P.; Wang, H.; Klein, A.; Biewer, C.; Stirnat, K.; Yamaguchi, Y.; Xu, L.; Gomez-Benitez, V.; Vicic, D.A. A Five-Coordinate Nickel(II) Fluoroalkyl Complex as a Precursor to a Spectroscopically Detectable Ni(III) Species. J. Am. Chem. Soc. 2013, 135, 8141–8144. [Google Scholar] [CrossRef]
- Tyrra, W.E. Oxidative perfluoroorganylation methods in group 12–16 chemistry: The reactions of haloperfluoroorganics and In and InBr, a convenient new route to AgRf (Rf = CF3, C6F5) and reactions of AgRf with group 12–16 elements. J. Fluor. Chem. 2001, 112, 149–152. [Google Scholar] [CrossRef]
- Wessel, W.; Tyrra, W.; Naumann, D. Synthese und Eigenschaften von Diethyldithiocarbaminsäureperfluororganylestern, (C2H5)2NC(S)SRf (Rf = CF3, C2F5, i-C3F7, n-C4F9, C6F5). Z. Anorg. Allg. Chem. 2001, 627, 1264–1268. [Google Scholar] [CrossRef]
- Harrison, D.J.; Daniels, A.L.; Guan, J.; Gabidullin, B.M.; Hall, M.B.; Baker, R.T. Nickel Fluorocarbene Metathesis with Fluoroalkenes. Angew. Chem. Int. Ed. 2018, 57, 5772–5776. [Google Scholar] [CrossRef]
- Kremlev, M.M.; Tyrra, W.; Mushta, A.I.; Naumann, D.; Yagupolskii, Y.L. The solid complex Zn(CF3)Br·2DMF as an alternative reagent for the preparation of both, trifluoromethyl and pentafluoroethyl copper, CuCF3 and CuC2F5. J. Fluor. Chem. 2010, 131, 212–216. [Google Scholar] [CrossRef]
- Wiemers, D.M.; Burton, D.J. Pregeneration, Spectroscopic Detection, and Chemical Reactivity of (Trifluoromethyl)copper, an Elusive and Complex Species. J. Am. Chem. Soc. 1986, 108, 832–834. [Google Scholar] [CrossRef]
- Demonti, L.; Saffon-Merceron, N.; Mézailles, N.; Nebra, N. Cross-Coupling through Ag(I)/Ag(III) Redox Manifold. Chem.-Eur. J. 2021, 27, 15396–15405. [Google Scholar] [CrossRef]
- APEX4—Software Suite for Crystallographic Programs; Bruker AXS, Inc.: Madison, WI, USA, 2021.
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. A Found. Adv. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Gomes, C.S.B.; Costa, S.I.; Silva, L.C.; Jiménez-Tenorio, M.; Valerga, P.; Carmen Puerta, M.; Gomes, P.T. Cationic R-Substituted-Indenyl Nickel(II) Complexes of Arsine and Stibine Ligands: Synthesis, Characterization, and Catalytic Behavior in the Oligomerization of Styrene. Eur. J. Inorg. Chem. 2018, 2018, 597–607. [Google Scholar] [CrossRef]
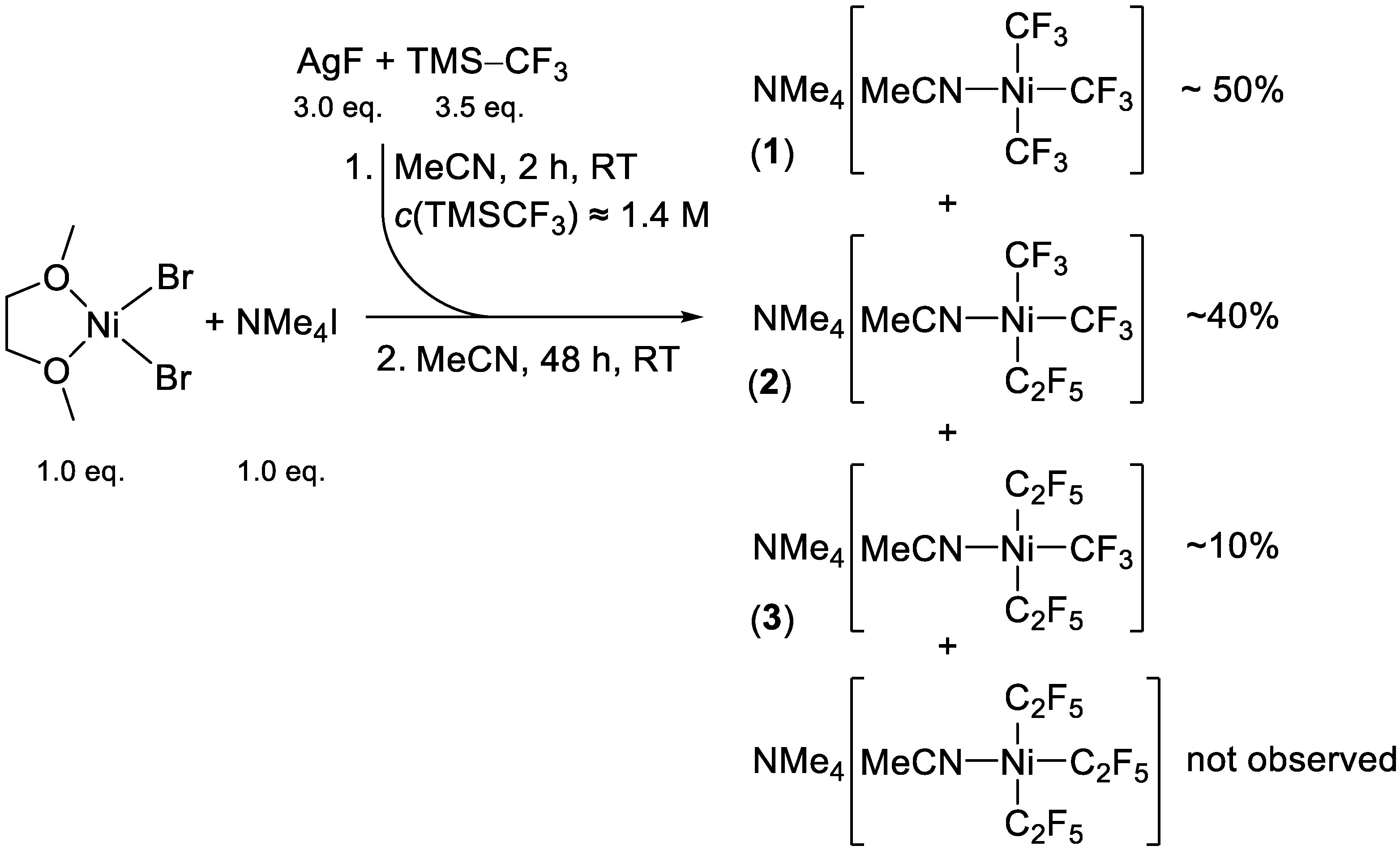


| 2 | P1 | 2 | P1 | ||
|---|---|---|---|---|---|
| Distances (Å) | Angles (°) | ||||
| Ni–C1 | 1.959(2) | 1.953(2) | N1–Ni–C1 | 88.86(6) | 89.43(8) |
| Ni–C4 | 1.948(2) | 1.938(2) | C1–Ni–C3 | 92.71(6) | 90.15(9) |
| Ni–C3 | 1.897(2) | 1.885(2) | C4–Ni–N1 | 89.48(6) | 90.28(8) |
| C3–Ni–C4 | 90.41(6) | 90.06(9) | |||
| Ni–N1 | 1.895(1) | 1.882(1) | N1−Ni1−C3 | 170.75(6) | 177.6(1) |
| C4−Ni1−C1 | 170.69(6) | 177.9(1) | |||
| 361.46(6) | 359.92(9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schäfer, S.A.; Jordan, R.; Klupsch, K.M.; Herwede, F.C.-H.; Klein, A. Synthesis of Tris(trifluoromethyl)nickelates(II)—Coping with “The C2F5 Problem”. Inorganics 2024, 12, 187. https://doi.org/10.3390/inorganics12070187
Schäfer SA, Jordan R, Klupsch KM, Herwede FC-H, Klein A. Synthesis of Tris(trifluoromethyl)nickelates(II)—Coping with “The C2F5 Problem”. Inorganics. 2024; 12(7):187. https://doi.org/10.3390/inorganics12070187
Chicago/Turabian StyleSchäfer, Sascha A., Rose Jordan, Katharina M. Klupsch, Felix Carl-Heinz Herwede, and Axel Klein. 2024. "Synthesis of Tris(trifluoromethyl)nickelates(II)—Coping with “The C2F5 Problem”" Inorganics 12, no. 7: 187. https://doi.org/10.3390/inorganics12070187
APA StyleSchäfer, S. A., Jordan, R., Klupsch, K. M., Herwede, F. C.-H., & Klein, A. (2024). Synthesis of Tris(trifluoromethyl)nickelates(II)—Coping with “The C2F5 Problem”. Inorganics, 12(7), 187. https://doi.org/10.3390/inorganics12070187








